Abstract
Whether emotional distracters call for attentional resources has been discussed in several studies. Previously we have shown that brief unpleasant distracters captured right hemisphere attentional resources as evidenced with reduced ERP responses and increased reaction times (RT) to non-emotional left visual field/right hemisphere (LFV/RH) targets. The aim of this study was to investigate whether emotional distracters selectively interfere with processes predominantly relying on the RH such as processing global visual features. Evoked potentials were recorded from eighteen subjects carrying out a visual discrimination task engaging global RH and local left hemisphere (LH) dependent processes. Unpleasant distracters reduced global target detection related right parietal activity. We conclude that brief unpleasant distracters compete for right hemisphere attentional resources with global level processing.
Keywords: Affect, Emotion, Event-Related Potential, EEG, P3, Cerebral Dominance, Hemisphere, Global, Local, Attention
Introduction
Hemispheric asymmetries in emotional and cognitive functions have been extensively studied. The degree of hemispheric asymmetry varies from highly lateralized functions such as language to functions with a lower degree of lateralization. However, both hemispheres contribute to nearly all cognitive and affective functions, even to those highly lateralized [1]. While it is evident there is a lower degree of lateralization in emotional processes, the literature supports an overall right hemisphere bias for emotional processing [2, 3] and a valence dependent bias, with unpleasant emotions engaging the right hemisphere and pleasant emotions engaging the left [4, 5].
While the right hemisphere is thought to predominate in visuospatial processing [2], the spatial frequency of visual stimuli may determine which hemisphere is primarily engaged. The left hemisphere is thought to predominate in high spatial frequency analysis while right hemisphere predominates in low spatial frequency processing [6, 7]. This hemispheric specialization may underlie the right hemisphere’s advantage for perceiving and attending to the whole stimuli (global) [8, 9] and left hemisphere to its parts (local) [10, 11]. Like emotional lateralization, global-local hemispheric asymmetry is relative rather than absolute, and has been observed in neuropsychological [12] and functional imaging studies [11, 13]. Patients with right hemisphere parieto-temporal lesions tend to have more difficulties in perceiving global level information in comparison to local level information while the opposite is true for left hemisphere lesions [12]. Patients with depression tend to have difficulty perceiving global level information [14]. The mechanisms of such mood-related attentional bias has not been established.
Attentional resource competition could account for unpleasant emotions leading to disadvantage in global processing. Unpleasant emotional information would capture right hemisphere processing resources leading to reduced available resources for other right hemisphere dependent functions such as global level processing. We have previously reported both behavioral and electrophysiological evidence for attentional resource competition favoring emotional stimuli in the right hemisphere with task irrelevant emotional stimuli leading to compromised left visual field (LVF) attention performance [15, 16]. Similarily, task-irrelevant emotional facial expressions have been shown to compete with task-related stimuli [17].
The aim of this study was to assess whether attentional competition would lead to compromised global processing due to preceding unpleasant stimuli. We used event related potentials (ERP) and behavioral responses to test the attentional competition hypothesis as a plausible mechanism for emotions directing focus of attention at lower or higher hierarcical levels of information. Specifically, we predicted that unpleasant emotional stimuli selectively interfere with global processing resulting in both behavioral impairment as well as diminished brain responses to global targets preceded by emotional stimuli. As target detection related N2-P3 peak-to-peak amplitude is assumed to reflect the amount of attentional resource allocation [18, 19], we predicted reduced N2-P3 amplitude to global targets in context of preceding unpleasant emotional stimuli.
Methods
Participants
Eighteen healthy right-handed volunteers (11 females, 7 males; mean age 26 years, range 18–48) with normal or corrected vision and no history of neurological or psychiatric abnormalities were recruited, and paid for their participation. Subjects gave their consent according to the University Guidelines. The study was approved by the Ethics Committee of the Institutional Review Board.
Stimuli
Three sets (pleasant, unpleasant and neutral) of 48 colored photos were chosen from the International Affective Picture System [20].
Paradigm
The subjects were seated in a sound attenuated booth facing a computer screen at a distance of one meter. Subjects were asked to discriminate between centrally presented Navon-type hierarchical letters [21] that were flashed for 150 ms. Targets occurred either at the local or global level of the stimuli and subjects were asked to attend to both local and global levels simultaneously. A brief emotional (pleasant or unpleasant; 150ms stimulus duration, IASP) or neutral picture was presented 300 ms prior to target onset. Subjects were told to ignore the flashing photos (IAPS) and respond to the target letters as quickly and accurately as possible. Subjects were instructed to keep their eyes on a fixation cross in the middle of the screen throughout the experiment.
Half of the subjects discriminated between the letters E and H using their index and middle fingers to indicate which letter they saw independent of whether their saw the target at the local or global level. Half of the subjects discriminated between the letters T and L. Thus, the exact same stimuli that served as local targets for half of the subjects served as global targets for the other half and vice versa. There were eight different local-global levels where there was always an E or H at the local or global level of the letter. The local-global letters consisted of global E/local T, global E/local L, global H/local T, global H/local L, global T/local E, global L/local E, global T/local H, global L/local H. Global letters extended 1.6° horizontally and 3.2° vertically of visual angle. Correspondingly, local letters extended 0.3 ° horizontally and 0.6 vertically of visual angle.
The affective stimuli were presented centrally and extended 13 ° horizontally of visual angle. The experiment was divided into four blocks, each lasting about ten minutes. The initial response hand was counterbalanced across subjects and after each block. Twelve pictures from each category were randomly presented eight times in each block. In each block there was a new set of pictures. Each affective picture was paired once with each letter type. The presentation of stimuli was randomized. The inter-target interval varied depending on RT between 1300–2600ms. After a response there was a 650 ms fixation cross before the next new trial. Eighteen percent of the targets were not preceded by any stimuli and 27 % of the pictures were not followed by a target.
Event-related potential and analysis
Electroengephalography (EEG) was recorded with Ag-AgCl electrodes placed at 30 scalp sites and referred to the linked mastoids. Horizontal electro-oculograms were recorded from the outer canthi of each eye and vertical EOG from beneath the left eye and Fp1. Impedances were maintained below 5 kΩ. The EEG was amplified (band pass 0.1–80 Hz) and sampled at 250 Hz. Trials containing blinks, horizontal eye movements or EMG artifacts were excluded from analysis. Four of the subjects were excluded from ERP analysis. Two were excluded due to excessive blinks time-locked to stimuli or responding and two due to high voltage periodic activity. EEG signals were averaged offline time-locked to stimulus onset. Epoch length was 1600 ms including 100 ms baseline before stimulus presentation.
Difference Waveforms
ERP difference waveforms (DW) were used to assess attentional competition between task-irrelevant affective stimuli and task-relevant global and local targets. ERPs to identical sets of IAPS stimuli presented alone were subtracted from those followed by a target. Thus, Target-DWs allow control for any physical aspects of IAPS stimuli. Furthermore, Target-DWs remove large IAPS evoked ERP waveforms and reveal smaller Target related ERP waveforms and how they have been modified by preceding emotional stimuli.
As follows, three different emotional categories (Neutral, Pleasant, Unpleasant) and two different target levels (Global, Local) resulted in six Target-DWs that were created to explore the modulatory effects of emotion on local and global processing. For example, UnpleasantGlobalDW = ERPs to unpleasant stimuli followed by a target at a global level - ERP to unpleasant stimuli not followed by any target. The remaining five difference waves were derived accordingly.
ERP measurements
As a reflection of attentional allocation to target processing we measured the N2-P3 peak to peak amplitude from Target-DWs. Since the P3 potential has its maximum in the parietal region, the N2-P3 peak to peak amplitudes for the targets were measured at one left and one right centro-parietal electrode, CP3 and CP4. The amplitude of the Target N2 was defined as the lowest negative peak within a time window of 270 – 350 ms and the amplitude of P3 as the highest positive peak 300 – 800 ms after the target onset.
Statistical Analysis
SPSS software program was used for statistical analysis. Repeated measures Analysis of Variance (ANOVA) or independent sample’s t test was performed on ERPs, reaction times (RT) and accuracy. Any significant interaction effects were decomposed by additional ANOVAs. Post-hoc tests were carried out by further ANOVAs or independent sample’s t test.
Target difference waveforms
Target-DWs were analyzed by measuring N2-P3 peak-to-peak amplitudes at CP3 for the left hemisphere and CP4 for the right hemispheres. The factors for Target-DWs were emotional Valence (Pleasant, Unpleasant, Neutral) and Target Level (Global, Local) and Hemisphere (Left (CP3), Right (CP4)).
Behavioral Analysis
For RT and response accuracy the factors were Emotional Valence (Pleasant, Unpleasant, Neutral) and Target Level (Global, Local).
Results
Event related potential results
Targets
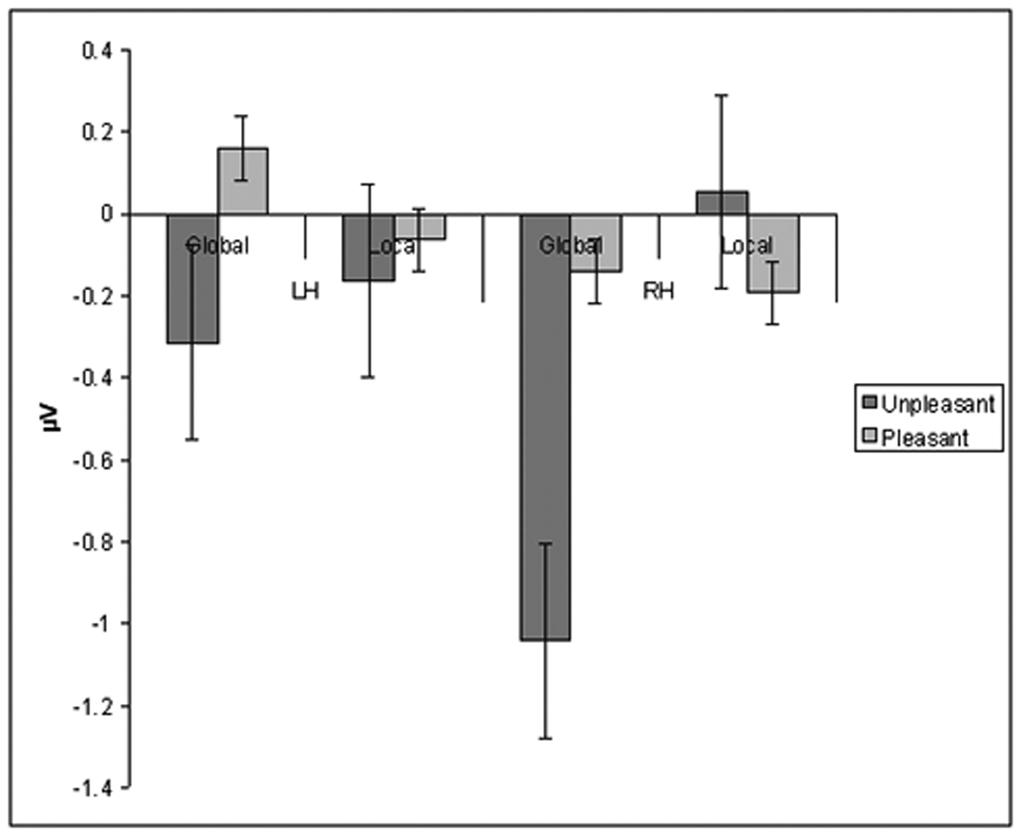
ANOVA with Valence (Pleasant, Unpleasant, Neutral), Target Level (Global, Local) and Hemisphere (Left (CP3), Right (CP4)) as within subjects factors was performed on N2-P3 peak-to-peak amplitude from Target-DWs. No significant main effects were observed. There was a significant three-way interaction effect on the Main ANOVA: Valence × Target Level × Hemisphere; F(2,34) = 3.3, p < 0.05. Post-hoc ANOVAs separately done on each hemisphere with Valence and Target Level as within subject factors revealed a significant interaction effect of Valence × Target Level F(2,34) = 5.9, p < 0.01. for the right hemisphere. No significant main or interaction effects were observed for the left hemisphere, specifically there was no significant interaction of Valence × Target Level F(2,34) = 0.42, p=0.67. Further post-hoc analysis examining interaction of Valence and Target Level in the Right Hemisphere was done performing t-test separately for each Valence. T-test examining effects of Unpleasant stimuli in the Right hemisphere to target processing revealed a significant effect of Target Level; t(1,17) = 2.3, p < 0.041. Thus, unpleasant distracters reduced detection-related right parietal activity (N2-P3 peak-to-peak amplitude) to Global targets as opposed to Local targets (Fig 1). Global and Local target evoked right parietal activity did not differ significantly when preceded by a neutral t(1,17) = 0.74, p = 0.52 or a pleasant distracter t(1,17) = 0.54, p = 0.61.
Fig 1.
Unpleasant stimuli reduce detection related ERP response to global targets, especially over the right hemisphere. Change in N2-P3 peak-to-peak amplitude for global and local targets over left (LH) and right hemisphere (RH) in comparison to targets preceded by non-affective stimuli.
Behavioral results
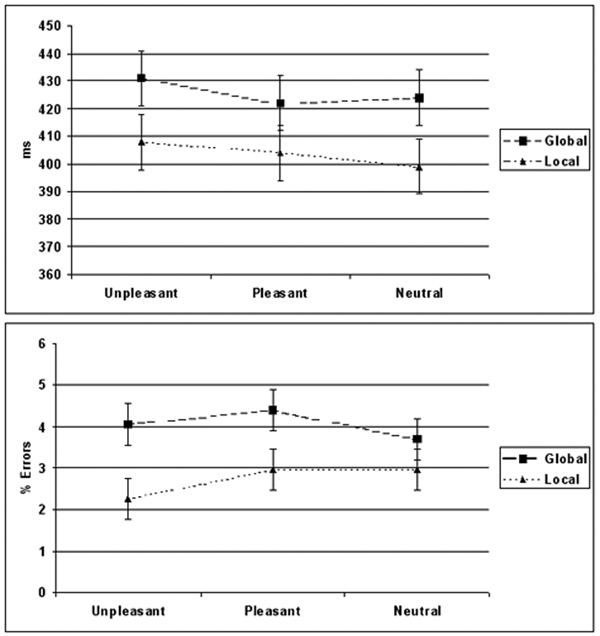
ANOVA was performed on RTs to targets preceded by an IAPS distracter with Valence (Pleasant, Unpleasant, Neutral) and Target Level (Global, Local) as within subject factors. In context of any IAPS distracter responses were slower to Global targets (420 ms ± 87) in comparison to Local targets (406 ms ± 83) [Main ANOVA: Target Level; F(1,17) = 6.3, p < 0.018]. The valence of the distracter had an effect on response speed [Main ANOVA: Valence; F(1,17) = 6.72, p < 0.028] with slower response speed when unpleasant distracter preceded any target as opposed to when preceded by a neutral distracter [Post-hoc ANOVA comparing Neutral and Unpleasant stimuli: Valence; F(1, 17) = 6.7, p < 0.02, Fig. 2.]. Post-hoc analysis comparing pleasant stimuli to neutral stimuli F(1,17) = 0.37, p = 0.56 and pleasant to unpleasant stimuli F(1,17) = 3.26, p = 0.09 did not show significant effect of valence.
Fig 2.
Effect of emotional distracters on global and local target detection performance. As opposed to neutral stimuli the effects of unpleasant stimuli on number of errors depended on the processing level of the target with less errors committed to local and more errors to global targets. Unpleasant distracters were associated with slower and less accurate responding to global targets than local targets. While global performance in context of visual distracters was overall worse than local performance, pleasant and neutral distracters impaired either speed or accuracy to global targets in comparison to local targets, but not both like unpleasant distracters did.
ANOVA was performed on the percent errors with Valence (Pleasant, Unpleasant, Neutral) and Target Level (Global, Local) as within subject factors. In context of any IAPS distracter global targets were associated with higher error rate than local targets [Target Level F(1,17) = 7.8, p < 0.011]. Due to a prior hypothesis of distinct emotional effect on global vs. local processing we analyzed separately each valence using a t-test. The difference in percentage errors differed between global and local targets only when preceded by an emotional distracter [Post-hoc t-test on Unpleasant stimuli: Target Level; t(1,17) = 3.4, p < 0.003, Post-hoc t-test on Pleasant stimuli: Target Level; t(1,17) = 2.13, p < 0.05; F, Post-hoc t-test on Neutral stimuli: Target Level; t(1,17) = 0.61, p =0.6, Fig 2].
Discussion
This study showed that very brief unpleasant emotional distracters reduced detection related evoked response amplitude to global targets, especially over the right parietal region. This finding supports an attentional resource competition model where unpleasant emotional stimuli capture right hemisphere processing resources and render subsequent right hemisphere dependent processing vulnerable to interference due to reduction in available resources. Further, these results, along with pervious studies, suggest emotional stimuli have priority for right hemisphere attentional resources [15, 16, 22].
It has been suggested in dissimilar dual tasks that if processing is performed in functionally close brain regions (within a hemisphere) performance suffers from interference due to activity spreading from one region to another, whereas if processing is performed in functionally distant regions (different hemispheres) performance improves [23]. Smaller detection related parietal brain potentials along with lower behavioral accuracy to global targets as opposed to local targets in context of unpleasant emotional stimuli are in line with this providing some evidence that processing unpleasant emotional information and global targets is predominantly performed within the same (RH) hemisphere leading to interference.
In this study, we employed a divided attention paradigm at both global and local levels with neutral and emotional distracters (IAPS images). Even though there was no task associated with the IAPS picture and the subjects were told to ignore them, processing visual emotional scenes automatically captured attentional resources. All IAPS pictures, independent of valence, seemed to capture right hemisphere attentional resources interfering with right hemisphere dependent global processing as evidenced by slowed RTs to Global targets when preceded by any IAPS picture. As the right hemisphere is also dominant in visuospatial processing, it is not surprising that complex colorful IAPS pictures of any valence would capture and engage right hemisphere processing resources. Furthermore, with rapid stimulus presentation, as well as a higher proportion of emotional than neutral stimuli, it is possible that some effect of emotional scenes may extend to neutral scenes. However, the ERP difference waveforms used in this study that subtract the effect of complex visual scenes showed a reduction in right parietal responses to global targets when preceded by unpleasant stimuli as opposed to pleasant or neutral stimuli. This suggests that unpleasantness on its own, independent of a visual scene, engage and capture right hemisphere processing resources.
With rapid presentation of emotional stimuli and targets there was a temporal overlap of emotional and target-related processing. Therefore, competition for temporarily and spatially limited resources lead to diminished responses to task-related global stimuli while emotional stimuli were prioritized in attentional resource allocation. These results resemble our previous findings of reduced detection related potentials to left visual field (RH) targets in context of unpleasant emotional stimuli [16]. Here the reduction was seen to centrally presented Global-targets presumed to predominantly depend on right hemispheric processing. Thus, the consequences of emotional capture of right hemisphere resources is not limited to left visual field attention but extends to other right hemisphere dependent functions as well. This was also reflected in impaired right hemisphere dependent performance with greater error rate to global targets than to local (left hemisphere dependent) targets when preceded by unpleasant emotional stimuli as opposed to neutral stimuli. Local targets did not compete for the same right hemisphere attentional resources, rather, based on fewer errors, there seemed to be an attentional bias to left hemisphere dependent local targets. It seems as if the processing is biased to whichever hemisphere has more resources available for processing information at the level of its preference (right global, left local). With left hemisphere resources unengaged by unpleasant emotional state, left hemisphere resources are readily available for visual processing of its preference, i.e. local.
The results from this study fit with attentional resource competition model and are in accordance with reports on mood and attentional bias with local bias in sad moods, depression and anxiety [24, 25]. Furthermore, based on the electrophysiological results we suggest a plausible explanation for previous behavioral observations on negative emotions creating a greater focus on the trees rather than forest.
Conclusion
The findings of this study are in agreement with the notion that unpleasant emotions bias attention to trees over the forest. Furthermore, we have suggested a plausible mechanism for such bias in the framework of attentional resource competition model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hellige JB. Hemispheric asymmetry: What's right and What's left (Perspectives in Cognitive Neuroscience) Cambridge: Harvard University Press; 1993. [Google Scholar]
- 2.Heilman KM, Bowers D, Valenstein E, Watson R. The right hemisphere: neuropsychological functions. Journal of Neurosurgery. 1986;64(5):693. doi: 10.3171/jns.1986.64.5.0693. [DOI] [PubMed] [Google Scholar]
- 3.Ahern GL, Schomer DL, Kleefield J, Blume H, Cosgrove GR, Weintraub S, et al. Right hemisphere advantage for evaluating emotional facial expressions. Cortex. 1991;27(2):193–202. doi: 10.1016/s0010-9452(13)80123-2. [DOI] [PubMed] [Google Scholar]
- 4.Killgore WD, Yurgelun-Todd DA. The right-hemisphere and valence hypothesis: could they both be right (and sometimes left) Soc Cogn Affect Neurosci. 2007;2(3):240–250. doi: 10.1093/scan/nsm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport. 1998;9(14):3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- 6.Peyrin C, Schwartz S, Seghier M, Michael C, Landis T, Vuillemier P. Hemispheric spezialization of human inferior temporal cortex during course-to-fine and fine-to-coarse analysis of natural visual scenes. Neuroimage. 2005;1;28(2):464–473. doi: 10.1016/j.neuroimage.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Peyrin C, Chauvin A, Chokron S, Marendaz C. Hemispheric specialization for spatial frequency processing in the analysis of natural scenes. Brain and Cognition. 2003;53(2):278–282. doi: 10.1016/s0278-2626(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 8.Shulman GL, Sullivan MA, Gish K, Sakoda WJ. The role of spatial frequency channels in the perception of local and global structure. Perception. 1986;15(3):259–273. doi: 10.1068/p150259. [DOI] [PubMed] [Google Scholar]
- 9.Doyon J, Milner B. Right temporal-lobe contribution to global visual processing. Neuropsychologia. 1991;29(5):343–360. doi: 10.1016/0028-3932(91)90024-3. [DOI] [PubMed] [Google Scholar]
- 10.Lamb MR, Robertson LC, Knight RT. Component mechanisms underlying the processing of hierarchically organized patterns: inferences from patients with unilateral cortical lesions. J Exp Psychol Learn Mem Cogn. 1990;16(3):471–483. doi: 10.1037//0278-7393.16.3.471. [DOI] [PubMed] [Google Scholar]
- 11.Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature. 1996;382(6592):626–628. doi: 10.1038/382626a0. [DOI] [PubMed] [Google Scholar]
- 12.Robertson LC, Lamb MR, Knight RT. Effects of lesions of temporal-parietal junction on perceptual and attentional processing in humans. J Neurosci. 1988;8(10):3757–3769. doi: 10.1523/JNEUROSCI.08-10-03757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120(Pt 10):1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- 14.Dreben EK, Fryer JH, McNair DM. Perceptual and conceptual information processing in schizophrenia and depression. Percept Mot Skills. 1995;80(2):447–465. doi: 10.2466/pms.1995.80.2.447. [DOI] [PubMed] [Google Scholar]
- 15.Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia. 2000;38(12):1576–1580. doi: 10.1016/s0028-3932(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 16.Hartikainen KM, Ogawa KH, Soltani M, Knight RT. Emotionally arousing stimuli compete for attention with left hemispace. Neuroreport. 2007;3;18(18):1929–1933. doi: 10.1097/WNR.0b013e3282f1ca18. [DOI] [PubMed] [Google Scholar]
- 17.Preston SD, Stansfield RB. I know how you feel: task-irrelevant facial expressions are spontaneously processed at a semantic level. Cognitive, Affective and Behavioral Neuroscience. 2008;8(1):54–64. doi: 10.3758/cabn.8.1.54. [DOI] [PubMed] [Google Scholar]
- 18.Daffner KR, Mesulam MM, Scinto LF, Cohen LG, Kennedy BP, West WC, et al. Regulation of attention to novel stimuli by frontal lobes: an event-related potential study. Neuroreport. 1998 Mar 30;9(5):787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- 19.Solbakk AK, Reinvang I, Svebak S, Nielsen CS, Sundet K. Attention to affective pictures in closed head injury: Event-related brain potentials and cardiac responses. Journal of Clinical and Experimental Neuropsychology. 2005;27:205–223. doi: 10.1080/13803390490515739. [DOI] [PubMed] [Google Scholar]
- 20.Lang PJ, Bradley MM, Cuthbert BN. Instruction manual and affective ratings. University of Florida, Gainesville, Florida: Center for Research in Psychophysiology; 1999. International Affective Picture System (IAPS) [Google Scholar]
- 21.Navon D. Forest before trees: the precedence of global features in visual processing. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- 22.Vuilleumier P, Schwartz S. Beware and be aware: capture of spatial attention by fear-related stimuli in neglect. Neuroreport. 2001;12(6):1119–1122. doi: 10.1097/00001756-200105080-00014. [DOI] [PubMed] [Google Scholar]
- 23.Liederman J. Subtraction in Addition to Addition: Dual Task Performance Improves When Tasks Are Presented to Separate Hemispheres. Journal of Clinical and Experimental Neuropsychology. 1986;Vol.8(No.5):486–502. doi: 10.1080/01688638608405172. 1986. [DOI] [PubMed] [Google Scholar]
- 24.Basso MR, Schefft BK, Ris MD, Dember WN. Mood and global-local visual processing. J Int Neuropsychol Soc. 1996;2(3):249–255. doi: 10.1017/s1355617700001193. [DOI] [PubMed] [Google Scholar]
- 25.Gasper K, Clore GL. Attending to the big picture: mood and global versus local processing of visual information. Psychol Sci. 2002;13(1):34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]