Abstract
Aldehydes such as acrolein are ubiquitous pollutants present in automobile exhaust, cigarette, wood, and coal smoke. Such aldehydes are also constituents of several food substances and are present in drinking water, irrigation canals, and effluents from manufacturing plants. Oral intake represents the most significant source of exposure to acrolein and related aldehydes. To study the effects of short-term oral exposure to acrolein on lipoprotein levels and metabolism, adult mice were gavage fed 0.1 to 5 mg acrolein/kg bwt and changes in plasma lipoproteins were assessed. Changes in hepatic gene expression related to lipid metabolism and cytokines were examined by qRT-PCR analysis. Acrolein feeding did not affect body weight, BUN, plasma creatinine, electrolytes, cytokines or liver enzymes, but increased plasma cholesterol and triglycerides. Similar results were obtained with apoE-null mice. Plasma lipoproteins from acrolein-fed mice showed altered electrophoretic mobility on agarose gels. Chromatographic analysis revealed elevated VLDL cholesterol, phospholipids, and triglycerides levels with little change in LDL or HDL. NMR analysis indicated shifts from small to large VLDL and from large to medium-small LDL with no change in the size of HDL particles. Increased plasma VLDL was associated with a significant decrease in post-heparin plasma hepatic lipase activity and a decrease in hepatic expression of hepatic lipase. These observations suggest that oral exposure to acrolein could induce or exacerbate systemic dyslipidemia and thereby contribute to cardiovascular disease risk.
Keywords: acute phase response, aldehyde, cholesterol, hepatic lipase, lipoproteins, triglycerides
Introduction
Aldehydes such as acrolein are toxic components of automobile exhaust and smog, and have been detected in high concentrations in cigarette, cotton, wood and coal smoke. They constitute 1 to 2 % of the volatiles generated from automobile exhaust and the burning of fossil fuels (Feron et al., 1991). Volatile aldehydes are also present in drinking water. Several aldehydes are generated upon storage of carbonated or non-carbonated water in plastic bottles (Nawrocki et al., 2002). At least 36 different aldehydes are found in water of which acrolein and endrin aldehyde have been classified as the two highest priority pollutants (Committee on Aldehydes, 1981). The recommended maximum concentration of acrolein in water is 65 µg/L and that of glutaraldehyde is 70 µg/L, but these limits are often exceeded (Beauchamp, Jr. et al., 1985; Ghilarducci and Tjeerdema, 1995). High levels of acrolein (20 – 100 µg/L) have been detected in effluents of industrial wastes, particularly paper manufacturing plants and in irrigation canals in which acrolein is used as a fungicide and herbicide (Ghilarducci and Tjeerdema, 1995).
Food substances are an additional source of aldehyde exposure. More than 300 different aldehydes have been detected in different food substances (Feron et al., 1991; Committee on Aldehydes, 1981; Ghilarducci and Tjeerdema, 1995). Typically, aldehydes are found at a concentration of 10 to 20 mg/kg, although very high concentrations of specific aldehydes are present in some foods and spices. High levels of acrolein have been detected in beer, wine, rum, bread and other foods (Feron et al., 1991). The formation of acrolein in foods, especially cooking oils, is further increased by cooking and frying and re-heating (Fullana et al., 2004). Furthermore, the concentration of non-volatile aldehydes increases when foods are heated in oil. Several of the aldehydes that appear on heating are unsaturated like acrolein because the process of frying or heating decreases the cis-double bond content of triglycerides and increases the formation of trans-unsaturated aldehydes (Fullana et al., 2004). Other aldehydes present in food are furfural, formaldehyde, and acetaldehyde (Committee on Aldehydes, 1981). Furfural has been detected in 150 types of food. It is particularly abundant in white bread and coffee. Crotonaldehyde is another, acrolein-like α,β-unsaturated aldehyde present in several natural foods. Overall, our estimates indicate that maximal daily human consumption of unsaturated aldehydes is nearly 5 mg/kg and the total aldehyde consumption (unsaturated and saturated) is about 7 mg/kg (Wang et al., 2008).
Despite high levels of estimated exposure, little is known in regard to the in vivo effects of aldehydes. In vitro experiments with cell culture systems show that exposure to low concentrations of acrolein or related aldehydes depletes reduced glutathione, which in turn, induces oxidative stress. Acrolein also reacts readily with nucleophilic DNA bases and protein side chains, and these reactions of acrolein have been linked to DNA modification and the modification of several transcription factors and phospholipids (Feng et al., 2006; Zemski Berry and Murphy, 2007). Exposure to high concentrations of acrolein, however, triggers necrotic or apoptotic cell death (Li et al., 1997). Previous studies have shown that low doses of acrolein elicit vasopressor effects suggesting changes in systolic blood pressure (Egle, Jr. and Hudgins, 1974; Green and Egle, Jr., 1983), and repeated exposure to α-ethylacrolein has been found to cause mild cardiac hypertrophy in rats (Appelman et al., 1981). Continuous 1 year oral exposure to acrolein in dogs revealed little toxicity except for a mild and persistent depression of serum albumin, calcium, and decrease in protein values (Parent et al., 1992). Also, variable changes in coagulation times were observed, but the significance of these findings remains uncertain. Our studies show that acrolein feeding in rodents suppresses phenylephrine-mediated vasopressor responses, exacerbates cardiac ischemia/reperfusion-induced injury, and blocks NO donor-mediated cardioprotection (Tsakadze et al., 2003; Luo et al., 2007; Wang et al., 2008).
In the current study, we examined the effects of acrolein on circulating lipoprotein levels and metabolism. Plasma lipoprotein levels are exquisitely sensitive to systemic inflammation and injury and previous studies have shown that exposure to reactive toxicants or infectious agents induces dyslipidemia (Khovidhunkit et al., 2004;Tous et al., 2005;Kitagawa et al., 1992). Given that ingesting water and food represents an abundant source of environmental aldehydes, we tested whether shortterm oral exposure to acrolein affects plasma lipoproteins. Our results show that acrolein feeding increases total plasma cholesterol and VLDL levels and induces lipoprotein modification. Such changes in plasma lipoproteins could contribute to cardiovascular disease risk due to environmental exposure to acrolein.
Methods
Animals
Adult C57BL/6 and male apoE-null mice (7–16 weeks old; Jackson Laboratories, Bar Harbor, ME) and male Sprague-Dawley rats (10–12 weeks old; Harlan, Indianapolis, IN) were housed, fed, and watered ad libitum according to animal care guidelines and UofL IACUC-approved protocols. Animals were fed autoclaved standard Rodent Diet 5010® (LabDiet, PMI Nutritional International).
Acute Acrolein Experiments
Dose-, Time-, and Route-Dependent Protocols
Mice were gavage-fed water (control) or acrolein in water (treated) at indicated doses in 100 µl water and euthanized 24h later with sodium pentobarbital in distilled water (60 mg/kg i.p. in 0.1 ml). Naïve, age- and sex-matched mice served as additional controls. To examine time-dependent changes, mice were gavage-fed water or acrolein (5 mg/kg) and euthanized at indicated after gavage. To test whether oral administration was essential for acrolein action, mice were also treated with acrolein by intraperitoneal injection and euthanized at indicated times after treatment. Mice were allowed free access to food and water throughout the treatment.
Stress Response Protocols
C57BL/6 mice were pretreated with corn oil or RU486 (mifepristone; 25 mg/kg, in corn oil, i.p.; Sigma) 1h before water or acrolein (5 mg/kg) oral exposure. This protocol is effective in blocking hypercorticoid-induced responses in mice (Nakamura et al., 2004). Plasma corticosterone levels were measured by ELISA (OCETIA; IDS Inc., Fountain Hills, AZ). The role of catecholamine release was tested by pre-treating mice with hexamethonium bromide (30 mg/kg, i.p.; Sigma) 1h before oral water or acrolein exposure to induce ganglionic blockade (Chen et al., 2005). To evaluate the contribution of HMG-CoA reductase, mice were pretreated with simvastatin (40 or 80 mg/kg; i.p.; Calbiochem) or vehicle, 1h before treatment.
Analytical Measurements
Blood and Urine Parameters
Blood was withdrawn from the right ventricle of pentobarbital-anesthetized mice using 0.2 M Na4·EDTA-coated syringes (25 Ga.; 1 ml) and placed in precoated Eppendorf tubes (20 µl/ml blood). Fresh blood was used for measurement of blood glucose (Hemocue Glucose 201, HemoCue, Inc., Mission Viejo, CA) and hematocrit. Standard plasma and urine parameters in pooled urine samples collected from 3 mice housed in standard metabolic cages for 24h following treatment (Bayer Clinitek 50, GMI, Inc., Ramsey, MN) were measured by the Clinical Pathology Core Laboratory at University of Louisville.
Measurement of Acrolein in Rodent Chow
Acrolein in rodent food was measured by GC-MS analysis (Vesely et al., 2003). For this, standard rodent chow (as above; 12 % kcal fat) samples were ground in water. Using a manual SPME holder (Supelco, USA), a 65 µm PDMS/DVB (Supelco, USA) fiber was placed for 10 min in the head space of 1 mL of a O-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine hydrochloride (PFBHA) solution (17 mg/ ml) in a 5 mL vial at an incubation temperature of 60 °C. The fiber loaded with derivatizing reagent (PFBHA) was then exposed for 10 min in the head space of a solution of 1–10 µM crotonaldehyde (internal standard) and then exposed for 20 min to the head space of rodent food samples (50 mg–300mg) in water under constant stirring (60 °C). Desorption of the SPME fiber was performed at 270 °C in the vaporizing injector, using a dedicated SPME liner in splitless mode (Agilent 5181-3316, USA) in a HP-5 capillary column (30 m x 0.25 mm, film thickness 0.25 µm). Helium was used as the carrier gas at a constant flow of 1 ml/min. The GC column temperature was set at 60 °C, increased by 8 °C/min until 150 °C and then by 100 °C/min until 270 °C and kept at this temperature for 6 min. The retention times for acrolein and crotonaldehyde were 8.30 and 8.56 min and 10.70 and 10.86 minutes, respectively (doublet observed for α,β-unsaturated aldehyde stereoisomers). The total analysis time was 18.5 min. The gas chromatograph was coupled to a 5973 detector for mass spectrometry analysis. Mass spectra were recorded in the electron impact (EI) ion mode at electron energy of 70 eV. For quantitative analysis by selective ion monitoring (SIM) was used. For acrolein and crotonaldehyde, the mass spectrometer was set to monitor molecular ion at m/z 181 to scan chromatograms for aldehyde derivatives. The m/z 181 ion was highly reproducible and the most intense ion of aldehydes observed.
Lipoprotein Electrophoresis
Individual plasma samples (300–500 µl) were mixed with Optiprep and overlayed with HEPES-buffered saline on top (50 µl; 0.85% NaCl; 10 mM HEPES; NaOH, pH 7.4) in Beckman Polyallomer centrifuge tubes and centrifuged at 350,000xg for 3h at 16 °C. Undiluted plasma and aliquots of ultracentrifugation fractions (2 µl) were loaded on agarose lipoprotein gels and separated at 100V for 45 min (LIPOGEL, Beckman-Coulter). For undiluted plasma analyses, an equivalent number of control and treated samples were loaded on each gel to normalize inter-gel differences. Gels were fixed in a fixing solution for 5 min (fixative: 180 ml reagent alcohol; 90 ml H2O; 30 ml glacial acetic acid), stained overnight with 0.070% staining solution (165 ml reagent alcohol, 3 ml Paragon Lipo Stain, 135 ml H2O), and destained for 2h (450 ml reagent alcohol, 550 ml H2O). The electrophoretic mobility (EPM) was calculated by measuring the distance (in mm) from the loading origin to the leading margin of the band front after migration.
Plasma Lipids and Lipoprotein Subclass Profiles
Total cholesterol, triglycerides, and phospholipids in plasma were determined using the Cholesterol CII Enzymatic Kit (Wako #276–64909), the L-Type TG-H Kit (Wako #432–40201), and the Phospholipids B Kit (Wako #990–54009), respectively. All lipoprotein values were calculated using calibrated standards (Wako). In addition, plasma (120 µl) was diluted in FPLC buffer and fractionated using a Beckman Biosys 510 FPLC (1 ml/min; 2 – Superose 6 columns in series) and collected in 0.5 ml fractions for measurement of total cholesterol, triglycerides, and phospholipids as above. For characterization of lipoprotein subclasses, plasma (100 µl) from treated mice was frozen (−80 °C) and analyzed within 2 weeks) by NMR spectroscopy (LipoScience, Inc., Raleigh, NC)(Otvos et al., 2002;Hammad et al., 2003). Plasma serum amyloid A (SAA) was measured spectrophotometrically by ELISA kit (SAA, BioSource, Invitrogen). Plasma HDL-associated lecithin-cholesterol acyl transferase (LCAT) activity was measured fluorimetrically using a commercial kit (Roar Biochemical, New York, NY). Hepatic and plasma apoB100 and apoB48 levels were determined by Western blot in 5 µl plasma run on SDS-PAGE (5% gel; Criterion) and blotted with an apoB100 (s-18) affinity-purified goat polyclonal antibody (1:500; Santa Cruz Biotech) in 2.5% BSA. A 1:5000 dilution of AB ECL plus specific donkey anti-goat IgG horseradish peroxidase-linked secondary antibody in 2.5% BSA was used and the blot was developed using an ECL plus kit and read on a Typhoon 9400 variable image scanner. Densitometric values of apoB bands were normalized to total amido black staining. Hepatic LDLR protein was measured using an anti-LDLR antibody (R&D Systems, Minneapolis, MN).
Lipoprotein Exposure to Acrolein in vitro
Human lipoproteins (9 µl; human LDL and HDL; 6.89 and 8.1mg/ml, respectively; Calbiochem) were incubated with acrolein (1 µl; 100–10,000 µM) in phosphate buffered saline (PBS) for 30 min at 37 °C. The lipoprotein samples or differentially-sedimented mouse plasma samples (2 µl) were separated on lipogels. Free acrolein was removed by overnight dialysis against PBS at 4 °C.
Lipoprotein (LPL) and Hepatic (HL) Lipase Measurements
For measuring LPL activity, mice were injected with heparin (10 U/g, i.p.; Sigma) in sterile saline 30 min before euthanasia and the blood was collected via cardiac puncture after anesthesia. For measuring HL activity, control and acrolein-treated mice were injected with heparin (5 U/g, tail vein; Sigma) 10 min before euthanasia and the blood was collected as described above. Total plasma LPL and HL activities were measured fluorimetrically using commercial kits (CONFLUOLIP, Cat. #: PR2003 or PR2004, respectively; Progen Biotechnik GmbH; Heidelberg, Germany).
Organ Analyses
Body, heart, liver, kidney, stomach, and gastrointestinal tract were weighed, and formalin-fixed or snap frozen in liquid nitrogen and stored at −80 °C until used. Glutathione (total GSH) and thiobarbituric acid (TBARS) levels were measured spectrophotometrically using previously published methods (Srivastava et al., 2002). For hepatic histology, sections of the liver were stained with H&E, Oil Red O, PAS±α-amylase, or PCNA. For hepatic cholesterol and triglycerides measurements, lipids were extracted by the Bligh and Dyer (1959) procedure and measured using commercial kits (from Wako) as described above.
Hepatic qRT-PCR Analyses
RNA isolation and qRT-PCR
Hepatic RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s protocol, and RNA pellets in ethanol were additionally washed with 75% ethanol, dried and dissolved in 100 µl of RNAse-free water. Nanodrop and Bioanalyzer (Agilent) analyses showed that RNA was of good quality and purity (ratio 260/280 and 260/230 > 1.8). Equal amount (2 µg) of RNA from each sample was converted into cDNA with AMV reverse transcriptase (Promega) followed by real-time PCR analysis. Primers for murine apoB100, hepatic lipase (Lipc), HMG-CoA reductase, LDLR, SREBF-1 and SREBF-2 were from SABiosciences. GAPDH mRNA level was used for normalization (primers: 5'-AGATCCACAACGGATACATT-3' (sense) and 5'-TCCCTCAAGATTGTCAGCAA-3' (antisense)). Real-time PCR was carried out in an iCycler iQ™ (Biorad, CA) thermocycler using SYBR green/Fluorescein PCR master mix (SABiosciences) and the following program: 95 °C for 5 min followed by 45 cycles of 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s, followed by melting curve analysis. The ΔΔC(T) method was used for quantitation (Livak et al., 2001).
qRT-PCR Cytokine Gene Array
The Mouse Inflammatory Cytokines and Receptors RT2 Profiler™ PCR Array and SYBR Green real-time PCR master mixes were from SABiosciences (Frederick, MD). For cytokine gene array, 1 µg total RNA/mouse was pooled for each group (control, n=3; acrolein, n=4) and RT-PCR was carried out according to the manufacturer’s protocol using iCycler iQ™ (Biorad, CA). A gene was eliminated from analysis if Ct exceeded 35 cycles, and of the 89 genes assayed, 25 genes were undetectable or expressed at low levels (i.e., Ct>35). Expression of 64 genes was analyzed using PCR Array Data Analysis Web Portal. The constitutive genes, Gusb, Hprt1, Hsp90ab1, Gapdh and Actb, were used for data normalization. The relative abundance of RNA was calculated by the ΔΔCt method.
Data Analysis
Values are reported as mean ± standard error (SEM). For statistical comparison between two groups, Student’s paired or unpaired t-test was used where appropriate. For comparing more than two groups, one-way ANOVA and Student-Newman-Keuls post-hoc test was used. For all statistics, type I error rate was controlled at α=0.05.
Results
Systemic toxicity of acrolein feeding
To study the systemic effects of acrolein, mice were gavage-fed 0.1 to 5 mg acrolein/kg body weight. Acrolein level in autoclaved rodent chow was 0.15±0.02 µg/g food as measured by GC-MS. Thus the background level of acrolein contributes to a negligible amount of acrolein at the highest dose used (5 mg/kg; <1 %), but significant amount (~30 %) at the lowest dose (0.1 mg/kg) used. Treatment with this dose-range of acrolein did not lead to overt toxicity. No mouse died during the protocol and no changes in animal activity or body weights were observed. At the highest dose, there was no change in plasma liver enzymes (ALT, AST) or alkaline phosphate activity levels 24h after acrolein-feeding, indicating no gross injury to the liver (Table 1). No change in blood hematocrit was detected (control: 38.7±1.5, n=12; acrolein: 38.4±1.3, n=11). Urine analysis of pooled samples from control and acrolein-treated mice (5 mg/kg; 24h post-treatment) revealed no difference in urine glucose, protein, ketones, specific gravity, pH, nitrates, creatinine, or any macroscopic variables (data not shown), indicating the lack of general toxicity. No changes in plasma Na+ or K+ levels were observed, and while a small increase in plasma chloride was observed, the significance of this finding is unclear (Table 1). At the 5 mg/kg dose, consistent decreases were, however, observed in plasma albumin and plasma total protein. Collectively, these observations suggest that exposure to acrolein within the dose-range studied did not induce lethality or result in non-specific toxicity.
Table 1.
Plasmatic changes in acrolein-fed mice.
| Control ApoE −/− |
ACRO ApoE −/− |
Control C57BL/6 |
ACRO C57BL/6 |
|
|---|---|---|---|---|
| Cholesterol1 | 207.4±17.1 (8) | 269.8±63.5 (4)* | 64.4±2.1 (10) | 115.3±9.0* (9) |
| Phospholipids1 | 185.3±30.5 (8) | 190.0±16.9 (4) | 132.5±5.9 (10) | 230.7±30.4* (7) |
| Triglycerides1 | 75.9±37.1 (8) | 144.3±64.0 (4)* | 67.5±6.3 (10) | 382.1±114.3* (9) |
| BUN1 | 21.3±1.5 | 19.8±2.7 | 19.8±2.8 | 20.5±4.4 |
| Creat1 | 0.2±0.0 | 0.2±0.0 | 0.3±0.0 | 0.3±0.0 |
| Alb2 | 21.7±1.5 | 16.0±0.7* | 21.3±1.9 | 13.3±1.0* |
| AlkP3 | 80.0±9.5 | 64.7±12.5 | <20 | <20 |
| AST3 | 132.7±56.8 | 48.6±47.5 | 49.8±18.1 | 53.5±18.0 |
| ALT3 | 32.7±4.7 | 43.8±22.2 | 46.0±2.9 | 47.2±6.1 |
| Na+1 | 181.3±4.5 | 176.8±5.1 | 160.0±2.2 | 157.7±3.1 |
| K+1 | 3.3±0.1 | 4.0±0.2* | 4.3±0.2 | 4.3±0.2 |
| Cl−1 | 70.3±2.3 | 86.6±6.1* | 102.8±0.7 | 106.2±0.6* |
| TP1 | 3.0±0.2 | 2.7±0.3 | 4.4±0.2 | 3.2±0.1* |
Adult C57BL/6 mice were gavage fed either water (Control) or acrolein (ACRO; 5 mg/kg) and changes in plasma constituents were measured 24h after exposure.
Units: =[mg/dl],
=[g/L],
=[U/L];
Values = mean ± S.D.; (n) = number of mice;
Abbreviations: BUN, blood urea nitrogen; Creat, creatinine; Alb, albumin; AlkP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TP, total phosphorus; ND, not determined.
= significant difference with appropriate control by unpaired t-test (P<0.05). For non-lipid plasma parameters: Control ApoE−/−, n=3 pooled samples from 6 mice; ACRO-treated ApoE−/−, n=5 samples (2 pooled) from 7 mice.
No difference in liver, heart, or kidney GSH was detected 24h after exposure (data not shown). Hepatic TBARS content was not different between acrolein-treated and control mice at 4h and 24h after gavage. Similarly, no obvious differences in hepatic triglycerides content, histological staining for fat content (Oil Red O staining), and overt injury (H&E staining) were observed between control and acrolein-treated mice at 24h post-exposure (data not shown). Collectively, these data indicate that acrolein treatment was not associated with sustained systemic or hepatic oxidative stress.
Acrolein-induced dyslipidemia: hypercholesterolemia and hypertriglyceridemia
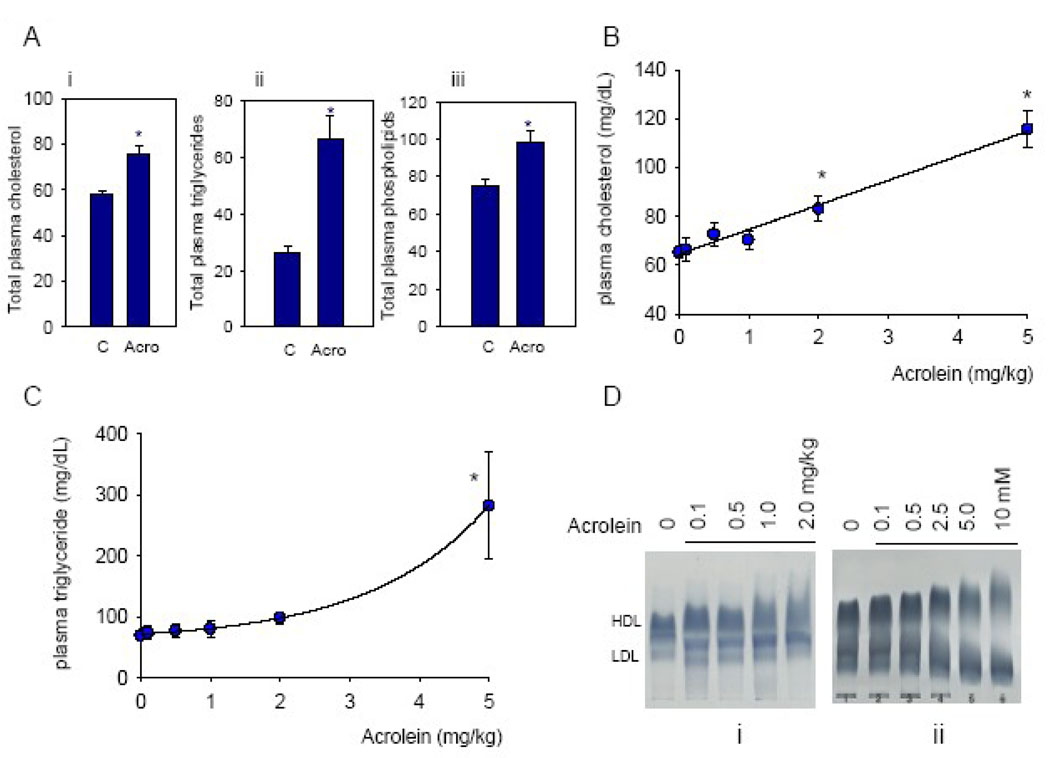
To examine how acrolein affects lipoproteins, changes in total plasma cholesterol were measured. Treatment with acrolein resulted 24h later in significant increases in total plasma cholesterol, triglycerides and phospholipids (Fig. 1A). Total cholesterol increased in a dose-dependent manner with no discernable threshold (Fig. 1B). Similar increases in triglycerides and phospholipids were observed, however, in the case of triglycerides the increase was more pronounced at doses exceeding 2 mg/kg (Fig. 1C), indicating that increases in total cholesterol and triglycerides may be due to mechanisms with different dose dependencies. Lipoproteins in the plasma of acrolein-fed animals displayed altered electrophoretic mobility. As shown in Fig. 1D, lipoproteins in the plasma from acrolein-fed mice show greater inhomogeneity of migration than those from water-fed mice. Changes in lipoprotein mobility were observed even with a dose of 0.1 mg/kg and were progressively increased at higher doses. Collectively, these data suggest that acrolein feeding increases total cholesterol, triglycerides and phospholipids and induces changes in lipoprotein charge and/or structure.
Figure 1.
Acrolein feeding induces dyslipidemia. Adult male C57BL/6 mice were fed acrolein and changes in plasma lipids were measured 24h after treatment. (A) Changes in total plasma cholesterol (i), triglycerides (ii) and phospholipids (iii) (all in mg/dL) in mice fed 5 mg acrolein/kg; Dose-dependent changes in plasma cholesterol (B) and triglyceride (C). Data are shown as discrete points. (D) Agarose gel electrophoresis of plasma lipoproteins isolated from mice-fed the indicated concentrations of acrolein (i), or lipoproteins incubated with the indicated concentrations of acrolein ex vivo (ii). Dose-response relationship for cholesterol (B) was the best fit of a linear equation (y=mx+c), where m=10.5±0.7 and c=64.5±1.5; R2=0.99. For triglycerides, (C) the relationship plotted was the best fit of an exponential equation (y=a+b.exp(k.x)) where a=60.2±3.3, b=11.8±2.3 and k=0.59±0.04; R2=0.99). Data in Fig. 1B, 1C and 1D are from same mice. Data are shown as mean ± S.E.M., * P<0.05; n=7–9.
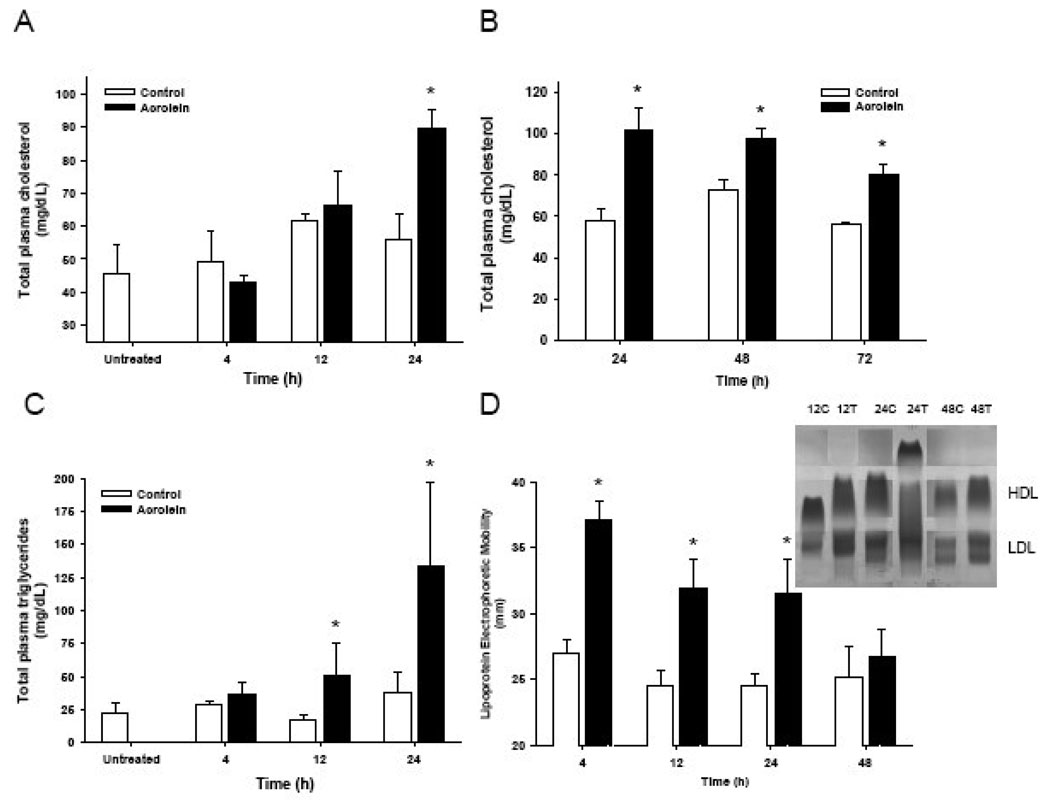
Time-course studies with C57BL/6 mice indicated no increase in plasma cholesterol 12h after treatment (Fig. 2A). Maximal levels were observed 24h after feeding and the cholesterol levels remained elevated for 72h (Fig. 2AB). Changes in plasma triglycerides were more rapid. Significant elevation of plasma triglycerides was observed 12h after treatment. Maximal triglyceride levels were observed 24h after treatment (Fig. 2C). Thereafter triglyceride levels returned to baseline values within 72h (not shown in Fig. 2C). In contrast to triglycerides and cholesterol levels, changes in lipoprotein electrophoretic mobility were maximal within 4h of acrolein exposure. The mobility remained elevated for 24h and returned to baseline 48h after treatment (Fig. 2D). These observations suggest that acrolein feeding induces early changes in lipoprotein structure and that these changes are followed by an increase in the levels of plasma triglycerides followed by cholesterol. Levels of lipoproteins remain elevated after a single dose of acrolein. A similar time course and degree of change were observed in Sprague-Dawley rats. In these rats acrolein dosing at 2 mg/kg body weight/day induced a sustained increase in plasma cholesterol levels over 5 days (Control: 65.0±5.4; acrolein: 102.3±1.5 mg/dL; n=5, 4 rats, respectively).
Figure 2.
Time course of acrolein-induced dyslipidemia. Plasma cholesterol (A, B) and triglyceride (C) levels and (D) electrophoretic mobility of lipoprotein isolated from adult C57BL/6 mice at indicated times after a single gavage dose of 5 mg acrolein/kg. Inset in Panel D shows electrophoretic separation of lipoproteins isolated from control (c) or treated (T) mice, 12, 24, and 48h after treatment. Data are shown as mean ± S.E.M., * P<0.05; n=5–7.
Lipoprotein modifications
Plasma lipoproteins carry cholesterol and triglycerides in particles of discrete size classes (VLDL, LDL and HDL), hence to identify which specific lipoproteins were affected, lipoproteins were analyzed by FPLC, ultracentrifugation and NMR. In comparison with lipoproteins of untreated mice, those of acrolein-exposed mice had significantly altered VLDL profiles with little or no change in HDL and LDL profiles (Fig. 3). Acrolein treatment increased VLDL cholesterol, phospholipids, and triglycerides levels by 100-, 30-, and 7.2-fold, respectively, compared with control levels (Table 2). Moreover, acrolein treatment altered VLDL lipid composition ratio (cholesterol:phospholipids:triglycerides) from 1:4:216 in controls to 1:1:12 indicating a shift toward increased cholesterol content in VLDL (Table 2). NMR data further support the FPLC results of enhanced VLDL triglycerides largely as a specific function of increased medium VLDL with an ~20% increase in overall particle size from 40.6±1.3 nm to 47.6±1.7 nm (Table 3). Similarly, acrolein increased total LDL by increasing the quantity of large LDL (~3x) and small LDL (~4x) classes without a change in overall particle size (Table 3). To a lesser degree, acrolein also increased large HDL (~1.3) and HDL cholesterol (~1.2x) while significantly, albeit modestly, decreasing HDL particle size (~4 %; Table 3). Ultracentrifugation studies revealed selective acrolein-induced inhomogenities in electrophoretic mobility both in HDL and VLDL components on agarose gels (Fig. 3C). Specifically, HDL mobility generally was enhanced by acrolein in C57BL/6 and apoE-null mice (Fig. 3C and Fig. 4B, left hand side) perhaps as a function of particle size, which was slightly decreased (Table 3), or direct structural modification as in Fig. 1D. In contrast, VLDL staining in Lipogels was more smeared rather than appearing as a uniformly increased mobility front as for HDL (Fig. 3C and Fig. 4B, right hand side). Moreover, plasma serum amyloid A, a known modifier of HDL function, was significantly elevated by acrolein treatment 24h after exposure (Fig. 3D), whereas plasma levels of two major inflammatory cytokines, IL-6 and TNF-α, were not altered at this time point (Table 4).
Figure 3.
Dyslipidemic changes in acrolein-fed C57BL/6 mice. Adult (8–12 week old) male mice were gavage-fed acrolein (5 mg/kg) and 24h later plasma lipoproteins were separated by FPLC and ultracentrifugation. FPLC elution profile of (A) cholesterol and (B) triglycerides in plasma isolated from water-fed (black line) or acrolein-fed (dotted line) mice. Individual lipoprotein fractions are indicated. (C) Agarose gel electrophoresis of ultracentrifugation fractions isolated from water-fed (control) or acrolein-fed (treated) mice. HDL-rich lipoproteins displayed the highest mobility as indicated. (D) Serum amyloid A levels in control and acrolein-treated mice. Data are shown as mean ± S.E.M., * P<0.05; n=5.
Table 2.
Acrolein-induced changes in plasma lipoproteins.
| Control | Acrolein | Change | ||
|---|---|---|---|---|
| Fraction | VLDL | |||
| Cholesterol1 | 0.1±0.2 | 11.5±14.2 | ↑115 | |
| Phospholipids1 | 0.5±0.9 | 11.5±15.9 | ↑23 | |
| Triglycerides1 | 26.6±4.7 | 160.4±183.6* | ↑6 | |
| LDL | ||||
| Cholesterol | 12.4±1.0 | 24.5±14.9 | ↑2 | |
| Phospholipids | 3.6±3.7 | 8.1±7.8 | ↑2.25 | |
| Triglycerides | 32.4±26.9 | 65.0±43.9 | ↑2 | |
| HDL | ||||
| Cholesterol | 58.3±6.4 | 55.2±26.5 | ↔ | |
| Phospholipids | 83.5±20.8 | 83.1±59.5 | ↔ | |
| Triglycerides | 0.3±0.6 | 1.2±2.4 | ↑4 |
Adult C57BL/6 mice were gavage-fed either water (Control; n=3) or acrolein (5 mg/kg; n=4) and changes in different lipoprotein subclasses were measured 24h after exposure.
Units: =[mg/dl], Values = mean ± S.D.;
= significant difference between Control and acrolein values (P=0.057).
Table 3.
Nuclear magnetic resonance spectroscopy (NMR) analysis of lipoproteins in acrolein-fed mice.
| VLDL | Control |
Acrolein 5 mg/kg bwt |
|---|---|---|
| Particle Concentration (nmol/L) | ||
| Total VLDL | 10.9±4.2 | 199.9±27.9*** |
| Large VLDL | 0.0±0.0 | 3.8±2.2* |
| Medium VLDL | 7.0±3.1 | 194.4±28.9*** |
| Small VLDL | 4.0±1.4 | 1.6±1.6 |
| Triglycerides1 | 10.8±5.0 | 317.1±45.6*** |
| Size (nm) | 40.6±1.3 | 47.6±1.7* |
| LDL | ||
| Particle Concentration (nmol/L) | ||
| Total LDL | 107.9±19.8 | 364.9±50.3** |
| IDL | 1.3±1.0 | 0±0 |
| Large LDL | 67.0±10.2 | 210.6±49.2** |
| Small LDL | 39.6±13.6 | 154.2±66.9 |
| Medium Small | 10.8±4.7 | 48.4±31.9 |
| Very Small | 28.8±11.5 | 105.9±37.5* |
| Size (nm) | 22.1±0.2 | 21.7±0.3 |
| HDL | ||
| Particle Concentration (µmol/L) | ||
| Total HDL | 16.5±1.1 | 21.2±0.4** |
| Large HDL | 16.5±1.1 | 21.2±0.4** |
| Medium HDL | 0±0 | 0±0 |
| Small HDL | 0±0 | 0±0 |
| Cholesterol1 | 45.6±2.8 | 55.8±1.5* |
| Size (nm) | 9.5±0.1 | 9.1±0.0* |
Adult male C57BL/6 mice were gavage fed either water (Control; n=6) or acrolein (5 mg/kg; n=4). Twenty four hours after treatment, blood was collected and particle concentration in each of the lipoprotein subclasses was estimated by NMR as described under Materials and Methods.
Units: = [mg/dl] for lipids values provided by LipoScience as estimates from particle # and % composition. Values = mean ± S.E.M.;
, significant difference between Control and acrolein values (p<0.05),
, significant difference between Control and acrolein values (p<0.01),
, significant difference between Control and acrolein values (p<0.001) as indicted by One Way ANOVA and Bonferroni post-test.
Figure 4.
Acrolein-induced dyslipidemia in apoE-null mice. Adult (8–12 week old), male apoE-null mice maintained on normal chow were gavage-fed acrolein and 24h later plasma was isolated and used for analysis. (A) Plasma levels of cholesterol and triglycerides in water (control) and acrolein-fed (acrolein) mice. (B) Agarose gel electrophoresis of ultracentrifugation fractions isolated from water-fed (control) or acrolein-fed (treated) mice. HDL-rich lipoproteins displayed the highest mobility as indicated. Data are shown as mean ± S.E.M., * P<0.05; n=6–8.
Table 4.
Effect of oral water (Control), oral acrolein exposure (5 mg/kg) or lipopolysaccharide (LPS, 100 µg/kg, i.p.) in male C57BL/6 mice at 24h post-exposure on plasma cytokine levels.
| Parameter | Control | ACRO | LPS |
|---|---|---|---|
| IL-61 | 2.6±0.9 (5) | 2.2±0.7 (5) | 727.4±39.4* (4) |
| TNF-α1 | 6.1±1.5 (9) | 14.5±5.3 (7) | 32.9±17.2* (4) |
Values = mean ± S.D.;
Units: = [pg/ml], number of mice (n) used in each group is shown in parenthesis.
P<0.05 for LPS treatment versus control.
To examine whether acrolein-induced dyslipidemia was due to a corticoid- or autonomic nervous system (ANS)-mediated stress response, C57BL/6 mice were pretreated with the glucocorticoid receptor antagonist, RU486, or hexamethonium bromide, respectively. RU486 had no effect on acrolein-induced elevation of cholesterol and phospholipid levels, but reduced the overall increase in plasma triglycerides by ~50%. However, the resultant mean triglyceride values were neither significantly different from acrolein treatment alone nor from control mean values indicating only part of the triglycerides response was glucocorticoid receptor-mediated (Table 5). RU486 treatment did not affect acrolein-induced changes in the electrophoretic mobility of lipoproteins isolated from acrolein-fed mice (data not shown). Acrolein-induced decreases in blood glucose and plasma albumin were not affected by RU486 treatment (Table 5). RU486 treatment alone significantly increased plasma corticosterone levels, which demonstrated effective receptor blockade (data not shown). Additionally, neither treatment with the ANS ganglionic blocker hexamethonium bromide nor with simvistatin affected acrolein-induced elevation of plasma lipids or changes in electrophoretic mobility (data not shown). Similarly, neither fasting nor sex (data not shown) of the mice affected acrolein-induced elevation of plasma lipids (water+fasting: cholesterol, 83±4 mg/dL; triglycerides, 40±4 mg/dL; ACRO+fasting: cholesterol, 103±6 mg/dL; triglycerides, 132±25 mg/dL; n=5,5, p<0.05). Interestingly, intraperitoneal administration of acrolein also increased plasma cholesterol (from 50±3 to 70±4 mg/dL; n=6,6), but did not affect triglycerides (or plasma albumin) at 24h post-exposure, indicating that acrolein-induced hypercholesterolemia was independent of the route of exposure and that changes in plasma cholesterol and triglycerides likely were due to different mechanisms.
Table 5.
Effect of RU486 on acrolein toxicity.
| Treatment | ||||
|---|---|---|---|---|
| Parameter | Control (n) | RU486 (n) | ACRO (n) | RU486+ACRO (n) |
| Cholesterol1 | 65.7±6.7 (13) | 67.1±8.9 (11) | 110.7±24.6* (12) | 102.1±25.4* (12) |
| Phospholipids1 | 132.6±17.0 (13) | 126.0±15.8 (11) | 218.2±70.0* (10) | 194.6±59.6* (10) |
| Triglycerides1 | 63.3±19.1 (13) | 49.0±10.0 (11) | 333.4±307.0* (12) | 195.7±229.4a (12) |
| Glucose1 | 172.2±19.2 (9) | 167.9±25.5 (9) | 113.1±19.1* (9) | 124.2±23.1* (10) |
| Protein2 | 40.6±1.7 (6) | 43.1±2.0 (6) | 33.9±2.9* (6) | 33.4±3.0* (6) |
Adult C57BL/6 mice were gavage fed either water (Control) or acrolein (ACRO; 5 mg/kg) and changes in the indicated plasma constituents were measured 24h after treatment. Values = mean ± S.D.;
Units: =[mg/dl],
=[g/L]; n = number of mice.
= significant difference between ACRO or RU486+ACRO treatment and Control values.
= not significantly different from ACRO alone (P=0.086), Control (P=0.092), or RU486 alone (P=0.172) treatment.
Role of hepatic synthesis and uptake
Changes in plasma lipoprotein levels by acrolein could be due to an increase in lipoprotein synthesis or a decrease in lipoprotein uptake. To examine uptake, we used apoE-null mice. In these mice, deletion of the apoE gene results in decreased lipoprotein clearance. We reasoned that if uptake is affected, these mice, with a basal decrease in uptake, should show less change in plasma lipoproteins than background (C57BL/6) mice. Treatment with acrolein did not affect BUN and plasma liver enzymes in apoE-null mice (Table 1). Mild increases in plasma potassium and chloride levels were observed. As in C57BL/6 mice, there was a significant decrease in plasma albumin, but not in total protein (Table 1). Levels of plasma cholesterol and triglycerides were significantly increased (Fig. 4A). The absolute magnitude of the changes in the cholesterol and triglycerides levels was similar to that observed in C57BL/6 mice, and the time course of the response was similar. Feeding acrolein induced distinct changes in the electrophoretic mobility of both the HDL and VLDL fractions isolated by ultracentrifugation and run on agarose gels (Fig. 4B). These data suggest that acrolein-induced dyslipidemia stimulated in apoE-null mice is not attenuated by apoE-deficiency or by high background levels of VLDL and LDL.
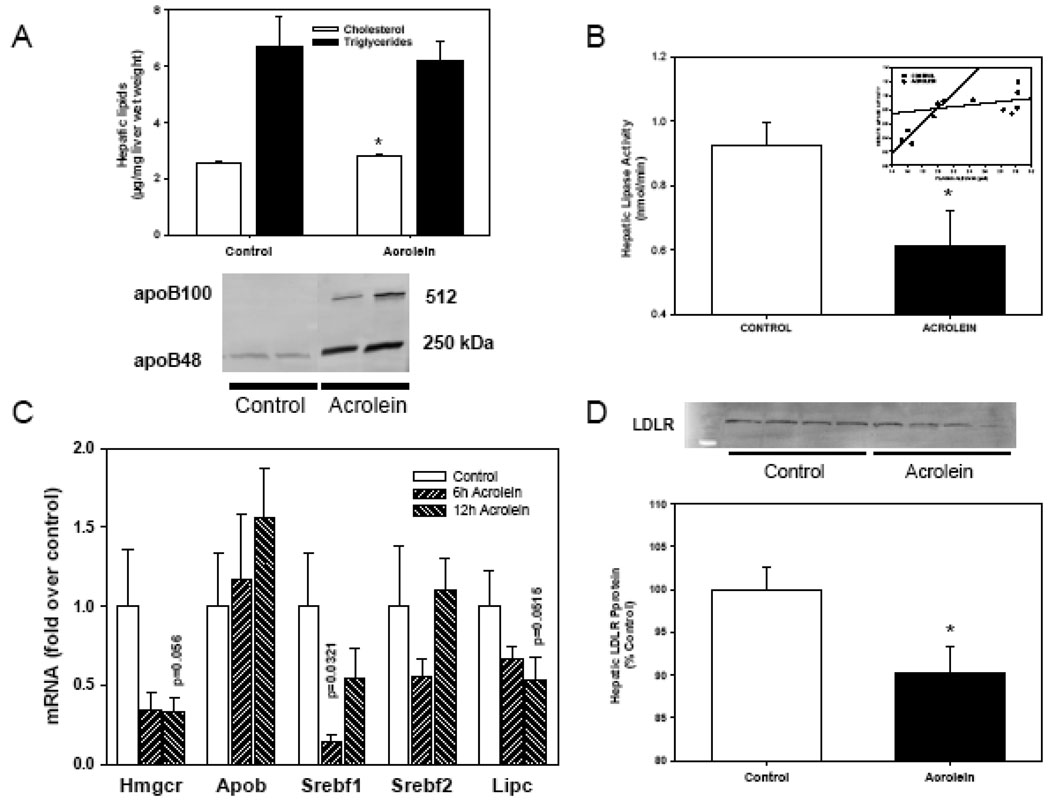
To examine whether hepatic metabolism of cholesterol is affected, we measured acrolein-induced changes in hepatic lipids and mRNA levels. Acrolein enhanced hepatic cholesterol level (Control, 2.65±0.09; ACRO, 3.03±0.03 µg/mg wet weight; n=4,4; p=0.0083), but not triglycerides level at 6h (Control, 7.9±2.4; ACRO, 4.8±1.6 µg/mg wet weight; n=4,4) while no change in either lipid was detected at 12h (data not shown). As shown in Fig. 5A, acrolein treatment led to a significant increase in hepatic cholesterol with no change in triglycerides at 24h (Fig. 5A). This increase in liver cholesterol was accompanied by a significant increase in the plasma apoB100 and apoB48 proteins (Fig. 5A). To test for a role of HMG-CoA reductase, we pretreated mice with simvastatin and assessed lipids at 24h post-acrolein exposure. Simvastatin pretreatment neither affected acrolein-induced dyslipidemia nor hepatic lipid levels (data not shown).
Figure 5.
Hepatic changes in acrolein-fed mice. Adult male C57BL/6 mice were gavage-fed acrolein (5 mg/kg) or water and hepatic changes were estimated at 6, 12 or 24 h after feeding. (A) Hepatic cholesterol and triglyceride levels (i) and plasma apoB100 and apoB48 24h after acrolein exposure. Control mice were fed water (n=6). (B) Hepatic lipase (HL) activity measured 12h after water (control) or acrolein gavage in mice (n=6). (C) Hepatic gene expression at 6h and 12h after water (control) or acrolein exposure. Changes in the mRNA levels of SREBF1, HMG-CoA reductase and HL (Lipc) were obtained using qRT-PCR (n=4). Changes in HMG-CoA mRNA levels were confirmed by normalization to stable levels of GAPDH mRNA. (D) Western blot of hepatic LDLR protein at 24h after water or acrolein exposure. Data are presented as mean ± S.E.M. * P < 0.05.
To assess whether the increase in plasma cholesterol was due to alterations in cholesterol removal, changes in plasma hepatic lipase and lipoprotein lipase were measured. Treatment with acrolein did not affect post-heparin lipoprotein lipase activity in the plasma (Control: 12.4±2.2 pmol/min; ACRO: 13.2±2.1 pmol/min; n=6,6). Similarly no changes in HDL-associated lecithin-cholesterol acyl transferase (LCAT) activity were detected (data not shown). In contrast, post-heparin hepatic lipase activity was significantly suppressed 12h after acrolein exposure (Fig. 5B). At 12h post-treatment, hepatic lipase activity suppression was strongly correlated with decreased plasma albumin level (R2=0.874) indicating a relationship between acrolein-induced injury and hepatic lipase regulation that was not present in water-fed matched controls (R2=0.024)(Fig. 5B, inset).
Hepatic gene analysis in acrolein-treated mice
Lipoprotein assembly and uptake
Acrolein treatment altered exchange of cholesterol between liver and plasma because plasma apoB protein was increased as shown by Western blot analysis while hepatic apoB gene level was unaltered (Fig. 5A). Similarly, qRT-PCR analysis of hepatic gene changes showed significant decrease in the mRNA level of hepatic lipase (Lipc), HMG-CoA reductase, Srebf1 and Ldlr (Fig. 5C). The decrease in Ldlr mRNA was correlated with a decrease in hepatic LDLR-protein by Western blot (Fig. 5D). Collectively, these data indicate that acrolein feeding suppresses hepatic cholesterol biosynthesis and decreases the clearance of plasma lipids with a prominent change in SREBF-dependent genes.
Inflammatory and cytokine genes
To understand if oral acrolein affects hepatic cytokine gene expression, we examined gene changes using a cytokine gene array at 6h post-treatment. Of the 89 genes assayed, 25 had mRNA levels too low to detect and of the remaining 64 genes: 9 genes were up-regulated and 12 genes were down-regulated by acrolein (Table 6). Interestingly, the anti-inflammatory cytokine gene, IL-10, was up-regulated 4.2 times while genes for IL-10 receptors, Il10ra and Il10rb, were both down-regulated by −2.5 fold (Table 6). The most significantly up-regulated genes were the MCP-3 (Scya7; +8.5-fold), CD128 (CDw128; +7.36-fold) and Interleukin 1 receptor, type II (Il1r2; +6.9-fold) while the integrin beta 2 gene (Itgb2) was the most down-regulated gene (−16.2-fold).
Table 6.
Changes in the expression of hepatic cytokine-related genes in acrolein-fed mice at 6h.
|
Up-regulated Genes |
||||
|---|---|---|---|---|
| Gene Symbol | Gene Description |
Log2 ratio 6h |
Fold Change 6h |
GenBank Accession |
| Ccl7 | Chemokine (C-C motif) ligand 7 | 3.08 | 8.46 | NM_ 013654 |
| Il8rb | Interleukin 8 receptor, beta | 2.88 | 7.36 | NM_009909 |
| Il1r2 | Interleukin 1 receptor, type II | 2.78 | 6.87 | NM_010555 |
| Ccl17 | Chemokine (C-C motif) ligand 17 | 2.28 | 4.86 | NM_011332 |
| Il10 | Interleukin 10 | 2.08 | 4.23 | NM_010548 |
| Cxcl1 | Chemokine (C-X-C motif) ligand 1 | 1.98 | 3.94 | NM_008176 |
| Ccl6 | Chemokine (C-C motif) ligand 6 Tumor necrosis factor receptor |
1.78 | 3.43 | NM_009139 |
| Tnfrsf1b | superfamily, member 1b | 1.58 | 2.99 | NM_ 011610 |
| Ccr1 | Chemokine (C-C motif) receptor 1 | 1.38 | 2.60 | NM_009912 |
|
Down-regulated Genes |
||||
| Gene Symbol | Gene Description |
Log2 ratio 6h |
Fold Change 6h |
GenBank Accession |
| Ccl2 | Chemokine (C-C motif) ligand 2 | −1.32 | −2.50 | NM_011333 |
| Ccr3 | Chemokine (C-C motif) receptor 3 | −1.32 | −2.50 | NM_009914 |
| Il10ra | Interleukin 10 receptor, alpha | −1.32 | −2.50 | NM_008348 |
| Il10rb | Interleukin 10 receptor, beta | −1.32 | −2.50 | NM_008349 |
| Il15 | Interleukin 15 | −1.42 | −2.68 | NM_008357 |
| Xcr1 | Chemokine (C motif) receptor 1 | −1.42 | −2.68 | NM_011798 |
| Ccr5 | Chemokine (C-C motif) receptor 5 | −1.72 | −3.29 | NM_009917 |
| Ccr9 | Chemokine (C-C motif) receptor 9 | −1.72 | −3.29 | NM_009913 |
| Ccl8 | Chemokine (C-C motif) ligand 8 | −1.92 | −3.78 | NM_021443 |
| Ccr2 | Chemokine (C-C motif) receptor 2 | −2.12 | −4.35 | NM_009915 |
| Cxcl11 | Chemokine (C-X-C motif) ligand 11 | −2.32 | −4.99 | NM_019494 |
| Itgb2 | Integrin beta 2 | −4.02 | −16.22 | NM_008404 |
Changes in gene expression were measured using a cytokine gene array (SA Biosciences). Differentially expressed genes (≥2.5-fold change) were identified using the ΔΔCT method.
Discussion
The major finding of this study is that oral exposure to acrolein elicits dyslipidemia in mice. Acrolein induced dyslipidemia independent of strain and gender and this was characterized by changes in the lipoprotein composition especially in VLDL cholesterol and triglycerides. The increase in VLDL cholesterol was associated with a decrease in the activity and expression of hepatic lipase, suggesting that acrolein exposure decreases cholesterol clearance and induces pro-atherogenic changes in lipoproteins.
Hepatic lipase and acrolein-induced dyslipidemia
The mechanism of acrolein-induced dyslipidemia appears to involve the parallel processes of lipid synthesis and clearance. Our data indicate that acrolein feeding promotes the accumulation of VLDL. Increases in VLDL and VLDL modifications were evident upon FPLC and ultracentrifugation studies, and Western blot analyses of apoB100 and apoB48, and NMR spectroscopy. These approaches collectively demonstrate quantitative enhancement of plasma cholesterol and triglycerides predominantly in VLDL fraction, and suggest that exposure to acrolein induces pro-atherogenic changes in plasma lipids status. Our qRT-PCR and lipase activity data indicate that plasma VLDL accumulation is likely a consequence of decreased VLDL clearance due to hepatic lipase and LDLR down-regulation more so than a consequence of increased lipid synthesis in part because several genes of cholesterol biosynthesis, including HMG-CoA reductase, also were down-regulated. LDLR down-regulation, however, may be less important than the decrease in hepatic lipase activity because acrolein feeding also increased plasma cholesterol in apoE-null mice, which are deficient in LDLR-mediated cholesterol clearance. Additionally, hepatic lipase-deficient humans and mice are dyslipidemic, and some hepatic lipase-deficient humans have elevated levels of triglycerides in both VLDL and LDL fractions (Santamarina-Fojo et al., 2004). Moreover, hepatic lipase deficiency in mice elevates plasma total and HDL cholesterol (~30%) without affecting triglycerides, however, when challenged with excess lipid these mice become hypertriglyceridemic (Homanics et al., 1995). These data indicate that acute down-regulation of hepatic lipase gene compromises lipolysis and lipoprotein uptake (Gonzalez-Navarro et al., 2004; Santamarina-Fojo et al., 2004). Based on these considerations it appears likely that acrolein-induced dyslipidemia could, in part, be attributable to lipase deficiency due to hepatic lipase inactivation – an effect also observed during acute phase response of inflammation/infection (Khovidhunkit et al., 2004).
Our analyses of hepatic gene and protein levels suggest a hepatic locus of acrolein-induced suppression of both cholesterol biosynthesis and lipid uptake. Specifically, our results show that acrolein down-regulates both hepatic lipase (Lipc) and Ldlr gene mRNAs at 6h, each of which is important in lipoprotein clearance, and acrolein decreases both hepatic lipase activity and hepatic LDLR protein levels at 12h -- the time point at which we first observe significant accrual of plasma triglycerides, followed by increased plasma cholesterol level. Acrolein appears to modulate the regulators of cholesterol metabolism, i.e., Srebf, and these changes may be sufficient to orchestrate downstream regulation of Ldlr and Lipc genes (Espenshade and Hughes, 2007). Similarly, SREBP regulate the levels of HMG-CoA reductase transcript and activity, and the former is clearly down-regulated by acrolein. Consistent with an acrolein-induced down-regulation of cholesterol biosynthesis, simvastatin, an HMG-CoA reductase inhibitor, had no effect on acrolein-induced plasma lipids. Collectively, these data indicate that acrolein feeding induces a transcriptionally-regulated decrease in cholesterol biosynthesis genes. Cholesterol is the primary negative feedback regulator of SREBP by binding to SREBP cleavage activating protein (Scap)(Espenshade and Hughes, 2007) and acrolein significantly increases hepatic cholesterol level at 6h and 24h after treatment (see Fig. 5). Interestingly, rats fed thermally-oxidized sunflower oil (~18 ml/kg; 6d) with significantly elevated carbonyl content (~32-fold) had significantly lower hepatic HMG-CoA reductase and LDLR mRNA levels compared with rats fed fresh sunflower oil (Koch et al., 2007). These data indicate aldehydes in food could, in general, suppress hepatic cholesterol biosynthesis and uptake via a common transcriptional mechanism. Moreover, we provide evidence that as in many other foods and beverages (Vesely et al., 2003) acrolein is present at low levels in the rodent food.
Role of plasma lipoprotein modifications
The mechanism of acrolein-induced dyslipidemia is likely to involve changes in the structure of plasma lipoproteins as well. Both time-dependent and dose-dependent changes in the electrophoretic mobility of the lipoproteins on agarose gels were found to precede hyperlipidemia. Our data indicate a strong association between the degree of structural modification in plasma lipoproteins and the level of hyperlipidemia – i.e., the more modified the lipoproteins, the greater the hyperlipidemia, indicating that structural changes may be related to dyslipidemia. Li et al., (2004) have reported the appearance of acrolein-modified plasma proteins of 100, 31, and 29 kDa, 1h after oral acrolein exposure in Sprague-Dawley rats (9.2 mg/kg)(Li et al., 2004). In our study, detailed analysis of the electrophoretic mobility changes shows that enhanced lipoprotein mobility is attributed to HDL changes because HDL migrates more than either LDL or VLDL/chylomicron fractions. Moreover, we found that modified lipoproteins circulate for almost 3 days in the plasma indicating that their clearance is decreased. It is known that structural modifications of either lipids or apoproteins can decrease the binding of lipoproteins to LDLR and/or lipases (Boren et al., 2001;Hevonoja et al., 2000;Knouff et al., 1999), and therefore, it is tempting to speculate that the increase in plasma lipoproteins in acrolein-treated animals is due to lipoprotein structural modifications that interfere with the mechanisms of lipoprotein clearance, as well as decreases in hepatic lipase and LDLR.
The overall systemic effects of acrolein exposure resemble the acute-phase response (APR) associated with infection, inflammation, burns, trauma, ischemic necrosis, and malignant growth Khovidhunkit et al., 2004; Tous et al., 2004; Kitagawa et al., 1992). The major overlapping features of acrolein exposure and a typical APR are hypercholesterolemia, hypertriglyceridemia, and hypoalbuminemia, all of which occur within 24h of exposure. The APR is a complex, coordinated, polygenic response involving multiple cytokines and stereotypical changes in hepatic-regulated positive acute-phase plasma proteins (e.g., increased levels of CRP, SAA, serum amyloid-P, fibrinogen) and negative acute-phase plasma proteins (e.g., decreased albumin, transferrin, α-fetoprotein). Chronic acrolein has been reported to induce hypoalbuminemia in beagles during a yearlong oral acrolein exposure (2 mg/kg/day)(Parent et al., 1992), and the degree of hypoalbuminemia was correlated with suppression of hepatic lipase activity in our current study (see Fig. 5B inset). Nevertheless, despite a strong APR response, we did not observe an increase in plasma or hepatic IL-6 or TNF-α level with acrolein treatment (see Table 4 and Table 6). Thus, it appears that acrolein affects a unique subset of APR proteins. The acrolein-induced APR lacks prominent systemic/hepatic cytokine elaboration, which is consistent with previously reported in vitro cell data where acrolein exerts anti-inflammatory action on NF-κB signaling and cytokine expression (Lambert et al., 2007;Lambert et al., 2005). The most salient outcome of oral acrolein exposure is that it induces profound dyslipidemia without increasing systemic or hepatic cytokines (Table 4 and Table 6).
Role of systemic oxidative stress
Because stress can induce dyslipidemia, we evaluated the role of several stress-dependent mechanisms. The results obtained, however, rule out the possibility that acrolein-induced dyslipidemia is dependent on corticosteroids, catecholamines, or fasting. Pretreatment with RU486, the steroid receptor antagonist mifepristone, at a dose (25 mg/kg) which blocks corticosteroid-mediated responses (e.g., fasting-induced immunosuppression in ICR mice; enhanced endotoxemia in rats )(Fan et al., 1994; Nakamura et al., 2004), had little to no effect on either acrolein-induced changes in the electrophoretic mobility of lipoprotein or plasma cholesterol levels. These data also rule out the possibility that the stress of fasting and/or the activation of the autonomic nervous system (with or without hypercorticism) mediate acrolein-induced dyslipidemia. Nonetheless, RU486 pretreatment had two subtle effects in acrolein-treated mice: 1) it attenuated the rise in plasma triglycerides by ~50% at 24h, and 2) it strengthened the correlative association between the change in plasma lipoprotein electrophoretic mobility and the change in plasma lipoprotein level. These effects indicate that changes in lipoprotein mobility are a direct manifestation of acrolein exposure (e.g., absorbed dose) and not due to stress (e.g., hypercorticism of fasting)(Ahima et al., 1996), and that changes in plasma lipids levels follow lipoprotein modification (vide supra).
Conclusions
Acrolein and cardiovascular disease (CVD) risk
Exposure to acrolein and related aldehydes is a major health concern (Committee on Aldehydes, 1981; Bhatnagar, 2004). Environmental sources of acrolein include industrial emission, automobile exhaust, wood, cotton or cigarette smoke. Aldehydes such as acrolein, crotonaldehyde and hexenal are, however, also natural constituents of several foods and spices and their concentration in food and drink is increased upon oxidation, brewing, heating, cooking, and frying. Therefore, aldehyde exposure via ingestion, even for acrolein, is likely to exceed that due to inhalation alone (Wang et al., 2008). Hence our observation that feeding acrolein induces dyslipidemia in mice raises important issues regarding pollutants and constituents of diet and cardiovascular risk. Although the human risk remains to be assessed, the results of the current study suggest the possibility that exposure to acrolein or similarly reactive constituents of foods and pollutants could contribute to dyslipidemia and inflammatory changes in HDL, thereby chronically increasing CVD risk or acutely triggering clinical CVD events.
In addition to foods and pollutants, acrolein is also a bioactive metabolite of chemicals such as allylamine, allyl alcohol, and the anticancer drug cyclophosphamide (Boor, 1983;Brock et al., 1979). Even though the role of acrolein in mediating the effects of these drugs and toxins remains unclear, metabolically-generated acrolein has been linked to cyclophosphamide-induced bladder hemorrhagic cystitis in humans (Cox, 1979). Significantly, treatment with cyclophosphamide inhibits LPL activity (Dousset et al., 1987;Loudet et al., 1985a;Loudet et al., 1985b), induces hypercholesterolemia and hypertriglyceridemia in rabbits and rats (Ilanchezhian et al., 1995;Dousset et al., 1987; Loudet et al., 1985b), and induces lipoprotein adducts of acrolein in CY-treated rats (Arikketh et al., 2004), indicating that as with ingestion, metabolic generation of acrolein increases plasma cholesterol and modifies plasma lipoproteins as well. This view is consistent with our observation that both gavage feeding and i.p. injection of acrolein increases plasma cholesterol. Based on these considerations, we speculate that the effects of acrolein on the liver are likely to be independent of the route of delivery. In this regard, it is important to point out that acrolein is also generated endogenously during lipid peroxidation, amine oxidase-mediated metabolism of polyamines, and myeloperoxidase (Alarcon, 1970;Anderson et al., 1997;Lovell et al., 2001;Sakata et al., 2003). Hence, dyslipidemia concurrent with several metabolic, pathologic or toxicological states could be, in part, and in common, linked to the effects of acrolein described here.
Despite unique insights offered by the study, the human significance of these findings remains unclear. Humans are exposed to unsaturated aldehydes such as acrolein through diet, water, and air. Our previous estimates suggest that the average daily consumption of unsaturated aldehydes from these sources is nearly 5 mg/kg (Wang et al., 2008), however, to avoid using a number of different aldehydes we used acrolein within this dose-range as a model unsaturated aldehyde. Hence, the effects reported here may not be the same in humans because of co-exposure to other aldehydes. Moreover, the results obtained from high-dose acrolein exposure in mice may not directly translate to human condition. Aldehydes, including acrolein, are extensively and rapidly metabolized in rodents (Parent et al., 1996), and therefore their toxicity will depend, in large measure, upon the metabolic capacity; which in humans may or may not be similar to mice, and may be affected by a variety of environmental factors (e.g., disease, age, gender, co-pollutant exposure, etc…) and genetic factors (levels of and polymorphic differences in aldehyde-metabolizing enzymes, such as GST and ALDHs). Thus quantitative extrapolation of the effects of metabolizable toxins across species is difficult. For instance, because of differences in metabolism, 1,3-butadiene is ~10-times more carcinogenic in mice than in rats (Henderson, 2001). Hence, humans may be more or less sensitive to aldehydes than mice and further studies are required to test whether the selective effects on cholesterol metabolism described here for mice are also elicited in humans exposed to acrolein and related aldehydes in the environment.
Acknowledgements
This work was supported in part by grants from NIH (ES11860, ES17260), NIEHS Center for Excellence P30ES014443, EPA, Philip Morris USA Inc. and by Philip Morris International, and STEROLTALK project, funded by the European Community as contract No. LSHG-CT-2005-512096 under 6th Framework Programme for Research and Technological Development in the thematic area of Life sciences, genomics and biotechnology for health. This paper reflects only the author's views and the European Community is not liable for any use that may be made of the information contained therein. We thank B. Bishop, D. Bolanowski, S. Brock, D. Clark, D. Hoetker, A. Tang, X-P. Li, C. Morehead, M. Peak, E. Werkman, and D. Young for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Alarcon RA. Acrolein. IV. Evidence for the formation of the cytotoxic aldehyde acrolein from enzymatically oxidized spermine or spermidine. Arch.Biochem.Biophys. 1970;137:365–372. doi: 10.1016/0003-9861(70)90450-9. [DOI] [PubMed] [Google Scholar]
- Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J.Clin.Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelman LM, Hendriksen CF, Feron VJ. Repeated exposure to alpha-ethylacrolein vapour: subacute toxicity study in rats. Toxicology. 1981;22:79–87. doi: 10.1016/0300-483x(81)90010-x. [DOI] [PubMed] [Google Scholar]
- Arikketh D, Niranjali S, Devaraj H. Detection of acrolein-lysine adducts in plasma low-density lipoprotein and in aorta of cyclophosphamide-administered rats. Arch.Toxicol. 2004;78:397–401. doi: 10.1007/s00204-004-0556-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp RO, Jr, Andjelkovich DA, Kligerman AD, Morgan KT, Heck HD. A critical review of the literature on acrolein toxicity. Crit Rev.Toxicol. 1985;14:309–380. doi: 10.3109/10408448509037461. [DOI] [PubMed] [Google Scholar]
- Boor PJ. Allylamine cardiotoxicity: metabolism and mechanism. Adv.Exp.Med.Biol. 1983;161:533–541. doi: 10.1007/978-1-4684-4472-8_32. [DOI] [PubMed] [Google Scholar]
- Boren J, Lookene A, Makoveichuk E, Xiang S, Gustafsson M, Liu H, Talmud P, Olivecrona G. Binding of low density lipoproteins to lipoprotein lipase is dependent on lipids but not on apolipoprotein B. Journal of Biological Chemistry. 2001;276:26916–26922. doi: 10.1074/jbc.M011090200. [DOI] [PubMed] [Google Scholar]
- Brock N, Stekar J, Pohl J, Niemeyer U, Scheffler G. Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide and sufosfamide. Arzneimittelforschung. 1979;29:659–661. [PubMed] [Google Scholar]
- Chen Y, Joaquim LF, Farah VM, Wichi RB, Fazan R, Jr, Salgado HC, Morris M. Cardiovascular autonomic control in mice lacking angiotensin AT1a receptors. Am.J.Physiol Regul.Integr.Comp Physiol. 2005;288:R1071–R1077. doi: 10.1152/ajpregu.00231.2004. [DOI] [PubMed] [Google Scholar]
- Committee on Aldehydes. Formaldehyde and other aldehydes. Washington D.C: National Academy Press; 1981. [Google Scholar]
- Cox PJ. Cyclophosphamide cystitis--identification of acrolein as the causative agent. Biochem.Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Dousset N, Loudet AM, Lespine A, Carton M, Douste-Blazy L, Chap H. Apolipoproteins induced by an antimitotic agent. Adv.Exp.Med.Biol. 1987;210:201–208. doi: 10.1007/978-1-4684-1268-0_29. [DOI] [PubMed] [Google Scholar]
- Fan J, Molina PE, Gelato MC, Lang CH. Differential tissue regulation of insulinlike growth factor-I content and binding proteins after endotoxin. Endocrinology. 1994;134:1685–1692. doi: 10.1210/endo.134.4.7511091. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc.Natl.Acad.Sci.U.S.A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutation Research. 1991;259:363–385. doi: 10.1016/0165-1218(91)90128-9. [DOI] [PubMed] [Google Scholar]
- Fullana A, Carbonell-Barrachina AA, Sidhu S. Comparison of volatile aldehydes present in the cooking fumes of extra virgin olive, olive, and canola oils. J.Agric.Food Chem. 2004;52:5207–5214. doi: 10.1021/jf035241f. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Navarro H, Nong Z, Amar MJ, Shamburek RD, Najib-Fruchart J, Paigen BJ, Brewer HB, Jr, Santamarina-Fojo S. The ligand-binding function of hepatic lipase modulates the development of atherosclerosis in transgenic mice. Journal of Biological Chemistry. 2004;279:45312–45321. doi: 10.1074/jbc.M406495200. [DOI] [PubMed] [Google Scholar]
- Hammad SM, Powell-Braxton L, Otvos JD, Eldridge L, Won W, Lyons TJ. Lipoprotein subclass profiles of hyperlipidemic diabetic mice measured by nuclear magnetic resonance spectroscopy. Metabolism. 2003;52:916–921. doi: 10.1016/s0026-0495(03)00058-1. [DOI] [PubMed] [Google Scholar]
- Henderson RF. Species differences in the metabolism of olefins: implications for risk assessment. Chemico-Biological Interactions. 2001;135–136:53–64. doi: 10.1016/s0009-2797(01)00170-3. [DOI] [PubMed] [Google Scholar]
- Hevonoja T, Pentikainen MO, Hyvonen MT, Kovanen PT, la-Korpela M. Structure of low density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochimica et Biophysica Acta. 2000;1488:189–210. doi: 10.1016/s1388-1981(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Homanics GE, de Silva HV, Osada J, Zhang SH, Wong H, Borensztajn J, Maeda N. Mild dyslipidemia in mice following targeted inactivation of the hepatic lipase gene. Journal of Biological Chemistry. 1995;270:2974–2980. doi: 10.1074/jbc.270.7.2974. [DOI] [PubMed] [Google Scholar]
- Ilanchezhian S, Thangaraju M, Sasirekha S, Sachdanandam P. Alpha-tocopherol ameliorates cyclophosphamide-induced hyperlipidemia in fibrosarcoma-bearing rats. Anticancer Drugs. 1995;6:771–774. doi: 10.1097/00001813-199512000-00009. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J.Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- Kitagawa S, Yamaguchi Y, Imaizumi N, Kunitomo M, Fujiwara M. A uniform alteration in serum lipid metabolism occurring during inflammation in mice. Jpn.J.Pharmacol. 1992;58:37–46. doi: 10.1254/jjp.58.37. [DOI] [PubMed] [Google Scholar]
- Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J.Clin.Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Konig B, Spielmann J, Leitner A, Stangl GI, Eder K. Thermally oxidized oil increases the expression of insulin-induced genes and inhibits activation of sterol regulatory element-binding protein-2 in rat liver. J.Nutr. 2007;137:2018–2023. doi: 10.1093/jn/137.9.2018. [DOI] [PubMed] [Google Scholar]
- Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, Harvey J, Freed BM. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol.Chem. 2007;282:19666–19675. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- Lambert C, McCue J, Portas M, Ouyang Y, Li J, Rosano TG, Lazis A, Freed BM. Acrolein in cigarette smoke inhibits T-cell responses. J Allergy Clin.Immunol. 2005;116:916–922. doi: 10.1016/j.jaci.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Li H, Wang J, Kaphalia B, Ansari GA, Khan MF. Quantitation of acrolein-protein adducts: potential biomarker of acrolein exposure. J.Toxicol.Environ.Health A. 2004;67:513–524. doi: 10.1080/15287390490276539. [DOI] [PubMed] [Google Scholar]
- Li L, Hamilton RF, Jr, Taylor DE, Holian A. Acrolein-induced cell death in human alveolar macrophages. Toxicology and Applied Pharmacology. 1997;145:331–339. doi: 10.1006/taap.1997.8189. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loudet AM, Dousset N, Carton M, Douste-Blazy L. Effect of an antimitotic agent (cyclophosphamide) on post-heparin plasma lipoprotein lipase activity in rabbit. Biochem.Pharmacol. 1985a;34:3597–3599. doi: 10.1016/0006-2952(85)90739-7. [DOI] [PubMed] [Google Scholar]
- Loudet AM, Dousset N, Perret B, Ierides M, Carton M, Douste-Blazy L. Triacylglycerol increase in plasma very low density lipoproteins in cyclophosphamide-treated rabbit: relationship with cholesteryl ester transfer activity. Biochimica et Biophysica Acta. 1985b;836:376–384. doi: 10.1016/0005-2760(85)90142-0. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol.Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Luo J, Hill BG, Gu Y, Cai J, Srivastava S, Bhatnagar A, Prabhu SD. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am.J.Physiol Heart Circ.Physiol. 2007;293:H3673–H3684. doi: 10.1152/ajpheart.00284.2007. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kouda K, Tokunaga R, Takeuchi H. Suppressive effects on delayed type hypersensitivity by fasting and dietary restriction in ICR mice. Toxicol.Lett. 2004;146:259–267. doi: 10.1016/j.toxlet.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Nawrocki J, Dabrowska A, Borcz A. Investigation of carbonyl compounds in bottled waters from Poland. Water Res. 2002;36:4893–4901. doi: 10.1016/s0043-1354(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am.J.Cardiol. 2002;90:22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- Parent RA, Caravello HE, Balmer MF, Shellenberger TE, Long JE. One-year toxicity of orally administered acrolein to the beagle dog. J.Appl.Toxicol. 1992;12:311–316. doi: 10.1002/jat.2550120504. [DOI] [PubMed] [Google Scholar]
- Parent RA, Caravello HE, Sharp DE. Metabolism and distribution of [2,3–14C]acrolein in Sprague-Dawley rats. J.Appl.Toxicol. 1996;16:449–457. doi: 10.1002/(SICI)1099-1263(199609)16:5<449::AID-JAT369>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem.Soc.Trans. 2003;31:371–374. doi: 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- Santamarina-Fojo S, Gonzalez-Navarro H, Freeman L, Wagner E, Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1750–1754. doi: 10.1161/01.ATV.0000140818.00570.2d. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chandrasekar B, Bhatnagar A, Prabhu SD. Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: role of aldose reductase. Am.J.Physiol Heart Circ.Physiol. 2002;283:H2612–H2619. doi: 10.1152/ajpheart.00592.2002. [DOI] [PubMed] [Google Scholar]
- Tous M, Ribas V, Ferre N, Escola-Gil JC, Blanco-Vaca F, onso-Villaverde C, Coll B, Camps J, Joven J. Turpentine-induced inflammation reduces the hepatic expression of the multiple drug resistance gene, the plasma cholesterol concentration and the development of atherosclerosis in apolipoprotein E deficient mice. Biochimica et Biophysica Acta. 2005;1733:192–198. doi: 10.1016/j.bbalip.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Tsakadze NL, Srivastava S, Awe SO, Adeagbo AS, Bhatnagar A, D’Souza SE. Acrolein-induced vasomotor responses of rat aorta. Am.J.Physiol Heart Circ.Physiol. 2003;285:H727–H734. doi: 10.1152/ajpheart.00269.2003. [DOI] [PubMed] [Google Scholar]
- Vesely P, Lusk L, Basarova G, Seabrooks J, Ryder D. Analysis of aldehydes in beer using solid-phase microextraction with on-fiber derivatization and gas chromatography/mass spectrometry. J.Agric.Food Chem. 2003;51:6941–6944. doi: 10.1021/jf034410t. [DOI] [PubMed] [Google Scholar]
- Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J.Mol.Cell Cardiol. 2008;44:1016–1022. doi: 10.1016/j.yjmcc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Zemski Berry KA, Murphy RC. Characterization of acrolein-glycerophosphoethanolamine lipid adducts using electrospray mass spectrometry. Chem.Res.Toxicol. 2007;20:1342–1351. doi: 10.1021/tx700102n. [DOI] [PMC free article] [PubMed] [Google Scholar]