Abstract
Background
Cafe-au-lait macules (CALMs) in NF1 are an early and accessible phenotype in NF1, but have not been extensively studied.
Objective
To more fully characterize the phenotype of CALMs in patients with NF1.
Methods
Twenty-four patients with a diagnosis of NF1 confirmed through clinical diagnosis or molecular genetic testing were recruited from patients seen in the Genetics Department at the University of Alabama at Birmingham. CALM locations were mapped using standard digital photography. Pigment intensity was measured with a narrowband spectrophotometer, which estimates the relative amount of melanin (M) based on its absorption of visible light. The major response was defined as the difference between the mean M from the CALM and the mean M from the surrounding skin. The major response for each spot was compared to spots within an individual and across individuals in the study population.
Results
There was significant variability of the major response, primarily attributable to intrapersonal variability (48.4%, <0.0001) and secondly to interpersonal variability (33.0%, <0.0094). Subsequent analysis based on genetic mutation type showed significantly darker spots in individuals with germline mutations leading to haploinsufficiency.
Limitations
The study was performed on a small population of patients and the method utilized has not yet been used extensively for this purpose.
Conclusions
CALMs vary in pigment intensity not only across individuals, but also within individuals and this variability was unrelated to sun exposure. Further studies may help elucidate the molecular basis of this finding, leading to an increased understanding of the pathogenesis of CALMs in NF1.
Introduction
Neurofibromatosis type 1 (NF1) is a relatively common autosomal dominant multisystem disorder that manifests with several skin findings, including café-au-lait macules (CALMs). The presence of 6 or more CALMs fulfills one of the seven NIH diagnostic criteria and is often the earliest sign of NF11,2; indeed, ninety-nine percent of patients with NF1 have fulfilled this criteria by age 13. CALMs appear shortly after birth and increase in number until 2 to 4 years of age4,5. CALMs are characteristically a uniform shade of light to dark brown and ovoid in shape, with smooth “coast of California” borders (Figures 1A & B). Most are between 5 and 30 mm, although they can involve entire anatomic regions. Their distribution appears random, sparing only the scalp, palms, and soles5,6.
Figures 1A & 1B.
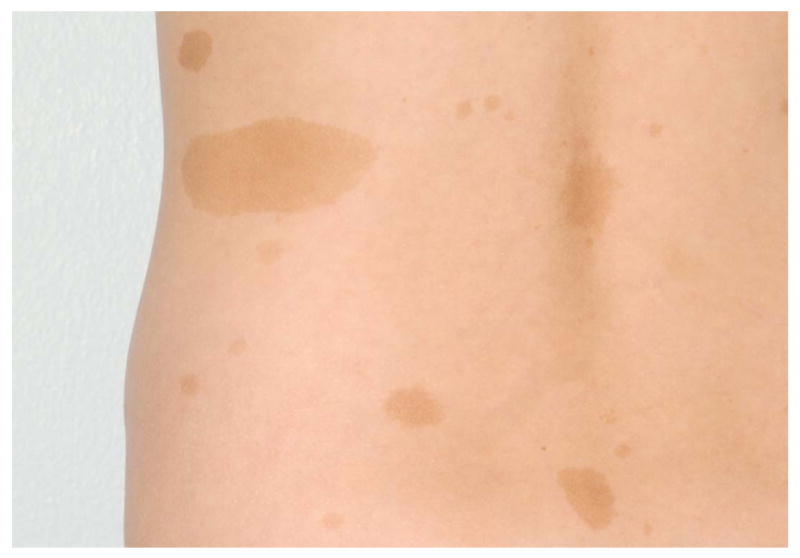
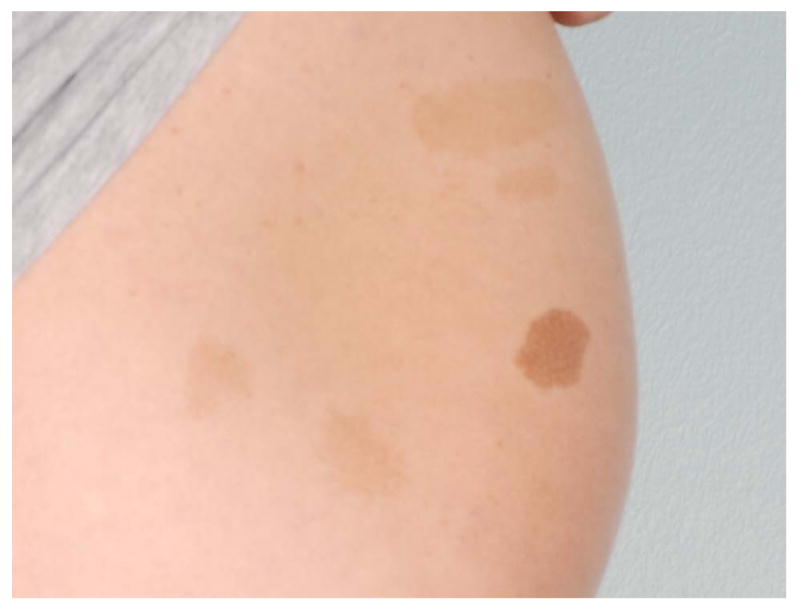
Café-au-lait macules in two children showing relative uniformity (A) and variability (B).
NF1 is caused by a mutation in the NF1 gene, which is located on chromosome 17q11.2. The gene encodes for neurofibromin, a ras guanosine triphosphatase (GTPase-activating protein, GAP) and as such serves as a regulator of signals for cell proliferation and differentiation7. Neurofibromin was demonstrated specifically as a regulator of melanogenic gene expression in murine melanocytes8. The primary tumor cell of the neurofibroma is a Schwann cell with a mutation in both NF1 alleles but may require additional molecular events for tumor formation9,10. In 2008, De Schepper et al. identified somatic or second hit NF1 mutations in 5/5 melanocyte cultures from CALMs in NF1 patients11; only germline mutations are found in the melanocytes of non-CALM skin12. Somatic mutations were not identified in either the keratinocytes or fibroblasts from the same CALMs or the melanocytes from uninvolved skin. This suggests that the melanocyte is the primary tumor cell in CALMs.
NF1 is known to display a wide range of phenotypic variability, both within and between families. In an individual, there is also variability in terms of rate of growth of specific tumors. Given that different lesions will have different “second hit” NF1 gene mutations, we hypothesize that rate of growth of specific tumors is correlated with the nature of the second hit mutation. Testing this hypothesis in neurofibromas, though, requires conducting a longitudinal study. Since the CALM also arises via a two-hit mechanism, the same hypothesis might be tested in CALM, using pigment intensity as a phenotype rather than rate of growth. Doing such a study, however, first requires demonstration of intra-individual variability in the pigmentation of CALM. This study reports on an approach to measurement of CALM pigmentation and explores the variability in pigmentation within an individual. We also present a preliminary test of the hypothesis in a small subset of patients whose NF1 gene mutation is known.
Methods
Patients and Materials
We obtained approval from our institution's IRB prior to conducting any study procedures. Prospective patients were identified from the electronic medical records of patients seen in the Department of Genetics at UAB. Inclusion criteria were: 1) ≥ 4 years of age; 2) diagnosis of NF1 based on NIH diagnostic criteria or a germline NF1 mutation identified by the Medical Genomics Laboratory at UAB; 3) presence of at least 6 CALMs; and 4) ability and willingness to cooperate with study-related procedures. We obtained informed consent and assent (ages 7 – 12) prior to study enrollment. Age, race, sex, and germline NF1 mutation (if known) were recorded. The UAB Medical Genomics Laboratory performed all mutational analysis using a multi-step detection protocol. This protocol has been shown to identify 95% of NF1 mutations in patients who fulfill NIH diagnostic criteria13. In cases where mutational testing performed, patients were classified according to the type of germline mutation: group 1 – nonsense, frameshift mutations; or group 2 – missense, in-frame splice mutations. Mutations in group 1 are expected to result in premature termination of translation with loss of neurofibromin production (haploinsufficiency) and group 2 mutations are expected to have a less deleterious effect on neurofibromin production, with possible either lower neurofibromin expression or altered function.
Total body digital photography was performed in 14 patients for determination of CALM distribution. Photography was performed with a system used in our dermatology clinic for mole-mapping. This consisted of a digital camera (D80, Nikon Inc.) mounted to a stand that allows for vertical and horizontal movement; to ensure consistent lighting, two lamps and ambient fluorescent lighting were used. We photographed patients using a series of standardized poses. The groin area was not photographed. We imported the pictures into Mirror Imaging Software (Canfield Scientific, Inc., USA) where they were stored for later use. For 10 of the patients, we manually plotted the location of their CALMs on a body diagram. At study's end, we plotted all CALMs on a single body diagram to ascertain the pattern of distribution.
Reflectance spectroscopy has been used extensively in dermatologic research for the objective measurement of skin color. One of the methods, narrowband spectrophotometry, takes advantage of the differences in absorption of visible light by hemoglobin and melanin and, thus, provides reasonable estimates of the amount of erythema and pigmentation, respectively. We used a narrowband spectrophotometer (DSM II, Cortex Technology, Denmark) to obtain all colorimetric data. The DSM II also records color using three values developed by the Commission International d'Eclairage (CIE): L*, light intensity; a*, amount of green or red; and b*, amount of blue or yellow14,15. A predecessor to this model demonstrated both reliability and efficacy for measuring skin pigmentation16.
We classified CALMs into three investigator-assigned categories based on estimated amount of sun exposure: (1) high (face, shoulders, posterior neck, distal dorsal arms); (2) medium (trunk, proximal dorsal arms), and low (buttocks, ventral arms, legs). To assess the reliability of the DSM II, we performed multiple measurements of 7 to 24 CALMs randomly scattered on the body in the first thirteen individuals. The same investigator (K.B.) obtained three to four measurements in succession; the probe was lifted and replaced in approximately the same spot in the center of the CALM between each measurement. Three readings of the surrounding, non-CALM skin were then taken at approximately 120-degree angles for each lesion to serve as the background/baseline measures for each spot. Both the E/M and CIE L*a*b* indices were recorded for each measurement; however, after discovering that the machine's values for both indexes were equally consistent (data analysis not shown) only the E/M values were recorded. To assess the homogeneity of CALMs, we measured 2 to 4 non-overlapping locations within 61 CALMs in five patients.
A retrospective review of the initial twenty patients showed that four had positive NF1 genetic testing. An additional four patients with positive NF1 germline mutations were then enrolled and CALMs were each measured once in the center and surrounding skin. These final four were included in the analyses of distribution and of genotype/phenotype only.
Statistical Analysis
Because of the biological relevance of the melanin (M) measurement, this parameter was chosen for all statistical comparisons. The major response was defined as the difference between the mean melanin at the center of the CALM and the mean melanin of the surrounding skin. This was done in an attempt to nullify the effect of sun exposure. Summary statistics were calculated to inspect the differences in the major response between different demographic groups. The measurements at the center and in the surrounding area were compared to ensure the consistency of two measurements at each spot. Generalized linear models17 were fitted to estimate the variance in the response explained by all potential fixed-effects factors, including gender, age, race, and sun-exposure status. Outliers and influential observations were inspected through model diagnostic plots and statistics. Apparent outliers were removed from the final analysis. Lastly, because the effects from individuals and spots are more likely to be random, we used linear mixed models18 to estimate the individual contribution and spot contributions to the major response by including age, gender, and sun exposure status as fixed-effect factors and individual and spot as random-effect factors. The random effects were assumed to follow normal distributions with zero means. Linear mixed models were used to assess homogeneity and to test the differences between the major responses in the two genetic groups, adjusting for the fixed effects of gender, age, and random effects from individuals.
Results
Patient Characteristics and CALM Distribution
Twenty-four patients were enrolled (14 males, 10 females), ranging in age from 4 to 48 years. The mean age of participants was 15.4 years and the median was 10 years; twenty-two of the patients were Caucasian and two were African-American (Table I). The mean number of CALMs per patient was 23.5, with a median of 24 and a range of 10-37. Analysis of CALM distribution was performed by calculating the ratio of % body area (estimated using Wallace's “Rule of Nines”) to the % of total CALMs in the respective region. Calculated ratios were 0.35 and 1.56 in the head and trunk regions and 0.70 and 0.78 in the upper and lower extremities, respectively. (Table I). The buttocks and genitalia were not examined in the majority of patients, thus diluting their contribution to overall distribution. The distribution and location of CALMs from all participants is shown in Figure 2.
Table I. Patient demographics and CALM distribution.
| Pt | Age | Sex | Race | # CALMs | Head | Trunk | U. Ext | L. Ext | Mutation | Mutation Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | F | C | 27 | 0 | 10 | 2 | 15 | ||
| 2 | 4 | M | C | 25 | 0 | 15 | 4 | 6 | ||
| 3 | 36 | M | C | 31 | 1 | 26 | 3 | 1 | c.3595dupA | truncating |
| 4 | 8 | M | C | 19 | 0 | 10 | 1 | 8 | ||
| 5 | 13 | M | C | 25 | 0 | 15 | 6 | 4 | ||
| 6 | 8 | M | C | 28 | 2 | 20 | 1 | 5 | ||
| 7 | 8 | M | C | 21 | 3 | 11 | 5 | 2 | ||
| 8 | 11 | F | C | 17 | 0 | 11 | 2 | 4 | ||
| 9 | 6 | F | C | 35 | 1 | 20 | 3 | 11 | R1748X | truncating |
| 10 | 6 | F | C | 19 | 1 | 12 | 2 | 4 | c.7272_7273delGT | truncating |
| 11 | 8 | M | C | 14 | 0 | 10 | 1 | 3 | ||
| 12 | 6 | F | C | 11 | 0 | 8 | 0 | 3 | ||
| 13 | 9 | F | C | 33 | 1 | 19 | 2 | 11 | ||
| 14 | 14 | M | C | 28 | 1 | 13 | 4 | 10 | ||
| 15 | 15 | M | C | 14 | 1 | 5 | 4 | 4 | ||
| 16 | 16 | F | AA | 19 | 2 | 6 | 6 | 5 | ||
| 17 | 14 | M | C | 37 | 0 | 26 | 6 | 5 | ||
| 18 | 11 | F | C | 10 | 0 | 7 | 2 | 1 | ||
| 19 | 5 | F | AA | 29 | 3 | 12 | 5 | 9 | ||
| 20 | 6 | M | C | 36 | 1 | 16 | 3 | 16 | ||
| 21 | 39 | M | C | 15 | 0 | 7 | 0 | 8 | c.2693T>C (p.L898P) | missense |
| 22 | 48 | M | C | 21 | 0 | 11 | 5 | 5 | c.3639_3641 delAAT (p. 1215delM) | amino acid deletion |
| 23 | 37 | F | C | 23 | 0 | 12 | 1 | 10 | c.3379delA | base pair deletion |
| 24 | 34 | M | C | 27 | 1 | 15 | 3 | 8 | c.288+1 G>T | splicing mutation (in frame) |
| Mean | 23.5 | 0.8 | 13.2 | 3.0 | 6.6 | |||||
| Median | 24.0 | 0.5 | 12.0 | 3.0 | 5.0 | |||||
| Range | 10-37 | 0-3 | 5-26 | 0-6 | 2-15 | |||||
| Total CALMs: | 564 | 18 | 317 | 71 | 158 | |||||
| % Total CALMs: | 3% | 56% | 13% | 28% | ||||||
| % Body Area: | 9% | 36% | 18% | 36% | ||||||
|
% Total CALMs / % Body Area: |
0.35 | 1.56 | 0.70 | 0.78 | ||||||
Figure 2.
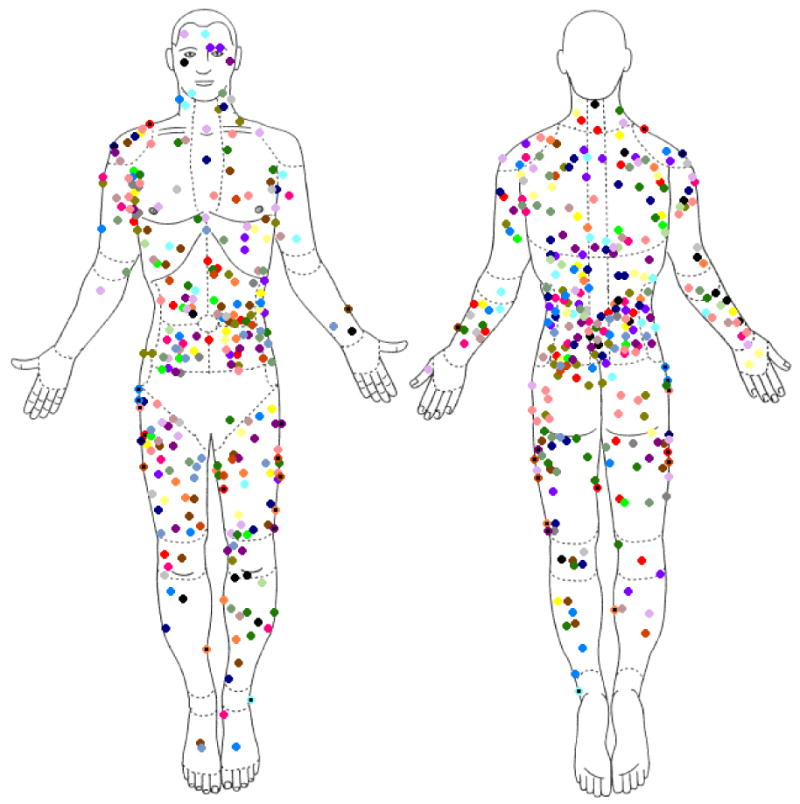
Distribution of café-au-lait macules in our series of 24 patients (each color represents a unique individual)
Validation of DSM II
The coefficient of variation across 145 spots from 13 patients ranged from 0% to 7.8%, with an average of 1.4% (± 1.2%).
Homogeneity of CALMs
Utilizing the linear mixed model to check the homogeneity of the measurements within CALMs, we used the measurements as a dependent variable, age and sex as fixed-effect variables, and individuals and spots within individuals as random effects. Both sex and age were found not significant in this model. Nearly two-thirds (61.62%, p<0.0001) of the total variance of the measurements after adjusting for sex and age comes from the CALMs, slightly more than one-third (34.53%, p<0.0001) comes from individuals, and 3.85% is random. Figure 3 shows that the relationship between multiple readings at one spot exhibit variation that is small compared with a larger variation between spots and between individuals.
Figure 3.
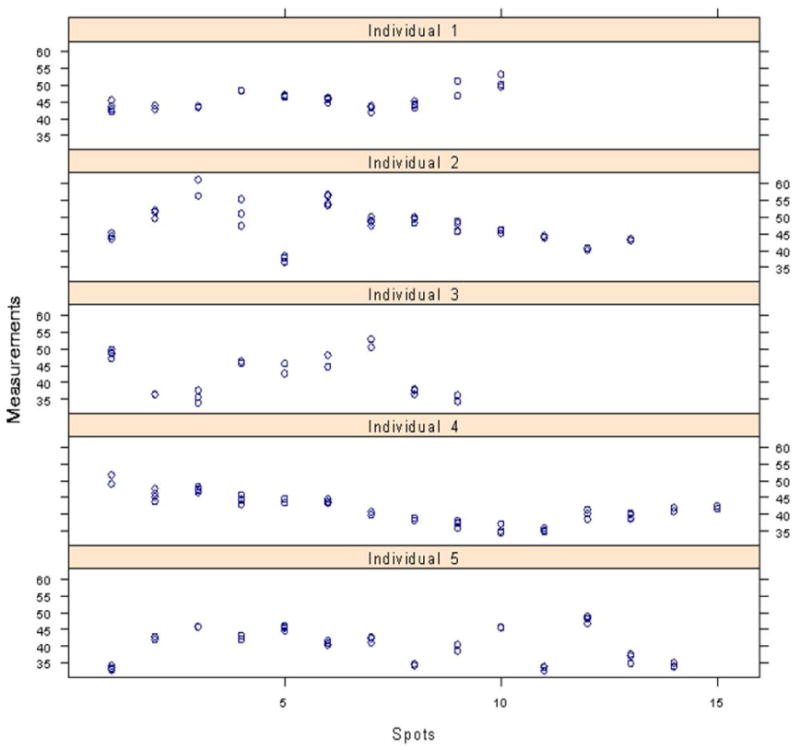
Results from analysis of CALM homogeneity displaying relative uniformity of readings within an individual spot as compared to variability from spot-to-spot.
Pigmentary Differences Among CALMs Within and Between Individuals
Table II provides summary data on the characteristics of the major response (melanin or M) in different groups based on age, gender, race, and sun exposure. The highest CALM means were seen in the two African-American patients and the lone 36-year old. The measurements at the center of CALMs and of the surrounding non-CALM skin were closely associated at each spot and their means and standard deviations across three different sun exposure areas are summarized in Figure 4. Even though the measurements at the center and in the surrounding area seemed associated to sun exposure status, the difference between the two did not vary significantly.
Table II. Summary data in different groups based on age, gender, race, and sun exposure. All values are melanin units (M).
| Group | Mean | Standard deviation | |
|---|---|---|---|
| Age | 4-9 | 5.037 | 2.879 |
| 11-16 | 5.5 | 3.038 | |
| 36 | 11.034 | 2.156 | |
| Sex | Male | 5.407 | 2.812 |
| Female | 5.365 | 3.375 | |
| Race | Caucasian | 4.967 | 2.561 |
| African American | 8.154 | 4.492 | |
| Sun exposure status | Low | 5.398 | 2.975 |
| Medium | 5.523 | 3.187 | |
| High | 4.863 | 2.854 | |
Figure 4.
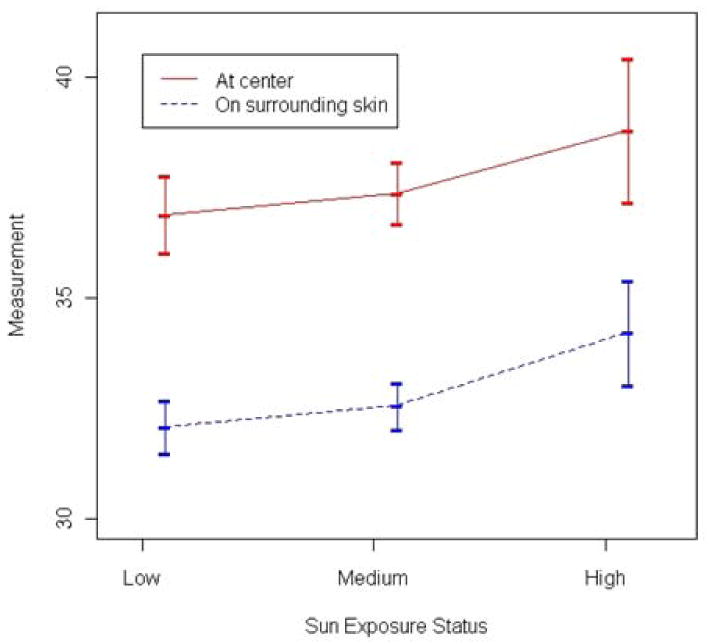
Relationship of sun exposure region to average melanin (M) reading.
Diagnostic plots as well as diagnostic statistics suggested that the measurements from two African-Americans interfered with data analysis by skewing results, an effect that might have been ameliorated had more dark-skinned individuals been enrolled. We thus excluded the two African Americans from further analysis and refit the generalized linear model with age, sex, and sun exposure status. The results were consistent with prior results, but sex and sun exposure status became less significant. All 3 factors together explained a small proportion of the total variation in the response.
Using the linear mixed model, we found that the variance between individuals was 2.461, the variance between spots was 3.610, and residual variance was 1.385. The variance in the major response after adjusting for age, sex, and sun exposure status was primarily explained by café-au-lait spots (48.42%, p<0.0001), secondly by individuals (33.01%, p=0.0094), and lastly by a random component from measurements (18.58%), which is consistent with the distribution of the response among individuals. Figure 5 shows the variability of readings within an individual as compared to those of the other study participants. The differences in the major response among high, medium, and low sun exposure groups were not significant after adjusting for age and sex.
Figure 5.
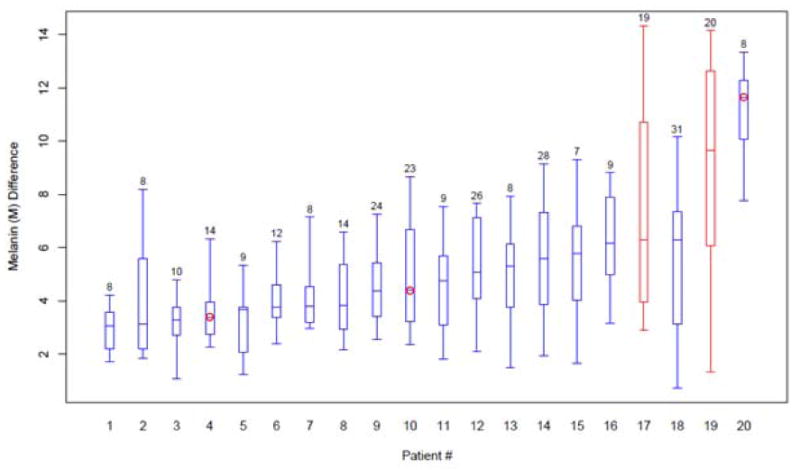
Range and mean of light meter readings in each individual with number of CALMs analyzed per patient. Circles indicate those in whom genetic mutation testing was available. The two African-American patients are shown in red.
Phenotypic correlation with germline NF1 mutation
Seven individuals had molecular analysis of the NF1 gene performed prior to study entry (Table I). Four of the mutations belonged to group 1 (nonsense/out-of-frame/frameshift) and three belonged to group 2 (missense/in-frame splice mutations). The CALMs of these seven patients were pooled and analyzed as a group, for a total of 66 in Group 1 and 62 in Group 2. Patients in group 1 had larger melanin (M) value differences compared to the individuals in group 2. The average difference in the M changes between the two groups was 5.780 units (95% CI 3.551-8.040), which is significantly different (p<0.0001). (Figure 6)
Figure 6.
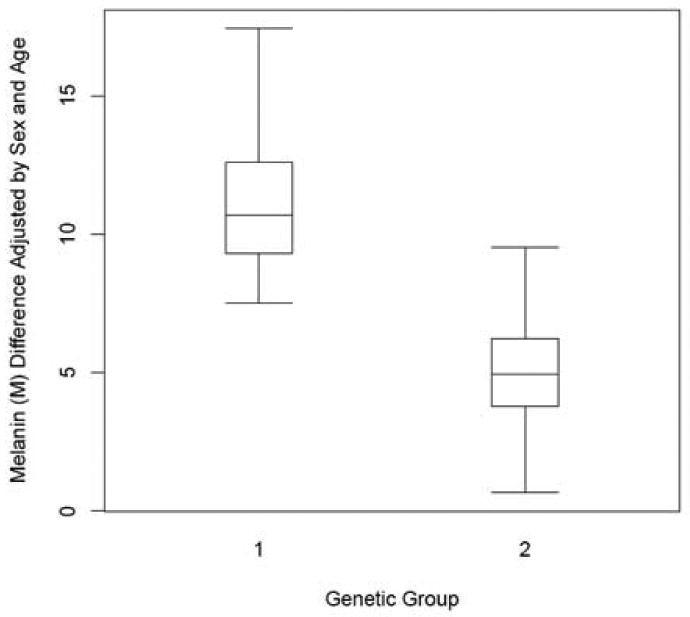
Range and mean of average melanin (M) differences in the two genetic groups. Group 1 = nonsense, frameshift mutations; Group 2 = missense, in-frame splice mutations.
A summary of the key findings from this study are presented in Table III.
Table III. Summary of Key Findings.
| Coefficient of Variation | p-value | ||
|---|---|---|---|
| Instrument Validation | Mean: | 1.4 ± 1.2% | n/a |
| Range: | 0 to 7.8% | n/a | |
| Source of Variation | |||
| Intrapersonal & Interpersonal CALM Variation | CALMs | 48.4% | <0.0001 |
| Individuals | 33.0% | 0.0094 | |
| Random | 18.6% | ||
| Source of Variation | |||
| Individual CALM Homogeneity | CALMs | 61.6% | <0.0001 |
| Individuals | 34.5% | <0.0001 | |
| Random | 3.9% | ||
| Observed Difference | |||
| Germline Mutation & Phenotype Correlation | Group 1 > Group 2 | 5.780 M units | <0.0001 |
Discussion
The CALM is a nearly universal finding in patients with NF1; however, there have been no publications reporting objective quantification of CALM pigmentation. We found the DSMII to be an effective and reliable method for evaluating the degree of CALM pigmentation. The average variation in repeated measures was small (1.4%), comparing favorably with previous studies looking at variability of readings; in a study involving the predecessor to this study's light meter the variability was 4%16.
We have found that there is significant phenotypic variability in CALM pigmentation both between individuals and also within an individual. The majority of variation is attributed to spot-to-spot variability, with less of an effect from person-to-person variability. We hypothesize that the differences in pigmentation of the CALM may be related to the combination of first and second hit NF1 mutations in the melanocytes, with the germline mutation determining the differences among individuals and the somatic mutation determining the spot-to-spot variability. This hypothesis was supported by observations in seven patients with known germline mutations. We found that patients with mutations resulting in haploinsufficiency of the NF1 gene product had statistically-significantly darker spots compared to patients with missense or in-frame splicing mutations. The number of patients (7) and lesions (128) studied in the genetic analysis was small; further studies should be performed to confirm or refute this finding.
The pathophysiology of NF1-associated CALMs is not fully understood. Early studies have consistently shown an increased number of epidermal melanocytes in CALM skin compared to the skin of normal individuals19-22. However, in some of these studies this increased melanocyte density was not restricted to the hyperpigmented skin, but was also found in the normal-appearing skin of patients with NF121,22. De Schepper et al. recently demonstrated, using a standardized software-assisted distance and surface measurement tool, a statistically significant increase in melanocyte density in NF1 CALM skin compared to NF1 normal skin23.
Of note, some patients had an overall uniformity to their CALM pigmentation while others displayed greater variation. A possible explanation for this “variable variability” is the interplay between the first and second hit mutations in the melanocytes. If a patient has a germline mutation causing absent neurofibromin expression, any second-hit's contribution might be minor, such that the patient's spots exhibit overall minimal variation. The cause and timing of the somatic mutation is unknown and one could speculate that the first mutation has an influence on the second mutation and, consequently, pigment variation. While not explored in melanocytes, this mutational relationship has been explored in the Schwann cell of neurofibromas9.
Two other possible contributors of CALM pigmentation were sun exposure and CALM location. When individual spots were stratified according to sun exposure, there was a direct relationship between the amount of sun exposure and the measured M value (Figure 4). This suggests that the function of melanocytes to darken in response to sunlight is unaffected by mutations in the NF1 gene and shows that CALM phenotypic variability is not due to sun exposure. Very few studies have looked at the relationships of cutaneous to manifestations to body segment24. Comparison of pigmentation values in 5 body segments (data not shown) showed no statistically-significant differences, effectively negating body site as an explanation for varied CALM pigmentation.
We have demonstrated a reliable tool and method to measure the phenotype of the CALM in patients with NF1; the light meter is non-invasive and simple to operate, making it an ideal tool especially for use in children. The genotype can be characterized through NF1 gene molecular analysis of the CALM melanocyte as described elsewhere11. Notably, café-au-lait macules provide an attractive model for studying the genotype-phenotype relationship in NF1. Unlike the neurofibroma, the CALM is an early and nearly-universal finding in patients with NF1 and does not require longitudinal observation for growth. The CALM may therefore provide an accessible phenotype for further studies of genotype-phenotype correlations in NF1.
Acknowledgments
Funding Sources: This study was funded in part by grants from the Department of Dermatology and Skin Diseases Research Center, University of Alabama at Birmingham and the Dermatology Foundation.
Abbreviations/Acronyms Used in this Text
- NF1
neurofibromatosis type 1
- CALM/CALMs
café-au-lait macule/s
- NIH
National Institutes of Health
- IRB
Institutional Review Board
- UAB
University of Alabama at Birmingham
- CIE
Commission International d'Eclairage
- M
melanin (value)
- CI
confidence interval
Footnotes
Contents of the manuscript have not been previously published and are not currently submitted elsewhere.
Conflict of Interest Disclosure: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anonymous. Arch Neurol; Neurofibromatosis. Conference Statement. National Institutes of Health Consensus Development Conference; 1988. May, pp. 575–8. [PubMed] [Google Scholar]
- 2.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997 Jul 2;278(1):51–7. [PubMed] [Google Scholar]
- 3.DeBella K, Szudek J, Friedman JM. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000 Mar;105(3 Pt 1):608–14. doi: 10.1542/peds.105.3.608. [DOI] [PubMed] [Google Scholar]
- 4.Korf BR. Clinical Features and Pathobiology of Neurofibromatosis 1. J Child Neurol. 2002;17(8):573–7. doi: 10.1177/088307380201700806. [DOI] [PubMed] [Google Scholar]
- 5.Landau M, Krafchik BR. The diagnostic value of café-au-lait macules. J Am Acad Dermatol. 1999 Jun;40(6 Pt 1):877–90. doi: 10.1016/s0190-9622(99)70075-7. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JM. Neurofibromatosis 1: clinical manifestations and diagnostic criteria. J Child Neurol. 2002;17(8):548–54. doi: 10.1177/088307380201700802. [DOI] [PubMed] [Google Scholar]
- 7.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001 Feb 23;104(4):593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 8.Diwakar G, Zhang D, Jiang S, et al. Neurofibromin as a regulator of melanocyte development and differentiation. J Cell Sci. 2008 Jan;121(Pt2):167–77. doi: 10.1242/jcs.013912. [DOI] [PubMed] [Google Scholar]
- 9.Maertens O, Brems H, Vandesompele J, et al. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat. 2006 Oct;27(10):1030–40. doi: 10.1002/humu.20389. [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski JL, Wu K, Gutmann DH, et al. Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1. Hum Mol Genet. 2000 Apr 12;9(7):1059–66. doi: 10.1093/hmg/9.7.1059. [DOI] [PubMed] [Google Scholar]
- 11.De Schepper S, Maertens O, Callens T, et al. Somatic Mutation Analysis in NF1 Café-au-lait Spots Reveals Two NF1 Hits in the Melanocytes. J Invest Dermatol. 2008;128(4):1050–3. doi: 10.1038/sj.jid.5701095. [DOI] [PubMed] [Google Scholar]
- 12.Maertens O, De Schepper S, Vandesompele J, et al. Molecular dissection of isolated features in mosaic neurofibromatosis type 1. Am J Hum Genet. 2007 Aug;81(2):243–51. doi: 10.1086/519562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messiaen LM, Callens T, Mortier G, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15(6):541–55. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Taylor S, Westerhof W. Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol. 2006;54(5):S282–S290. doi: 10.1016/j.jaad.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Stamatas GN, Zmudzka BZ, Kollias N, Beer JZ. Non-Invasive Measurements of Skin Pigmentation In Situ. Pigment Cell Res. 2004;17(6):618–26. doi: 10.1111/j.1600-0749.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 16.Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter, the DermaSpectrometer, and the Mexameter. Skin Res Technol. 2000 Nov;6(4):230–238. doi: 10.1034/j.1600-0846.2000.006004230.x. [DOI] [PubMed] [Google Scholar]
- 17.McCullagh P, Nelder JA. Generalized Linear Models. 2nd. Chapman & Hall/CRC; 1989. [Google Scholar]
- 18.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2000. [Google Scholar]
- 19.Takahasi M. Studies on café au lait spots in neurofibromatosis and pigmented macules of nevus spilus. Tohoku J Exp Med. 1976;118(3):255–73. [PubMed] [Google Scholar]
- 20.Johnson BL, Charneco DR. Café au lait spot in neurofibromatosis and in normal individuals. Arch Dermatol. 1970;102(4):442–6. [PubMed] [Google Scholar]
- 21.Benedict PH, Szabo G, Fitzpatrick TB, Sinesi SJ. Melanotic Macules in Albright's syndrome and in neurofibromatosis. JAMA. 1968 Aug 26;205(9):618–26. [PubMed] [Google Scholar]
- 22.Frenk E, Marazzi A. Neurofibromatosis of von Recklinghausen: a quantitative study of the epidermal keratinocyte and melanocyte populations. J Invest Dermatol. 1984;83(1):23–5. doi: 10.1111/1523-1747.ep12261648. [DOI] [PubMed] [Google Scholar]
- 23.De Schepper S, Boucneau J, Haeghen YV, et al. Café-au-lait spots in neurofibromatosis type 1 and in healthy control individuals: hyperpigmentation of a different kind? Arch Dermatol Res. 2006;297:439–449. doi: 10.1007/s00403-006-0644-6. [DOI] [PubMed] [Google Scholar]
- 24.Palmer C, Szudek J, Harry J, et al. Analysis of Neurofibromatosis 1 (NF1) Lesions by Body Segment. Am J Med Genet A. 2004 Mar;125A(2):157–61. doi: 10.1002/ajmg.a.20354. [DOI] [PubMed] [Google Scholar]


