Abstract
In many vertebrates, reproduction is regulated by social interactions in which dominant males control access to females and food. Subordinate males that displace dominant individuals must rapidly adopt behavioral and physiological traits of the higher rank to gain reproductive success. To understand the process of phenotypic plasticity during social ascent, we analyzed the temporal expression pattern of dominance behaviors and circulating androgen levels when socially-suppressed males of an African cichlid fish Astatotilapia burtoni ascended in status. These experiments tested a prediction of the ‘challenge hypothesis’ that, during periods of social instability, male androgen levels are higher than during socially stable times. We found that socially and reproductively suppressed males perform territorial and reproductive behaviors within minutes of an opportunity to ascend in status, and that animals switch from initial expression of territorial behaviors to more reproductive behaviors during territory establishment. Following this rapid response, social stability may be achieved within 1–3 days of social ascent. Consistent with predictions of the ‘challenge hypothesis’, circulating 11-ketotestosterone (11-KT) levels were elevated within 30 min following social opportunity, coincident with increased aggressive behavior. However, territorial behaviors and serum 11-KT levels were then dissociated by 72 hrs after social ascent, suggesting either rapid social stability and/or increased physiological potential for androgen production. This behavioral and physiological plasticity in male A. burtoni suggests that perception of social opportunity triggers a suite of quick changes to facilitate rapid transition towards reproductive success, and reveals important features of social ascent not previously recognized.
Keywords: 11-ketotestosterone, androgen, Astatotilapia burtoni, challenge hypothesis, dominance, reproductive behavior, social ascension, territorial behavior
Introduction
Social interactions can have profound effects on the behavior, physiology, and reproductive capacities of animals, especially in species where a dominance hierarchy is socially regulated. Typically in vertebrates, low social rank is associated with submissive behaviors and reduced reproductive opportunities, while high-ranking individuals show dominant behaviors and have high reproductive success (Creel et al., 2002; Ellis, 1995; Fernald, 2009; Ryder et al., 2009; Sapolsky, 2005). However, dynamic social interactions and changing physical habitats due to disturbance or variable environmental conditions offer subordinate individuals opportunities to displace higher-ranking dominant ones. Such shifts in social status can be accompanied by dramatic changes in the brain that may occur more slowly than changes in behavior and physiology (Burmeister et al., 2005; White et al., 2002). What is the precise timing of the changes in these phenotypic traits? Here we asked how quickly behavioral and physiological changes occur during social transitions. We exploited a particularly useful model system, the highly social African cichlid fish, Astatotilapia burtoni, to test how perception of social opportunity influences changes in behavior and circulating steroid levels.
Astatotilapia burtoni is endemic to Lake Tanganyika, Africa where it lives in shallow shore pools in a lek-like social system. Males exist in one of two phenotypes: 1) dominant territorial males (~10–30% of population) are brightly colored, aggressively defend a spawning territory, and actively court and spawn with females; and 2) subordinate non-territorial males resemble and school with females, express submissive behaviors, and do not court females (Fernald and Hirata, 1977). Males can rapidly and reversibly switch between dominant and subordinate states depending on the composition of the social environment, and such transformations produce a suite of behavioral and physiological changes (Fernald, 2009). When subordinate males ascend in social status, they intensify their body coloration and increase dominance behaviors within minutes (Burmeister et al., 2005). Further, within 20 minutes, the immediate early gene egr-1 is up-regulated in the gonadotropin-releasing hormone neurons (GnRH1) located in the preoptic area of the brain (Burmeister et al., 2005). These neurons are the neural gateway to the entire reproductive axis in all vertebrates. In contrast to this rapid behavioral and brain genomic response, other physiological changes such as increases in GnRH1 neuron size, GnRH1 synthesis, and testis growth can take up to a week or more to achieve dominant male levels (White et al., 2002). It may be advantageous for subordinate males to quickly display dominance coloration and behaviors to secure a territory in a dynamic social environment even though their reproductive axis may be up-regulated more slowly.
What role do androgens play in this status transformation? Androgens are known as critical regulators of male reproduction in all vertebrates, typically increasing concomitantly with reproductive-related behavior and physiology. However, circulating androgen levels are also influenced by social interactions with conspecifics, or by watching the interactions of others (Cardwell and Liley, 1991; Dzieweczynski et al., 2006; Hirschenhauser and Oliveira, 2006; Hirschenhauser et al., 2004; Oliveira et al., 2002; Oliveira et al., 2001). Generally, androgen levels are higher in dominant compared to subordinate individuals within a population (Oliveira, 2009; Oliveira et al., 2002; Parikh et al., 2006), but the relationship between androgen levels and social status also depends on other factors. For example, Wingfield et al (1990) proposed the ‘challenge hypothesis’ which predicts that androgen levels also depend on aggressive interactions over status rank and the stability of the social environment. In A. burtoni, dominant males have higher circulating levels of both testosterone (T) and the important fish androgen 11-ketotestosterone (11-KT) compared to subordinates (Parikh et al., 2006). Moreover, intramuscular injections of T can increase aggressive behaviors among males (Fernald, 1976). These data are consistent with the prediction that stable dominant males involved in more aggressive interactions defending their territory will have higher circulating androgens that can also directly influence behavior. However, previous studies measured androgen levels only in stable male phenotypes and little is known about how dominance behaviors and androgen levels change during times of social transition and instability.
Another prediction of the ‘challenge hypothesis’ is that male androgen levels should be higher during periods of social instability (i.e., during territory establishment and status changes within a dominance hierarchy) when compared to more stable conditions. We therefore predicted that aggression levels would be correlated with circulating androgen levels when fish recognize an opportunity to ascend in social rank, but that aggressive behaviors and androgens will be dissociated as the social environment temporally stabilizes. In A. burtoni, we can control precisely social stability and the timing of social transitions, making it an ideal model to test predictions of the ‘challenge hypothesis’. However, A. burtoni also differs from many previous models used to examine the ‘challenge hypothesis’ because 1) it is not a seasonal breeder, but rather, reproductive fitness is dictated by social status, and the ability to reproduce depends on both male-male territorial and male-female sexual interactions; and 2) subordinate males have small testes in relation to their body size, which poses a potential physiological constraint to androgen production. These variables represent two of the recently proposed factors to include in future tests of the ‘challenge hypothesis’, namely, the androgen response to male-female interactions, and the physiological potential to produce and secrete androgens (Goymann et al., 2007).
Although Burmeister et al. (2005) showed previously that ascending subordinate A. burtoni males display dominance behaviors within minutes, these data were sampled only during the 20 minutes following the first dominance behaviors because the newly ascended males were then sacrificed to measure immediate early gene mRNA levels. Thus, the expression of territorial and reproductive behaviors over subsequent days as the ascended males’ status stabilized was unknown. Burmeister et al. (2005) also described for the first time that even dominant males apparently began their typical aggressive behaviors anew each day. Therefore, the goal of this study was to examine in detail the temporal expression pattern of dominance behaviors and circulating androgen levels when socially-suppressed A. burtoni males ascended in status. This allowed us to test a prediction of the ‘challenge hypothesis’ that during periods of social instability and territory establishment, male androgen levels are higher than during times of social stability.
Methods
Animals
Laboratory-bred male cichlid fish Astatotilapia burtoni, derived from wild-caught stock collected in Lake Tanganyika, Africa, in the 1970’s were maintained in aquaria under environmental conditions that mimic their natural equatorial habitat (28°C; pH 8.0; 12h light:12h dark with full spectrum illumination; constant aeration), and fed cichlid pellets and flakes (AquaDine, Healdsburg, CA, USA) each morning. Aquaria contained gravel-covered bottoms with half terra cotta pots that served as spawning territories. All experimental procedures were approved by the Stanford Administrative Panel for Laboratory Animal Care.
Social manipulation
We used a paradigm similar to that described by Burmeister et al. (2005) to create an opportunity for social ascent by subordinate males, except that our subjects were previously suppressed for 4–5 weeks rather than 2 weeks (Fig. 1). The 4–5 week period was chosen to ensure full suppression of the entire reproductive axis, and mimics social situations in the wild and the laboratory (Fernald and Hirata, 1977; Hofmann et al., 1999). All subject males used in this study were dominant for at least 3 weeks prior to use in experiments, but other details on past breeding history was unknown. To create socially and reproductively suppressed males for the ascension paradigm, larger dominant males were used to suppress smaller subject males. Specifically, dominant subject males (standard length: 6.23 ± 0.52 cm; body mass: 7.52 ± 0.21 g) from community tanks were placed into aquaria for 4–5 weeks with several larger dominant suppressor males (2–4; SL ~7.5–9.0 cm), females (6–10; SL ~4.5–6.5 cm), and subordinate males (3–4; SL ~5.0–6.5 cm). Suppression of subject fish was verified by focal observations to confirm subordinate behaviors (e.g., fleeing) and submissive coloration as previously described (Burmeister et al., 2005). At the end of the suppression period, subjects were moved into the central compartment of an experimental tank that contained one larger resident dominant male (~7.5–9.0 cm) and 4 females (~5.0–6.5 cm). This central compartment was isolated from community tanks on either side that contained multiple dominant males, subordinate males, and females, with transparent acrylic barriers so that fish could interact visually but not physically. All dominant males in adjacent community tanks were smaller in size than the suppressed subject male to ensure his ascension upon presentation of social opportunity.
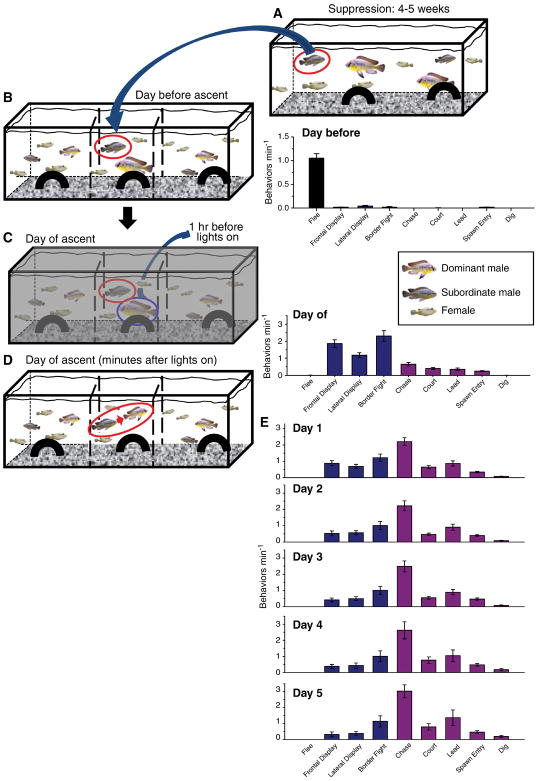
Fig. 1.
Schematic representation of the experimental paradigm and resulting expression of behaviors of suppressed Astatotilapia burtoni males before, during, and after social ascent. A) Previously dominant subject males (circled in red) were placed into community suppression tanks for 4–5 weeks that contained several large dominant males, subordinate males, and females. B) Suppressed subject males were then transferred to a central compartment of an experimental tank for 2 days prior to social opportunity. The central compartment contained a larger resident dominant male and 3–4 females, and was separated with transparent acrylic barriers (dashed lines) on either side from community tanks containing females, subordinate males, and dominant males that were smaller in size than the subject fish. Fish were filmed on the day before social ascent and showed primarily submissive fleeing behaviors (black bars). C) On the day of social ascent, the resident dominant male was removed from the central compartment 1 hr before the lights turned on in the morning. D) At light onset, ascension was filmed and submissive, territorial (blue bars) and reproductive (pink bars) behaviors were quantified. E) Number of behaviors min−1 for submissive, territorial and reproductive behaviors were quantified for 5 days after the day of social ascent. Data are plotted as mean behaviors min−1 ± SE.
Subject males remained in the experimental tank for 2 days during which time we confirmed with behavioral observations that they remained suppressed by the larger resident male (e.g., performed no territorial or reproductive behaviors, and fled from suppressor male). On the day of ascension, the resident suppressor male was removed with a net in the dark 1 hr prior to light onset using infrared night vision goggles. This protocol minimized disturbance in the tank and meant that visual absence of the suppressor occurred consistently only at light onset for all tested individuals.
Stable dominant and stable subordinate males were also used as control comparisons to males ascending in social status. Stable subordinate males were suppressed in community tanks for 4–5 weeks and transferred to the experimental tank as described above. On the day of ascension, the net was dipped into the tank prior to light onset to replicate any disturbance caused by catching the resident male. The dominant resident was not removed however, which kept the subject male in subordinate status. Stable dominants were dominant males that maintained their status in community tanks for 4–5 weeks, and were then placed in the experimental tank with four females but no larger resident male, where they maintained their dominance status. On the stimulus day, a net was dipped into the water prior to light onset to simulate resident male removal. Stable subordinate and dominant animals were all sacrificed on the simulated stimulus day at 30 min after they displayed dominance behaviors at a rate of 3 min−1, as described below for ascending males.
Behavioral analyses
Behavior in the experimental aquarium was recorded for 45 min for later quantification beginning at light onset in the morning (digital video camera, Sony HDR) on the day before, day of, and for each of 5 days after social ascent. Videos were scored by observers without knowledge of the fish identification number or time after ascent (e.g., day of or days 1–5). Latency to time of ascent was measured as the time between light onset and the time when males performed dominance behaviors at a rate of 3 behaviors min−1. The first type of behavior performed after light onset was also recorded for each individual.
Nine different behaviors were quantified (see (Fernald, 1977): one submissive behavior (fleeing); three territorial or agonistic behaviors (frontal displays, lateral displays, and border fights); and five reproductive behaviors (chasing, courtship displays, leading, spawn site entries, and digging). Fleeing was defined as a rapid retreat swim from an approaching dominant male or female. Frontal displays were defined as head-on encounters with neighboring males with associated flaring of the gill opercula. Lateral displays were classified as presentations of the side of the body to another fish with erect fins, flared opercula, and body quivering. Border fights were interactions with neighboring dominant males across the acrylic barrier typified by head-on lunges and rams against the barrier with an open mouth. Chasing was defined as rapid swims directed towards another fish, and was grouped with reproductive behaviors here because they were only directed towards females within the same compartment, and chases are a normal component of the courtship repertoire. Courtship displays were defined as rapid quivers by the male with presentation of the anal fin egg spots, and leading was defined as swimming towards the shelter accompanied by back-and-forth motions of the tail (waggles) as the male attempted to lead females towards the spawning territory. We defined spawning site entries as any time the subject male entered the half terra cotta pot that served as a spawning shelter at the center of his territory, and digging as any time the male emerged from the pot with a mouth full of gravel to deposit outside the shelter.
We quantified behavioral rate (behaviors min−1) for 35 min after light onset for each day and compiled them into 5-min bins to examine their temporal patterns. We also calculated mean behavior rates for each of the nine quantified behaviors per day to test whether there were overall differences in types of behavior on the days following social opportunity, or between ascending males and control stable dominant males.
Blood samples and androgen hormone measurement
All stable dominant, subordinate, and ascending males were anesthetized in ice-cold tank water, measured for standard length (±1 mm), weighed (± 0.001 g), and blood samples collected by caudal severance within 2 min of capture into 100 μl heparinized capillary tubes. Ascending males were sampled at 30 min, 6 hr, 24 hr, 72 hr, and 120 hr after social ascent. Blood samples were centrifuged for 10 minutes (8000 rpm) and plasma removed and stored at −80°C until assayed. Paired testes were also removed, weighed, and gonadosomatic index calculated [GSI = (gonad mass/body mass) *100]. Plasma samples (5 μl) were extracted three times with 200 μl of ethyl ether (extraction efficiency 87–89%), and then reconstituted in assay buffer (1:40 dilution) prior to analysis. Circulating levels of the fish androgen 11-ketotestosterone (11-KT) were then measured by strictly following the protocol from a commercially available enzyme-linked immunoabsorbant assay (EIA) kit (Cayman Chemical, Inc.). 11-KT was chosen for analysis because it is thought to be the behaviorally-relevant androgen in teleost fishes and is often tightly correlated with agonistic and reproductive behaviors (Hirschenhauser et al., 2004; Kime, 1993). Further, previous studies showed that 11-KT levels were higher in stable dominant male A. burtoni compared to subordinate males (Parikh et al., 2006), but circulating androgen levels during social ascent are unknown. All samples were assayed in duplicate, plates were read at 405 nm using a microplate reader (UVmax Microplate Reader, Molecular Devices), and hormone levels determined based on a standard curve. Intra-assay coefficients of variation (CV) were 4.0%, 3.2%, and 4.8%, inter-assay CV was 7.2%, and assay validation was performed previously (Maruska and Fernald, in review; Parikh et al., 2006).
Statistical analyses
Data that met the assumptions of parametric statistics (e.g., normal distribution and equal variance) were compared with either Student’s t-tests (two groups) or one-way analysis of variance (ANOVA) (three or more groups), while data that could not be normalized by transformation were compared with non-parametric Mann-Whitney rank sum tests or Kruskal-Wallis tests (KW). For consistency however, data are plotted and reported as means ± standard error of the mean with the appropriate statistical test indicated in the text. We chose to compare the behavior rates across the different sampling periods with the non-parametric KW rather than the repeated measures ANOVA because the data were not normally distributed, and behavioral observations were only repeated on some individuals, creating missing values on subsequent days that precluded use of non-parametric rank repeated measures tests. Statistical comparisons were performed with SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA.).
Results
Behavioral response to perception of social opportunity
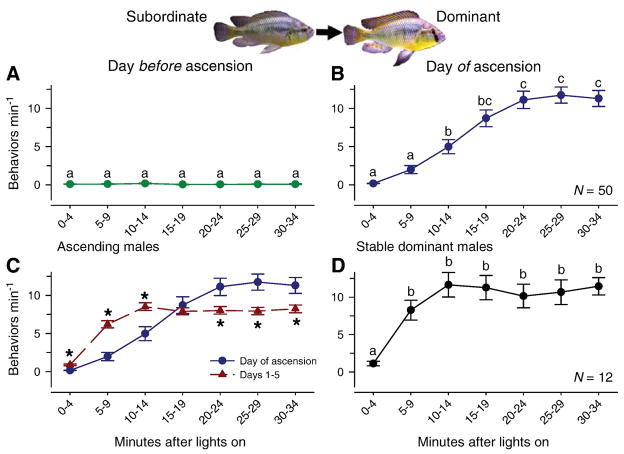
Suppressed subordinate subject fish displayed submissive behaviors (fleeing) and almost no dominance behaviors (frontal displays, lateral displays, border fights, chasing, courtship displays, leading, spawn site entries, or digging) on the day prior to social ascent (Figs. 1,2A). However, when the lights came on after removal of the dominant resident suppressor male, subjects showed a rapid and steady increase in rates of behavior that typically reached ~ 5 behaviors min−1 by 10–14 min, and further increased to over 10 behaviors min−1 by 20 min after light onset (Figs. 1,2B) (KW p < 0.001, H = 140.98, Dunn’s test p < 0.05). The mean latency to ascend (e.g., reach a rate of 3 behaviors min−1) was 12.73 ± 1.2 min (N = 50) after light onset.
Fig. 2.
Ascending A. burtoni males display dominance behaviors within minutes of the perception of social opportunity. A) Subordinate non-territorial males show essentially no territorial or reproductive behaviors on the day before ascension. B) Suppressed males rapidly increase dominance behaviors within minutes after light onset when presented with an opportunity to ascend in social status by removal of the dominant resident male. C) Ascending males show reduced rates of dominance behaviors on the day of ascension compared to subsequent days (days 1–5) for the first 15 min after light onset, but higher rates from 20–35 min. D) Stable dominant control males also showed a rapid increase in dominance behaviors by 5–9 min after light onset, which was then maintained throughout the sampling period. Data are plotted as mean dominance behaviors (reproductive and territorial behaviors combined) min−1 ± SE compiled into 5-min bins. Time points with different letters in A, B, and D are statistically different at p < 0.05, and asterisks in C indicate differences between behavior rates on the day of ascension compared to days 1–5 after ascension at each time period. Note that some error bars are obscured by symbols.
Since there was no difference in the rates of behavioral acts at different times after light onset across days 1 through 5 for ascending males (KW, p > 0.05), we pooled these data and compared them to behavior rates on the day of ascent (Fig. 2C). Ascended males showed a more rapid increase in behaviors on the days following the perception of social opportunity compared to the day of ascension from 0 to 14 min after light onset (Mann-Whitney Rank sum test, p < 0.001 for 0–4, 5–9, and 10–14 min). In contrast, dominance behavior rates on the day of ascent were greater than on days 1–5 from 20 to 35 min (20–24 min, p = 0.027; 25–29 min, p = 0.002; 30–34 min, p = 0.012).
Control stable dominant males also began their typical behaviors anew each day, as previously described by Burmeister et al. (2005), but they showed a more rapid increase in behavior rates compared to ascending males, typically reaching levels of 7–8 behaviors min−1 by 5–9 min after light onset (Fig. 2D). The mean latency for stable dominant males to achieve a rate of 3 behaviors min−1 was 5.3 ± 0.68 min (N = 12), which was about twice as fast as newly ascending males (Mann-Whitney, U = 53.5, p < 0.001).
On the day of ascent, the first behavior performed by the majority (88%) of ascending subject males was to enter the terra cotta pot that served as a spawning shelter in the center of the territory (Fig. 3). A few fish (10%) performed a courtship display as the first behavior, and only one (2%) performed any type of territorial behavior. Similarly, stable dominant males also entered the pot as the first behavior after light onset (75%), while the remaining 25% first performed a courtship display. Thus for both ascending and stable dominant males, 98–100% of the individuals first performed a reproductive behavior after light onset.
Fig. 3.
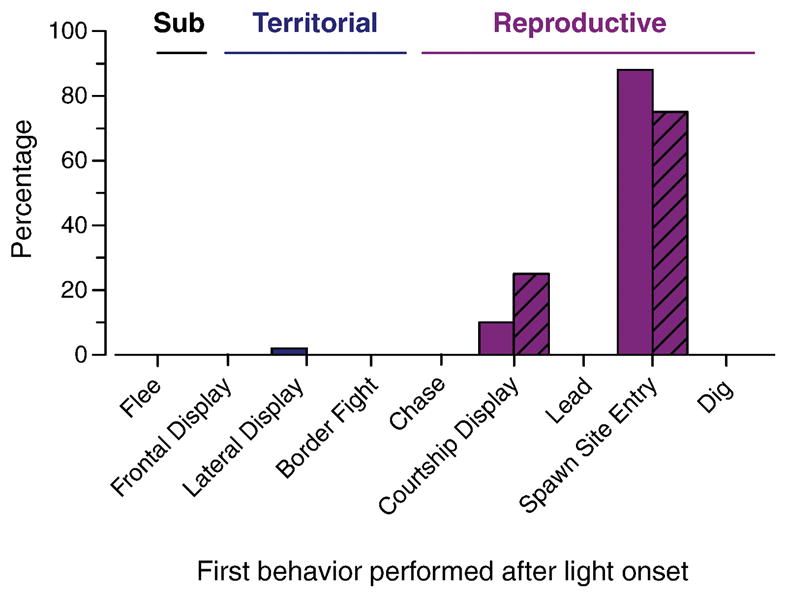
Ascending and control stable males both enter the spawning shelter as their first behavior performed after light onset. Approximately 88% of ascending fish and 75% of control stable dominant males first entered the terra cotta pot that serves as a spawning shelter. A smaller percentage of both ascending (10%) and stable dominant (25%) males first performed a courtship display, and only a single ascending male displayed any type of territorial behavior. Solid bars represent ascending males (N = 50) and hatched bars represent control stable dominant males (N = 12).
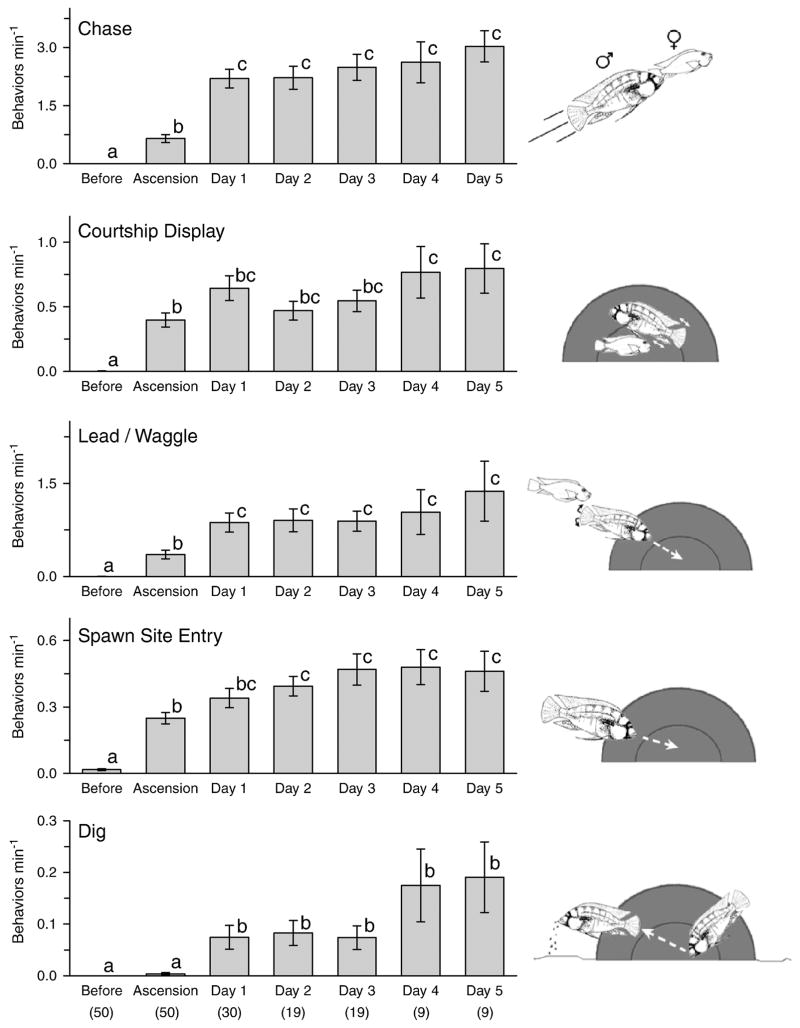
To test whether specific behavior rates changed over successive days after social ascent, we examined each of the nine quantified behaviors separately on the day before, day of, and for 5 days after ascension (Figs. 1, 4 and 5). Fleeing was the most common behavior on the day before social ascent, but was essentially absent on all subsequent days (KW, H = 153.40, p < 0.001, Dunn’s, p < 0.05). The territorial behaviors including frontal displays, lateral displays, and border fights, were elevated on the day of ascent compared to the day before, but then decreased by day 1 and were maintained at lower rates through day 5 as territorial defense stabilized (KW: frontal display, H = 83.16, p < 0.001; lateral display, H = 76.97, p < 0.001; border fight, H = 76.56, p < 0.001; Dunn’s, p < 0.05) (Fig. 4).
Fig. 4.
Total number of behaviors min−1 for each quantified submissive and territorial behavior on the day before, day of, and for 5 days after social ascent. Fleeing was the primary behavior on the day before ascension, while territorial frontal displays, lateral displays, and border fights increased dramatically on the day of ascent but decreased over subsequent days. Schematic line drawings at right illustrate each behavior. Bars with different letters are statistically different at p < 0.05. Data are plotted as mean ± SE and sample sizes are indicated in parentheses.
Fig. 5.
Total number of behaviors min−1 for each reproductive-related behavior on the day before, day of, and for 5 days after social ascent. All reproductive behaviors increased on the day of ascension, with the exception of digging which increased the following day (day 1). Several other behaviors (chasing, courtship displays, leading) also showed a further increase one day after ascension. Schematic line drawings at right illustrate each behavior, (gray area indicates the half terra cotta pot used as a spawning shelter). Bars with different letters are statistically different at p < 0.05. Data are plotted as mean ± SE and sample sizes are indicated in parentheses.
Reproductive behaviors including chasing (towards females), courtship displays, leading, and spawn site entries, were all higher on the day of social ascent than on the day before (Fig. 5). The rate of chasing also increased by two-fold on the day after ascension (day 1) and remained at this higher level through day 5 where rates achieved a three-fold increase over the day of ascension (KW, H = 124.50, p < 0.001, Dunn’s, p < 0.05). Courtship displays were also elevated on days 1–5, but were statistically higher than the day of ascension only on days 4 and 5 (KW, H = 100.19, p < 0.001, Dunn’s, p < 0.05). Tail waggles and leads towards the spawning shelter doubled on day 1 compared to the day of ascension, and remained at this rate or higher through day 5 (KW, H = 97.88, p < 0.001, Dunn’s, p < 0.05). Ascended males also showed increased rates of spawn site entries in the days following social ascent (KW, H = 107.57, p < 0.001, Dunn’s, p < 0.05). Digging was the only behavior that did not show a statistical increase between the day before and the day of ascension, but there was a ten-fold increase in this behavior on the day after ascension that persisted through day 5 (KW, H = 68.66, p < 0.001, Dunn’s, p < 0.05).
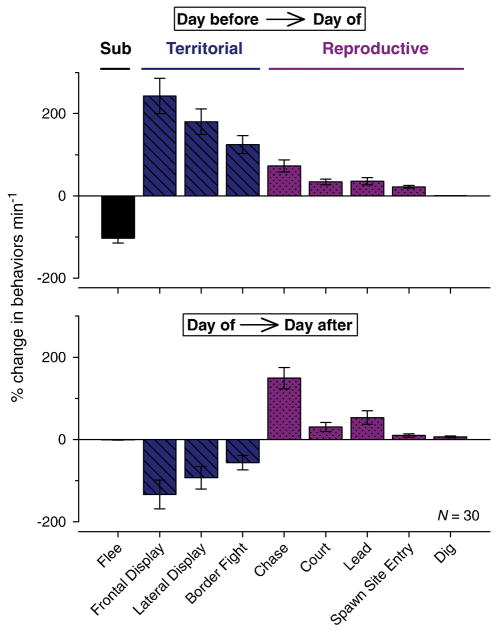
How do the rates of territorial versus reproductive behaviors change over time as newly ascended males achieve dominance status? To understand the transition from territorial defense to reproductive behavior in recently ascended males, we compared the percent change in rate of each behavior between the day before and the day of ascent, and also between the day of ascent and day 1 after ascent. This analysis showed that in addition to the rapid and dramatic increase in all dominance behaviors on the day of ascent compared to the day before, there was also a shift in behavior type on the first day after ascent (Fig. 6). The rate of territorial behaviors (frontal displays, lateral displays, border fights) showed a 50–125% decrease on the day after ascension (i.e., day 1), while reproductive behaviors (chasing, leading, spawn site entries, and digging) showed a 10–125% further increase on day 1 compared to the day of ascension. Thus the rate of territorial behaviors was highest on the day of ascension, while reproductive-related behaviors were more pronounced by only one day after the newly ascended fish achieved a higher social rank. There were no further significant behavioral shifts over the subsequent 4-day period (see Figs. 4, 5).
Fig. 6.
Ascension to dominance status is associated with a switch from initial territorial to more reproductive behaviors by one day after social ascent. There is a decrease in submissive fleeing (black bar) and an increase in all territorial (blue hatched bars) and reproductive (pink stippled bars) behaviors from the day before ascension compared to the day of ascension (top graph). However, there is a subsequent decrease in territorial frontal displays, lateral displays, and border fights, but a further increase in reproductive-related chasing, courtship, leading, spawn site entries, and digging between the day of ascension and one day after ascent. Data are plotted as the mean percent change in behaviors min−1 ± SE, where positive values indicate relative increases and negative values indicate relative decreases in behavior between days.
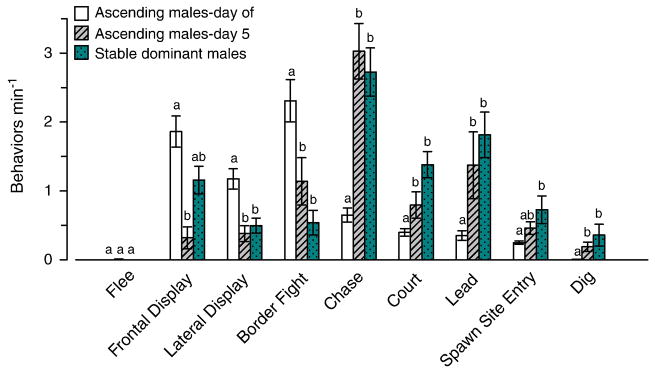
To test whether behavior patterns differed between ascending and stable dominant males, we compared the total rates of specific behaviors for stable dominant males to the behaviors of ascending males on the day of ascent and on day 5 after ascent (Fig. 7). This analysis showed that ascending males on the day of ascent displayed more territorial behaviors and fewer reproductive behaviors compared to both stable dominant males and ascending males on day 5 after ascent (KW, all p < 0.05, Dunn’s, all p < 0.05 except frontal display and spawn site entry). However, there was no difference in rates of any reproductive or territorial behavior between ascending males on day 5 after ascent compared to stable dominant males (all p > 0.05), suggesting stability of dominance behaviors by 5 days after ascent (Fig. 7).
Fig. 7.
Comparison of the total behaviors min−1 per day among stable dominant males, and individual ascending males on the day of and 5 days after ascent. Ascending males on the day of ascent displayed more territorial, but fewer reproductive behaviors compared to stable dominant males. There was also no difference in behavior rates between ascending males 5 days after ascent and stable dominant males, suggesting social stability was achieved by 5 days. Data are plotted as mean ± SE behaviors min−1 (behavior rates for the same individual ascending males on the day of ascent and on day 5 after ascent were used, N = 9; stable dominant males, N = 12). Bars with different letters within each behavior are statistically different at p < 0.05.
Gonadal and hormonal response to perception of social opportunity
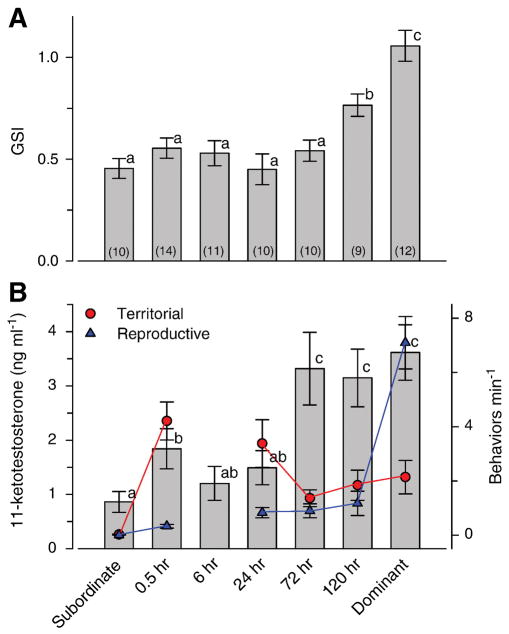
Gonadosomatic index, an indicator of reproductive investment, was elevated by 5 days after a suppressed male was given an opportunity to ascend, although it had not yet reached the higher level found in stable dominant individuals (ANOVA, F = 13.15, p < 0.001; Tukey’s test, p < 0.05) (Fig. 8A).
Fig. 8.
Gonadosomatic index, circulating 11-ketotestosterone levels, and territorial and reproductive behaviors for stable subordinate, stable dominant, and ascending males at different times after perception of social opportunity. A) Gonadosomatic index (GSI) is elevated by 120 hrs after perception of social opportunity, but has not yet reached the higher stable dominant levels. GSI is also two-fold higher in stable dominant compared to subordinate males. B) Circulating 11-ketotestosterone (11-KT) levels (bars) were elevated by 30 min after social ascent, and reached stable dominant levels by 72 hrs. The total territorial behaviors (frontal displays, lateral displays, border fights) min−1 (red circles) and reproductive behaviors (courtship displays, leads, spawn site entries, digs) min−1 (blue triangles) for each day from these same individuals are overlaid on this plot and referenced to the right y-axis (note there are no behavioral measures for the 6 hr blood sampling time). Bars for GSI and 11-KT with different letters are statistically different at p < 0.05. Data are plotted as mean ± SE and sample sizes are indicated in parentheses.
Circulating 11-KT levels were elevated above subordinate male levels at 30 min after ascent, remained relatively stable for the first 24 hrs, and then increased two-fold by 72 hrs after ascent to reach stable dominant levels (Fig. 8B) (ANOVA, F = 6.91, p < 0.001; Tukey’s test, p < 0.05). Stable dominant male 11-KT levels (3.62 ± 0.51 ng ml−1) were also approximately three-fold higher than those of subordinate males (0.86 ± 0.19 ng ml−1).
Relationship between circulating androgen levels and behavior
To determine whether the plasma 11-KT levels were related to aggressive territorial (pooled measures of frontal displays, lateral displays, and border fights) and reproductive (pooled measures of chases, courtship displays, leads, spawn site entries, digging) behaviors, we overlaid the androgen plot with the mean number of territorial and reproductive behaviors min−1 at each time point for these same individuals (Fig. 8B). This analysis showed that territorial behaviors, but not reproductive behaviors, were also elevated at 30 min after ascent (KW, H = 26.8, p < 0.001; Dunn’s test, p < 0.05), coincident with the increase in 11-KT levels. Territorial behaviors remained high at 24 hrs and then decreased from 72–120 hrs, despite the increase in circulating 11-KT levels during this same time. Reproductive behaviors did not differ from 30 min to 120 hrs (p > 0.05). In stable animals, subordinate males showed no aggressive or reproductive behaviors and had low 11-KT levels. Dominant males displayed higher rates of aggressive behaviors compared to subordinates, high 11-KT levels that were not different from ascending fish at 72–120 hrs, and the highest rate of reproductive behaviors measured for any group (KW, H = 26.8, p < 0.001; Dunn’s test, p > 0.05).
When data from all ascending and stable animals were analyzed together, there was an overall positive correlation between individual males’ circulating 11-KT levels and their rate of total dominance behaviors (pooled territorial and reproductive) (r = 0.41, p = 0.001), as well as total reproductive behaviors alone (chase, courtship display, lead, spawn site entry, dig) (r = 0.45, p < 0.001), but not total territorial behaviors alone (frontal display, lateral display, border fights). In addition, 11-KT levels were positively correlated with all individual reproductive-related behaviors: chasing (r = 0.36, p = 0.005), courtship displays (r = 0.40, p = 0.002), leading (r = 0.39, p = 0.002), spawn site entries (r = 0.40, p = 0.002), and digging (r = 0.35, p = 0.007), but were not correlated with any of the individual territorial behaviors (all r < 0.14, p > 0.05). However, since these patterns were likely driven by the near-zero levels in subordinates and very high levels in dominants, we also tested for correlations when stable subordinate and stable dominant animals were removed from the analysis. In this case, 11-KT levels were not correlated with total dominance behaviors or any single behavior (reproductive or territorial) in the individual ascending animals (all p > 0.05). Similarly, 11-KT levels were positively correlated with GSI in all animals together (r = 0.33, p = 0.004), but not in individual ascending animals alone (r = 0.14, p = 0.347).
Discussion
Our data show that socially and reproductively suppressed male A. burtoni begin territorial and reproductive behaviors within minutes of an opportunity to ascend in social status, that animals switch from initial expression of territorial behaviors to more reproductive behaviors during territory establishment, and that social stability may be achieved quickly within 1–3 days of social ascent. Further, measures of circulating 11-KT levels in both stable and socially transitioning males show that an individual’s androgen levels may result from diverse mechanisms at different time-points (e.g., social interactions immediately after opportunity vs. physiological potential of a larger more developed testis at later times that could support greater androgen production). These results can be understood in relation to the life-history traits of A. burtoni.
Temporal expression of behaviors in ascending males
The present study provides new information on several important aspects of the behavior of socially ascending male A. burtoni. First, we show that after the lights turn on in the morning, both newly ascended males and stable dominant males first enter the pot that serves as a spawning shelter before performing any other behaviors (see Fig. 3). For established males, pot entry may signal territory ownership. For ascending males, pot entry may also be an attempt to verify the absence of the resident dominant male, and in fact, many males emerged from their first pot entry with a visible increase in body coloration and their black eye-bar expressed.
Second, we also show that while both ascending and stable dominant males began typical behaviors anew each day as previously reported (Burmeister et al., 2005), the latency and rate of behaviors differed between them. For example, ascending males on the day of ascent showed dominant behavior rates after light onset that were slower than those of stable dominant males, but increased to near dominant male rates by the following day (i.e., day 1; see Fig. 2). This indicates that given the opportunity, subordinate males can acquire a territory extremely rapidly and that stability of social status may also be achieved quickly (i.e., within the first 1–3 days). However, this rapid temporal stability may be due in part to the fact that ascending males were prevented from physically interacting with neighbor males by the transparent barrier, or because of the size differential between the subject and neighboring males. In a previous study that used community tanks of many size-matched A. burtoni males, Fox et al. (1997) found that the stability index, reflecting stable defense of territories, was not elevated until 7 weeks after community establishment, and that stability depended on the density and composition of the tank. However, since the subject ascending males used in our study were always larger than the neighboring dominant males, and the bordering community tanks were stable for > 7 weeks, it is likely that the ascended male would still have quickly achieved dominance and held his territory even if physical contact were allowed.
Third, we found that stable dominant A. burtoni males also achieved a rate of 3 behaviors min−1 twice as quickly after light onset than did ascending males. This distinction was not observed in the previous study by Burmeister et al. (2005), and the mean latency to ascend was shorter in that study (~7 min) compared to our mean of ~13 min. These differences may be due to either the larger sample size used in the present study, or longer suppression time (4–5 weeks vs. 2 weeks). Nevertheless, it is clear that suppressed subordinate males are capable of quickly perceiving a social opportunity and taking advantage of it within minutes.
Finally, our study showed that ascending males on the day of ascent performed more territorial aggressive behaviors but fewer reproductive behaviors compared to stable dominant males (see Fig. 7). These results are similar to the Burmeister et al. (2005) study where ascending males showed higher rates of threat displays (frontal and lateral displays combined) compared to stable dominant animals, but our data differ from that study because they found no other differences in behaviors between ascending and dominant males, while we observed several (see Fig. 7). The higher levels of territorial behaviors seen here in ascending males suggests that they need to assert their dominance status quickly by aggressive interactions with neighboring males in order to secure the newly vacated territory. Once dominance is established, males then spend more time on reproductive activities, similar to that typically seen in stable dominant males. This is further supported by the overnight shift from territorial to reproductive behaviors observed between the day of and day after social ascent (see Fig. 6), and the similar behavior rates between ascended males on day 5 compared to stable dominants (see Fig. 7). Stable dominant males likely show lower rates of territorial behaviors because their status and social hierarchy has already been established and they are less frequently challenged by familiar neighbors [i.e., Dear enemy effect;(Temeles, 1994)], therefore leaving more time for reproductive activities.
Gonadal and hormonal response to perception of social opportunity
Newly ascended A. burtoni males showed a rapid elevation in circulating 11-KT levels at 30 min following the first signs of dominance behavior (see Fig. 8). This increase was also associated with higher levels of territorial behaviors in these same fish, even though there was no direct correlation between aggressive behaviors and plasma 11-KT levels in individual fish. These data support the prediction of the ‘challenge hypothesis’ that male-male interactions can increase androgen levels in A. burtoni, as described in other fishes (Cardwell and Liley, 1991; Desjardins et al., 2006; Oliveira et al., 2002; Remage-Healey and Bass, 2005). Rapidly increased androgen levels may be an anticipatory mechanism to prepare ascending fish for future challenges they are likely to encounter to maintain their new dominance status (Oliveira, 2009). Importantly, ascending A. burtoni males attain this elevated aggression and androgen levels with low physiological potential because of their suppressed reproductive axis and small testis size. On the other hand, there is evidence for rapid activation of the entire reproductive axis from GnRH1 neurons in the brain (Burmeister et al., 2005), to the pituitary, and down to the testes within this same 30 min timeframe (Maruska et al., unpublished) suggesting that social modulation of androgens may be due to social regulation of the brain-pituitary-gonad axis. In fact, subordinate males that were previously dominant still maintain some level of spermatogenesis, and therefore have the potential for rapid gonadal androgen production during social transition (Kustan, Maruska, Fernald, unpublished). Alternatively, circulating androgens are not always required for expression of short-term behavioral plasticity since gonad removal does not prevent rapid display of male-typical territorial and reproductive behaviors in sex-changing fishes (Godwin et al., 1996), and castration does not inhibit aggressive behaviors in some mammals (Demas et al., 1999; Scotti et al., 2008). Another possibility is that social opportunity rapidly decreases brain aromatase activity, thereby leaving more testosterone available for quick conversion into 11-KT, as described for the sex-changing goby Lythrypnus dalli (Black et al., 2005). Thus, rapid behavioral responses may also be due to alternate sources of circulating androgens such as the interrenal gland, liver, or brain, variations in androgen receptor expression or steroid-binding proteins, fluctuations in neurosteroids, aromatase or neuropeptides within behaviorally-relevant brain regions, or possibly mediated via androgen-independent neural circuits in the brain.
We also found an increase in circulating 11-KT levels 72 hrs after social ascent, which was not related to dominance behaviors (see Fig. 8). Although the increase in testicular size, or GSI, was not detected until 120 hrs after suppressed males became dominant, there are changes in testis cell composition associated with early spermatogenesis (i.e., increased percentage of type B spermatogonia and spermatocytes) that occur at 72 hrs after ascent in these same animals, and even subordinate males with low GSI have a high percentage of steroid-producing interstitial (or Leydig) cells (Maruska and Fernald, unpublished). In another teleost, the gene that encodes 11β-hydroxylase, an enzyme involved in androgen synthesis, also peaks during these early stages of spermatogenesis (Vinas and Piferrer, 2008), thus providing a source of androgens to the general circulation. Moreover, it seems likely that the elevation in 11-KT levels at 30 min is caused by social interactions as predicted by the ‘challenge hypothesis’, but that the later increases at 72–120 hrs are due to testicular activity related to up-regulation of the reproductive axis. Future studies that examine androgen responsiveness due to social interactions versus physiological potential are needed to tease apart these contributing factors.
The prediction of the ‘challenge hypothesis’ that dominant individuals have high plasma androgen levels compared to subordinates was supported in A. burtoni, as previously reported (Parikh et al., 2006). However, in stable environments, the ‘challenge hypothesis’ also predicts lower aggression and decreased androgen levels, which has been demonstrated in several diverse taxa including fishes (Goymann et al., 2007; Hirschenhauser and Oliveira, 2006; Oliveira et al., 2002; Wingfield et al., 1990). Based on behavioral data in A. burtoni, the social environment for an individual ascending male appears to stabilize by as soon as 72 hrs after social ascent. However, despite the lower rates of territorial behaviors during this 72–120 hr period, and in stable dominant males, circulating 11-KT levels remained high (see Fig. 8). These data are not consistent with the ‘challenge hypothesis’, but may be a consequence of the life-history traits of this species as discussed below.
The ‘challenge hypothesis’ was originally proposed to explain male androgen levels in seasonally breeding birds based on mating system and the male’s role in parental care (Wingfield et al., 1990), but has since been applied to all vertebrates (Hirschenhauser and Oliveira, 2006). However, many original predictions of the ‘challenge hypothesis’ may not be directly applicable to A. burtoni for several reasons. First, A. burtoni males are polygynous with no paternal care, and thus the ‘challenge hypothesis’ would predict high levels of breeding baseline androgens (close to the physiological maximum) and low androgen responsiveness (androgen response to challenge above baseline). However, unlike many species previously used as models, A. burtoni is not a seasonal breeder. Rather, spawning occurs year-round and male reproductive opportunity is linked to social status and the successful defense of a territory via aggressive interactions. This lack of a distinct breeding season in A. burtoni may at least partially influence the dual androgen responsiveness pattern observed during social transition (i.e., quick 30 min response coincident with aggressive behaviors, and later dissociated response at 72 hrs). In other words, instead of seasonal androgen levels establishing the breeding baseline, variations in circulating androgen levels in A. burtoni may be coupled to different life stages (i.e., dominant vs. subordinate) with important reproductive consequences. These different life stage-related androgen levels may then serve as the baseline upon which androgen responsiveness and behavioral plasticity must act. Life-history related dissociations between androgens and aggressive behaviors have also been shown in other taxa, prompting several proposed modifications of the ‘challenge hypothesis’ that require future experimental testing (Gill et al., 2008; Goymann et al., 2007; Lynn and Wingfield, 2008; Moore et al., 2004; Muller and Wrangham, 2004).
Second, the ‘challenge hypothesis’ predicts that a correlation between androgen levels and social status will only emerge during periods of social instability, and that androgen levels become dissociated from status in stable social groups. A. burtoni, however, live in a dynamic environment where changes in status can occur every few weeks under both natural and laboratory conditions (Fernald and Hirata, 1977; Hofmann et al., 1999). Further, under semi-natural conditions, social stability in A. burtoni can depend on both the density and composition of tank inhabitants (Fox et al., 1997). Thus it can be argued that social stability may either occur quickly and last for only a relatively short period of time (i.e., days or weeks), or alternatively, that A. burtoni exists in a constant state of social instability. Our data that show rapidly elevated 11-KT levels and high aggressive behaviors during social transition (e.g., 30 min to 24 hrs), but dissociated androgen levels and aggression by 72 hrs after ascent, supports the hypothesis that social stability can be achieved quickly given the right conditions. If social stability is in fact achieved by 72 hrs, then the dissociation between 11-KT levels and territorial aggressive behaviors observed in A. burtoni does support the prediction of the ‘challenge hypothesis’ that aggression and androgen levels are less related in stable animals. Future studies that measure male androgen levels in response to experimental manipulation of social stability will provide further insight into the relationship between stability and androgen responsiveness. However, it is also important to note that the frequent temporal social instability for A. burtoni males may be very different from the seasonal social instability in birds for which the original ‘challenge hypothesis’ was proposed.
Finally, subordinate social status in A. burtoni is accompanied by dramatic changes in reproductive physiology, including reduced testis size, which may translate into fewer steroid-synthesizing cells in fish of lower social rank. Thus, A. burtoni may experience a physiological constraint on androgen availability, a variable also not considered in the original ‘challenge hypothesis’. For reasons discussed above, however, it is likely that suppressed males have enough testicular androgen potential to promote and sustain rapid expression of dominance behaviors while they await full up-regulation of the reproductive axis. The fact that 11-KT levels increased at 72 hrs after ascent and then remained at this high level in stable dominant animals suggests that androgens may also be required to maintain the higher rates of reproductive and aggressive behaviors typical of these territorial males. This is consistent with a previous study that showed intramuscular injections of androgens can increase aggressive behaviors among male A. burtoni (Fernald, 1976). Experiments that compare behavior rates in gonadectomized ascending males (along with androgen implants) could test whether gonadal androgens are required for behavioral transition and social stability. Circulating 11-KT levels in A. burtoni were also better correlated with reproductive behaviors than with territorial aggressive behaviors when all transitional and stable animals were examined together. Thus, androgen responsiveness may also depend on exposure to and appropriate behavioral feedback from conspecifics, especially receptive females. Exposure of males to receptive females causes increased androgen secretion in fishes, amphibians, reptiles, birds, and mammals (Wingfield et al., 1994), and was recently proposed as a key component for androgen responsiveness measures in all vertebrates (Goymann et al., 2007). In another territorial cichlid fish, Oreochromis mossambicus, males also respond to live intruders with an increase in androgen levels (Hirschenhauser et al., 2004), but show no androgen response when presented with a mirror (Oliveira et al., 2005), providing further evidence for context-specific androgen responsiveness. Recently, Goymann et al. (2007) have proposed the inclusion of additional measures of androgen responsiveness to the ‘challenge hypothesis’, such as responsiveness to receptive females (Rmale-female), non-social environmental cues (Renvironmental), and the physiological potential to release androgens to the circulation (Rpotential). Direct experimental tests of these response values should help determine how social, environmental, and physiological factors contribute to androgen responsiveness in A. burtoni and other species.
Acknowledgments
We thank Jackie Kustan, Anne Stake, April Zhang, and Helen McCurdy for help with behavioral analyses, and Julie Desjardins, Brian Grone, and two anonymous reviewers for valuable comments on the manuscript. This work was supported by National Institutes of Health (NIH) NRSA F32NS061431 to KPM and NIH NS034590 to RDF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proc Biol Sci. 2005;272:2435–40. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid Behavioral and Genomic Responses to Social Opportunity. PLoS Biol. 2005;3:e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell JR, Liley NR. Androgen control of social status in males of a wild population of stoplight parrotfish, Sparisoma viride. Horm Behav. 1991;25:1–18. doi: 10.1016/0018-506x(91)90035-g. [DOI] [PubMed] [Google Scholar]
- Creel SN, Wildt DE, Monfort SL. Behavioral and endocrine mechanisms of reproductive suppression in Serengeti dwarf mongooses. Animal Behavior. 2002;43:231–245. [Google Scholar]
- Demas GE, Moffatt CA, Drazen DL, Nelson RJ. Castration does not inhibit aggressive behavior in adult male prairie voles (Microtus ochrogaster) Physiol Behav. 1999;66:59–62. doi: 10.1016/s0031-9384(98)00268-6. [DOI] [PubMed] [Google Scholar]
- Desjardins JK, Hazelden MR, Van der Kraak GJ, Balshine S. Male and female cooperatively breeding fish provide support for the “Challenge Hypothesis”. Behavioral Ecology. 2006;17:149–154. [Google Scholar]
- Dzieweczynski TL, Eklund AC, Rowland WJ. Male 11-ketotestosterone levels change as a result of being watched in Siamese fighting fish, Betta splendens. Gen Comp Endocrinol. 2006;147:184–9. doi: 10.1016/j.ygcen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol. 1995;16:257–333. [Google Scholar]
- Fernald RD. The effect of testosterone on the behavior and coloration of adult male cichlid fish (Haplochromis burtoni, Gunther) Horm Res. 1976;7:172–8. doi: 10.1159/000178726. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Quantitative behavioral observations of Haplochromis burtoni under semi-natural conditions. Animal Behavior. 1977;25:643–653. [Google Scholar]
- Fernald RD. Social regulation of reproduction: what changes and why? Hormones, Brain and Behavior. 2009;1:683–691. [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: quantitative behavioral observations. Animal Behavior. 1977;25:964–975. [Google Scholar]
- Fox HE, White SA, Kao MH, Fernald RD. Stress and dominance in a social fish. J Neurosci. 1997;17:6463–9. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SA, Costa LM, Hau M. Males of a single-brooded tropical bird species do not show increases in testosterone during social challenges. Horm Behav. 2008;54:115–24. doi: 10.1016/j.yhbeh.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Godwin J, Crews D, Warner RR. Behavioural sex change in the absence of gonads in a coral reef fish. Proc R Soc Lond B. 1996:1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness-revisiting the Challenge Hypothesis. Horm Behav. 2007;51:463–76. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Animal Behaviour. 2006;71:265–277. [Google Scholar]
- Hirschenhauser K, Taborsky M, Oliveira T, Canario AV, Oliveira RF. A test of the ‘challenge hypothesis’ in cichlid fish: simulated partner and territory intruder experiments. Animal Behaviour. 2004;68:741–750. [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci U S A. 1999;96:14171–6. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime D. ‘Classical’ and ‘non-classical’ reproductive steroids in fish. Reviews in Fish Biology and FIsheries. 1993;3:160–180. [Google Scholar]
- Lynn SE, Wingfield JC. Dissociation of testosterone and aggressive behavior during the breeding season in male chestnut-collared longspurs, Calcarius ornatus. Gen Comp Endocrinol. 2008;156:181–9. doi: 10.1016/j.ygcen.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. doi: 10.1186/1471-2202-11-58. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore IT, Wada H, Perfito N, Buch DS, Hahn TP, Wingfield JC. Territoriality and testosterone in an equatorial population of rufous-collared sparrows, Zonotrichia capensis. Animal Behavior. 2004;67:411–420. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Animal Behavior. 2004;67:113–123. [Google Scholar]
- Oliveira RF. Social behavior in context: hormonal modulation of behavioral plasticity and social competence. Integrative and Comparative Biology. 2009;49:423–440. doi: 10.1093/icb/icp055. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Carneiro LA, Canario AV. Behavioural endocrinology: no hormonal response in tied fights. Nature. 2005;437:207–8. doi: 10.1038/437207a. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV. Social modulation of androgen levels in male teleost fish. Comparative Biochemistry and Physiology B Biochemistry and Molecular Biology. 2002;132:203–15. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Lopes M, Carneiro LA, Canario AV. Watching fights raises fish hormone levels. Nature. 2001;409:475. doi: 10.1038/35054128. [DOI] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behav Brain Res. 2006;166:291–5. doi: 10.1016/j.bbr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Ryder TB, Parker PG, Blake JG, Loiselle BA. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc Biol Sci. 2009;276:2377–84. doi: 10.1098/rspb.2009.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Scotti MA, Belen J, Jackson JE, Demas GE. The role of androgens in the mediation of seasonal territorial aggression in male Siberian hamsters (Phodopus sungorus) Physiol Behav. 2008;95:633–640. doi: 10.1016/j.physbeh.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Temeles EJ. The role of neighbors in territorial systems: when are they ‘dear enemies’? Animal Behavior. 1994;47:339–350. [Google Scholar]
- Vinas J, Piferrer F. Stage-specific gene expression during fish spermatogenesis as determined by laser-capture microdissection and quantitative-PCR in sea bass (Dicentrarchus labrax) gonads. Biol Reprod. 2008;79:738–47. doi: 10.1095/biolreprod.108.069708. [DOI] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J Exp Biol. 2002;205:2567–81. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner R, Dufty A, Jr, Ball GF. The “challenge hypothesis “: theoretical implications for patterns of testosterone secretion, mating system, and breeding strategies. American Naturalist. 1990;136:829–846. [Google Scholar]
- Wingfield JC, Whaling CS, Marler P. Communication in vertebrate aggression and reproduction: the role of hormones. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 303–342. [Google Scholar]