Abstract
Purpose
To investigate 24 h in vitro maturation (IVM) of cumulus-stripped immature oocytes from stimulated cycles.
Methods
263 oocytes identified as immature after cumulus stripping for ICSI were subjected to in vitro maturation (IVM). Fertilization rates and reproductive outcomes of matured oocytes were compared against 234 in vivo matured sibling oocytes (IVO-MII-Sib) from the same cycles (n = 41). Day 2 embryo development was compared against 116 embryos from ICSI cycles having no IVM (IVO-Ext controls).
Results
While fertilization rates were similar between IVM and IVO-MII-Sib oocytes (62.1% vs. 64.0%, p = 0.9909), day 2 embryo quality was reduced in the IVM group compared with IVO-Ext controls as evidenced by fewer embryos having 4 cells (28.3% vs. 54.3%, p = 0.0026), low fragmentation (30.0% vs. 65.2%, p < 0.0001) or perfectly symmetric blastomeres (28.6% vs. 46.9%, p = 0.0371). 0 of 17 IVM embryos with known fate implanted.
Conclusion
Efficacy of 24 h IVM for cumulus-stripped GV and MI oocytes for either clinical use or study of normal meiotic maturation is questionable.
Keywords: ICSI, Immature oocytes, In-vitro maturation, Ovarian stimulation
Introduction
Following ovarian stimulation, oocytes are retrieved at varying stages of meiotic maturity, with an overall average of 15–20% failing to extrude the first polar body [1–4]. Occasionally, however, retrieval of oocytes at the germinal vesicle (GV) or metaphase I (MI) stage represents a significantly larger portion of the oocytes retrieved. Over the last 20 years, much research has focused on in-vitro maturation (IVM) of such immature oocytes in order to gain better understanding of the molecular and genetic mechanisms of oocyte maturation, as well as to increase the yield of ova capable of fertilization and transfer.
The literature regarding in-vitro maturation, however, is clouded by the use of identical terminology to describe disparate practices and techniques; IVM of immature oocytes has been described in stimulated or unstimulated cycles [5–7], cumulus retained or cumulus stripped oocytes [8, 9], and for varying time periods of maturation, ranging from 4 to 48 h [2, 3, 10, 11]. The diverse situations in which in-vitro maturation has been employed makes comparison between studies difficult, especially when laboratory techniques, culture media, and stimulatory types are not explicitly delineated.
It is standard practice in intracytoplasmic injection (ICSI) cycles to remove the cumulus and corona cells shortly after retrieval in order to assess nuclear maturity prior to injection. Those oocytes lacking the first polar body are re-evaluated at the completion of injection of the initial cohort of metaphase II (MII) oocytes, with further injection of those initially immature oocytes that have achieved polar body extrusion in the subsequent brief duration (typically 3–6 h) since retrieval. The question remains, however, what fate should become those oocytes which remain immature at the completion of injections on the day of retrieval. We therefore pursued the present study to investigate the utility of an additional 24 h period of IVM for such immature oocytes.
Materials and methods
This study was approved by the Partners’ Healthcare Institutional Review Board for chart review.
Cycle inclusion criteria
The index cycles (n = 41) identified for study were culled following review of all ICSI cycles performed in the Brigham & Women’s Center for Infertility and Reproductive Surgery from March 2008 through June 2009. During this 15 month interval, IVM was applied either when deemed clinically necessary based on a patient’s prior history of a high incidence of immature oocytes or when feasible in the laboratory as determined by clinical case load. Forty one cycles were identified as having at least one immature oocyte (GV or MI) remaining after completion of day 1 injections.
Clinical protocols
Controlled ovarian stimulation was achieved with any one of six protocols. In the majority of cycles (23 of 41) luteal down-regulation was performed using leuprolide acetate (LA; Lupron; TAP Pharmaceuticals, Lake Forest, IL). Leuprolide acetate was started either 1 week following urinary LH surge or the day after midluteal progesterone determination, and was continued to at least the second day of menses after which the dose was decreased until hCG administration. The remainder of patients were stimulated using antagonist (7 cycles), microflare (8 cycles), or estrogen priming (4 cycles) protocols per routine clinical indication. Baseline ultrasonography and blood testing were performed before stimulation to document no complex ovarian cysts >3 cm, estradiol (E2) of <50 pg/mL, and progesterone (P) < 1.5 ng/mL.
When baseline criteria were met gonadotropin therapy (either Gonal-F, EMD Serono Laboratories, Inc., Rockland, MA or Follistim, Organon, Roseland, NJ) with or without hMG (Repronex or Menopur: Ferring Pharmaceuticals Inc., Suffern, NY) was begun. Stimulation was achieved using doses of between 150 and 600 total IUI of FSH per day, with or without LH, depending on patient age, basal FSH and anticipated response. Monitoring of follicles was achieved using transvaginal ultrasound in the standard fashion. Serum E2 levels were measured, in general, on stimulation day 6 and then every 1–3 days as indicated. When ≥ 4 follicles were documented on ultrasound with 2 lead follicles measuring ≥ 18 mm in mean diameter, and the E2 concentration was >500 pg/mL, patients were administered a 10,000 IU IM dose of hCG (Profasi: Serono, Geneva, Switzerland or Novarel: Ferring Pharmaceuticals Inc., Suffern, NY). Retrieval was performed 36 h following hCG administration in the standard fashion with intravenous general anesthesia using Propofol (AstraZeneca, Wilmington, DE).
The day after oocyte retrieval, luteal progesterone (P) supplementation was begun using either daily IM P (50 mg), daily 8% P vaginal gel (Crinone; Wyeth-Ayerst, Madison, NJ), or three times daily vaginal P suppositories (200 mg). Embryo transfer was performed with a Wallace catheter (Marlow/Cooper Surgical, Shelton, CT) except in one case in which a SureView (Smiths-Medical, Hythe, Kent, UK) embryo transfer catheter was employed.
Laboratory protocols
In order to assess oocyte maturation, cumulus-corona cells were removed from the cumulus-oocyte complexes (COCs) 2–4 h after retrieval by exposing the COCs to 80 IU/ML hyaluronidase (Sigma Aldrich, St. Louis, MO) for 30–60 s, followed by mechanical denudation using 150 um stripper pipettes (MidAtlantic Diagnostics Inc., Mount Laurel, New Jersey). Denuded oocytes were examined under a stereo microscope and classified according to meiotic maturity as GV (characterized by the presence of this defining structure), MI (characterized by the absence of both a GV and an extruded first polar body), or MII (characterized by the presence of an extruded polar body in the perivitelline space). Oocytes of a patient were then grouped into SAGE culture medium (CooperSurgical, Trumbull, CT) according to maturity (all MII oocytes in one dish; the GV and MI oocytes in another). Metaphase II oocytes underwent ICSI on average 3–6 h after retrieval.
When the last MII oocyte was injected, the GV and MI oocytes were again evaluated to determine which, if any, had completed the first meiotic division in the brief intervening hours. Those oocytes achieving MII status were then injected as well. These two groups of injected oocytes were then incubated in 25 μL drops of G1.5 medium (Vitro Life, Inc., Englewood, CO), overlain with 8 ml equilibrated mineral oil (Irvine Scientific, Santa Ana, CA). Oocytes which remained at the GV or MI stage subsequent to the second evaluation were placed in SAGE IVM medium (CooperSurgical, Trumbull, CT) with the addition of 75 mIU/mL of FSH and 75 mIU/mL of LH per the manufacturer’s recommendation. This medium itself contained 5% plasma fraction protein, and no further protein supplements were added. The time at which these immature oocytes were placed in IVM media was defined as time zero for IVM. After approximately 24 h of incubation in IVM medium (mean 23.1 h, range 18.7–27.5 h), oocytes were re-evaluated; those oocytes having achieved completion of the first meiotic division now underwent second day injection, while those which remained immature were discarded. In all cases, sperm from the original preparation used to perform ICSI on the day of oocyte retrieval was kept in the incubator overnight and used to perform the second day injections. In all cases, sufficient motile sperm of gross normal morphology were used for these injections.
Zygotes having two pronuclei (2PN) at the fertilization check 16–18 h after ICSI were cultured individually in fresh 25 μL drops of G1.5 medium, overlaid with 8 mL of equilibrated oil in Falcon 1007 culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ). All cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air.
Embryo assessment
On day 3 subsequent to retrieval, embryo morphology was assessed using standard criteria for cell number, fragmentation, and asymmetry, with fragmentation and asymmetry graded using numerical scores [12]. Fragmentation scores of 0 through 4 were ascribed to each embryo, where each score correlated to 0, 1–9, 10–25, 26–50, or >50% fragmentation, respectively. Blastomere asymmetry was determined using a numerical score of 1 through 3 based on uniformity in size and shape, where a score of 1 represented perfect symmetry, 2 represented moderate asymmetry, and 3 severe asymmetry. Due to the 24 h delay in injection, IVM zygotes ultimately underwent day 2 embryo evaluation subsequent to fertilization check. Embryo assessments were performed by a team of 6–8 embryologists who routinely participate in proficiency testing to ensure consistency in grading. Good quality embryos were defined as having at least 8-cells and <10% fragmentation.
Embryos with the lowest percentage of fragmentation, lowest asymmetry, and optimal number of cells were chosen for transfer. Preference was given to 8-cell embryos derived from in vivo matured sibling oocytes where possible, other characteristics being equal. The number of embryos transferred was determined based on number and quality of available embryos, patient age, and prior clinical history. To maximize the number of embryos for these patients, IVM embryos were frozen either at the 2PN stage or on day 2, provided they met criteria for freezing (at least 4-cells with <10% fragmentation and perfect or moderately symmetrical blastomeres). The decision regarding whether to freeze at the 2pn-stage was based on a clinical assessment of the total number of embryos predicted to be available for transfer arising from IVO-MII-Sib oocytes.
Control embryos
Two separate control groups were employed in our analysis. Maturation efficiency, fertilization rate, developmental competency, and ultimate reproductive outcomes were analyzed with respect to 234 in vivo matured sibling oocytes (IVO-MII-Sib) from the 41 cycles from which the 263 IVM oocytes arose. However, this group of sibling oocytes could not serve as a basis of comparison for embryo development (cell number, fragmentation, and asymmetry), given that during the time period of this study, our Center for Assisted Reproductive Technology had ceased performing standard day 2 embryo evaluations due to our recent findings that little benefit is accrued in performing day 2 evaluations for day 3 transfers [13]. Therefore, in order to comment on the day 2 development of the 53 IVM embryos from 22 of the 41 IVM cycles, a matched-pair control group was selected from 22 cycles performed during January 2006 through December 2007, during which time day 2 embryo evaluations were routinely performed and culture media, stimulation, and ICSI protocols were similar to those used in 2008/2009.
The control group was obtained by matching each of the 22 cycles having at least one day 2 IVM embryo with a non-IVM external control cycle (IVO-Ext), matching for age, attempt number, stimulation protocol, diagnosis, number of oocytes retrieved, and number of mature oocytes. Where number of oocytes retrieved and number of mature oocytes were unable to be sufficiently matched, percent oocyte maturity was selected for matching. By creating this matched control group, we eliminated a source of bias, given the inability to compare day 2 IVM embryo development with the day 3 embryo development of the IVO-MII-Sib oocytes.
In the text that follows, the sibling control group refers to IVO-MII-Sib oocytes which arose from the 41 index cycles. Discussion of the matched-pair control group refers to the 116 external control embryos (IVO-Ext) assembled for the purposes of comparing day 2 embryo development only.
Outcome variables assessed
Index embryos were compared with IVO-Ext controls in terms of distribution by cell number, fragmentation, and asymmetry scores on day 2. Implantation and ongoing pregnancy rates were compared between IVM embryos and IVO-MII-Sib embryos with known developmental fate. Moreover, implantation and ongoing pregnancy rates were analyzed after stratifying cycles according to the percentage of embryos transferred that were derived from IVM. Implantation rate was defined as the number of sacs detected by ultrasound at 5–6 weeks out of the total number of embryos transferred. Ongoing pregnancy rate was defined as the number of transfers resulting in at least one viable fetus (by ultrasound to at least 8 weeks) out of all transfers performed; this outcome variable was assessed as opposed to livebirth rate given the recent nature of the cycles included in this study.
Statistical analysis
StatView (version 5.0.1, SAS Institute, Cary, NC, USA) and StatXact (version 6.2.0, Cytel Software Corporation, Cambridge, MA, USA) statistical software were utilized to perform the data analysis for this study. Differences in continuous variables between the index population and matched-pair population were determined using the Wilcoxon Signed Rank test. Categorical variables were analyzed by Fisher’s Exact test or Fisher-Freeman-Halton test. In all cases, p < 0.05 was considered to be statistically significant.
Results
Patient and cycle demographics
The average age of the patients who underwent the 41 study cycles was 36.6 y, and the mean day 3 FSH of this population was 7.7 mIU/ml. Twenty-one of the 41 cycles involved patients on their first IVF/ICSI attempt; for the remainder of cycles, the average attempt number was 3.2. Mean attempt number for all index cycles combined was 2.1. Most cycles were undertaken for male-factor infertility, although other diagnoses included oligo-ovulation [1], PCOS [1], tubal factor [2], unexplained [4], and diminished ovarian reserve [5]. An average of 12.5 oocytes was retrieved per cycle, 6.6 of which were subjected to IVM for an average of an additional 23.1 h subsequent to completion of first day ICSI. In 26 of 41 cycles (63.5%), immature oocytes comprised >50% of oocytes retrieved, with 4 cycles (9.8%) notable for the absence of MIIs. For the remaining cycles, 3 (7.3%) were characterized by <20% immature oocytes, and 12 (29.3%) by immaturity ranging from 20–49% of the total oocytes retrieved.
Of the 41 study cycles in which extended duration IVM was employed, in 37 (90.2%), at least one oocyte had progressed to MII; 32 of these cycles (86.5%) subsequently achieved successful fertilization in at least one IVM oocyte. 10 of those 32 cycles (31.3%) involved day 1 freezing of all IVM zygotes. The remaining 22 cycles (68.8%) underwent day 2 embryo morphological assessment. The 53 resultant embryos from these 22 cycles were then compared with 116 embryos arising from the 22 IVO-Ext control cycles.
Of the 516 oocytes retrieved from the 41 study cycles, 19 (3.7%) were discarded immediately after exposure to hyaluronidase due to their grossly abnormal appearance, such as extensive vacuolization or extreme cytoplasmic granularity. Of the remaining 497 oocytes, 216 (43.5%) were mature, 185 (37.2%) were at the GV stage, and 96 (19.3%) were at MI. Of those MI oocytes, 18 had matured by the completion of initial ICSI and were thus injected with the initial cohort of IVO-MII-Sib oocytes. Therefore, a total of 263 oocytes were subjected to IVM.
Maturation, fertilization and fate of IVM versus in vivo matured sibling oocytes
Maturation and fertilization rates, as well as developmental fates were compared between the 263 IVM oocytes and 234 IVO-MII-Sib oocytes. As shown in Table 1, significantly more MII oocytes were obtained from the IVO-MII-Sib group than from IVM (5.7 versus 3.1; p = 0.0007). While the absolute number of 2PN zygotes resulting from IVM oocytes was significantly lower than that from IVO-MII-Sib oocytes (2.2 versus 4.0 oocytes, respectively; p = 0.0019), the fertilization rate (%2PN/MII) was not significantly different between the two groups. Conversely, although a similar number of embryos was frozen for the two groups, a higher percentage of embryos in the IVM group was frozen (33.6% versus 14.5%; p = 0.0097). This increased incidence of freezing in the IVM group was due to the fact that embryos derived from IVO-MII-Sib oocytes tended to be preferentially selected for transfer (a mean of 2.3 versus 1.0 embryos, respectively; p = 0.0016).
Table 1.
Maturation, fertilization and fate of IVM and IVO-MII-Sib oocytes
| IVM (N = 263) | IVO-MII-Sib (N = 234) | p-value | |
|---|---|---|---|
| # MII | 3.1 ± 2.2 | 5.7 ± 4.3 | 0.0007 |
| # 2PN | 2.2 ± 1.8 | 4.0 ± 2.7 | 0.0019 |
| % 2pn/MII | 62.1 ± 34.6 | 64.0 ± 23.4 | 0.9909 |
| # Frozen | 0.9 ± 1.6 | 0.8 ± 2.2 | 0.3022 |
| % Frozen | 33.6 ± 47.4 | 14.5 ± 32.7 | 0.0097 |
| # Embryos transferred | 1.0 ± 0.8 | 2.3 ± 1.0 | 0.0016 |
| % of all embryos transferred | 24.9 ± 30.8 | 75.1 ± 30.8 | 0.0001 |
Values represent mean ± standard deviation for the 41 study cycles.
GV vs MI oocytes: maturation, fertilization and fate
Collectively, 47.9% of immature oocytes subjected to IVM achieved MII status. As shown in Table 2, progression to MII was significantly more likely in oocytes subjected to IVM at the MI stage than those placed in IVM medium at the GV stage (78.2% vs. 35.1%, p = 0.0001). Fertilization rate did not differ between the two groups (67.2% vs. 60.0%, p = 0.8587), nor did the percentage of embryos frozen (34.2% vs. 41.0%, p = 0.6450). Twice as many embryos arising from MI oocytes were transferred as compared with those arising from GV oocytes, however the differences observed in the proportion of embryos selected for transfer did not reach statistical significance (39.0% versus 20.5%; p = 0.0899).
Table 2.
Maturation, fertilization and fate of GV and MI IVM oocytes
| GV (n = 185) | MI (n = 78) | p-value | |
|---|---|---|---|
| # MII | 65 (35.1%) | 61 (78.2%) | P = 0.0001 |
| # 2PN | 39 (60.0%) | 41 (67.2%) | P = 0.8587 |
| # Frozen | 16 (41.0%) | 14 (34.2%) | p = 0.6450 |
| # Transferred | 8 (20.5%) | 16 (39.0%) | P = 0.0899 |
Values represent mean ± standard deviation
Assessment of developmental competency of IVM oocytes
By nature of their delayed injection, IVM oocytes undergoing second day ICSI underwent day 2 embryo evaluation. In order to evaluate developmental competency of the embryos arising from the 22 cycles in which day 2 evaluations were performed, a non-IVM matched-pair control group (IVO-Ext) of 22 cycles from 2006–2007 in which day 2 embryo evaluation was routinely performed was selected for matched-pair analysis (see methods regarding selection of IVO-Ext controls). Table 3 shows the demographic similarities between these two populations, revealing statistical differences only in the number and percentage of mature oocytes retrieved.
Table 3.
Demographic similarities between IVM and IVO-Ext patients undergoing cycles with day 2 embryo evaluations
| IVM | IVO-Ext | p-value | |
|---|---|---|---|
| Age | 37.6 ± 5.0 | 37.6 ± 5.0 | 0.7224 |
| Attempt # | 2.5 ± 1.7 | 2.1 ± 1.1 | 0.2636 |
| d3 FSH | 7.9 ± 3.4 | 7.7 ± 3.1 | 0.7782 |
| Peak E2 | 1879 ± 909 | 1711 ± 1038 | 0.4925 |
| # Oocytes | 11.4 ± 6.4 | 12.9 ± 7.7 | 0.0950 |
| # MII retrieved | 4.6 ± 3.4 | 7.5 ± 4.9 | 0.0008 |
| % Maturity | 39.9 ± 19.8 | 57.9 ± 13.5 | 0.0005 |
Values represent mean ± standard deviation for the 22 IVM and 22 IVO-Ext cycles.
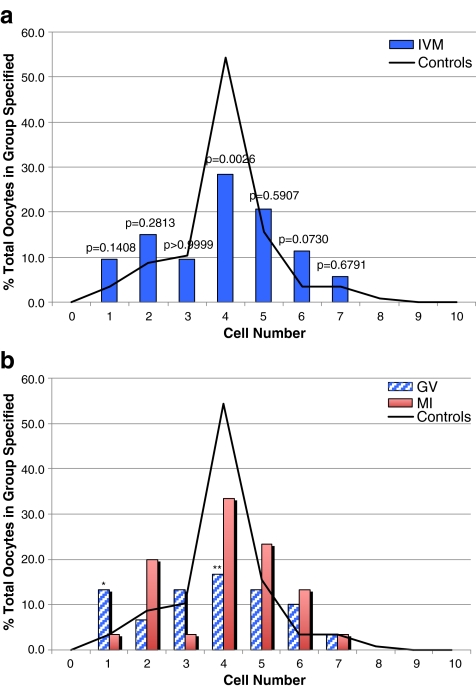
Figure 1a shows day 2 cell number for all IVM embryos with respect to IVO-Ext control embryos. A significant trend toward decreased cell number was noted for IVM versus control embryos (p = 0.0248), which manifested in a significant decrease in the proportion of 4 cell embryos (28.3% vs. 54.3%; p = 0.0026). Figure 1b further delineates cell number according to whether the embryos were derived from oocytes obtained from IVM of GV or MI oocytes. As shown, a proportionally worse developmental trend was observed in embryos from the GV-MII oocytes as compared with MI-MII (p = 0.0140), with significant differences observed at the 1 cell stage as well as 4-cell stage (p = 0.0258 and p = 0.0056, respectively). No significant difference was detected in embryos arising from MI-MII oocytes as compared with controls.
Fig. 1.
a Cell number in day 2 embryos derived from IVM oocytes matured from either the GV-stage or MI, compared with IVO-Ext controls. b Cell number in day 2 embryos derived from IVM oocytes matured from the GV-stage versus MI, compared with IVO-Ext controls. *p = 0.0258 (GV vs controls), **p = 0.0056 (GV vs controls). For all other comparisons, p > 0.05
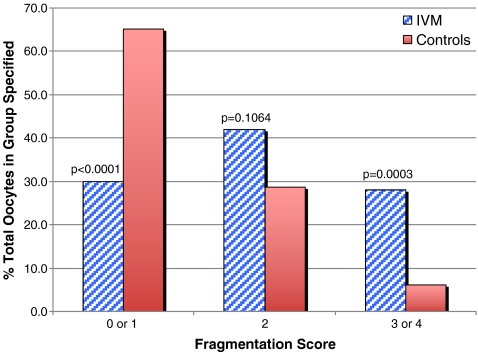
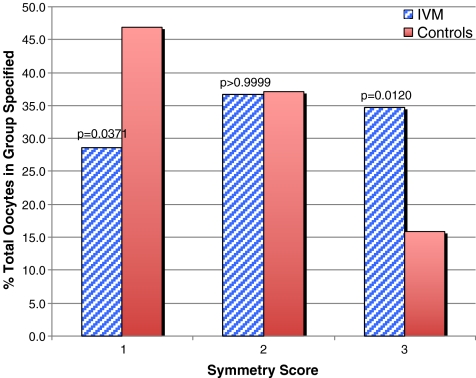
The fragmentation and symmetry distributions for IVM embryos and the IVO-Ext controls are compared in Figs. 2 and 3, respectively. In vitro maturation was associated with significantly worse trends in fragmentation (p < 0.00001) and asymmetry (p = 0.0188). There were no statistically significant differences detected in fragmentation or symmetry relative to degree of oocyte immaturity (i.e. GV vs. MI, data not shown).
Fig. 2.
Fragmentation in day 2 embryos derived from IVM oocytes matured from either the GV-stage or MI, compared with IVO-Ext controls
Fig. 3.
Symmetry in day 2 embryos derived from IVM oocytes matured from either the GV-stage or MI, compared with IVO-Ext controls
Implantation potential of embryos arising from IVM versus IVO-MII-Sib oocytes
In total, 24 of the 53 IVM embryos (45.3%) obtained in the 41 cycles were transferred, as well as 76 of the 129 embryos derived from IVO-MII-Sib oocytes (58.9%). Of the 55 IVO-MII-Sib embryos with known developmental fate, 10 (18.2%) successfully implanted. No implantation occurred for the 17 IVM embryos with known fate (where known fate was defined as transfer of only IVM embryos or the combined transfer of IVM/IVO embryos in which no implantation occurred). The remaining 7 IVM embryos were transferred in 5 cycles in which pregnancy did occur, however transfer occurred along with IVO-MII-Sib embryos, thus preventing determination of definitive developmental fate.
Cycle outcomes
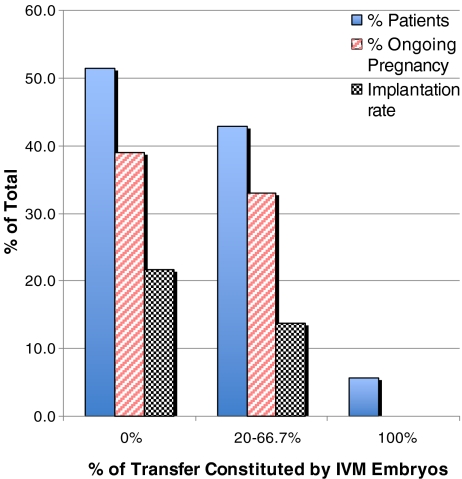
In order to make a more detailed comment on developmental competency, we analyzed implantation rate and ongoing pregnancy rate on a cycle population level with respect to the proportion of all embryos transferred that were derived from post-cumulus stripped IVM oocytes. As shown in Fig. 4, in cycles with a greater proportion of IVM embryos transferred, we observed reduced rates of implantation (p = 0.5239) and ongoing pregnancy (p = 0.8664), with complete IVM transfer associated with zero rates of implantation and ongoing pregnancy. None of these observations, however, reached statistical significance.
Fig. 4.
Implantation and ongoing pregnancy rate according to number of IVM embryos transferred
Discussion
In the present study, we set out to assess the developmental potential and clinical implications of IVM of immature oocytes retrieved from controlled ovarian stimulation IVF-ICSI cycles, specifically investigating 24 h of IVM with subsequent second day injection of matured ooocytes. We initially felt that 24 h of IVM, with second day ICSI of those oocytes with delayed polar body extrusion, would improve the ultimate yield of MII oocytes, thus optimizing patient cycles. Successful pregnancies from such extended incubation have been reported in the literature, albeit with low incidence [14–16].
While we implemented this practice as a means to optimize numbers of available embryos for transfer, we discovered that resultant embryos exhibited sub-optimal development as evidenced by slow cleavage and increased fragmentation and asymmetry. Moreover, reproductive outcomes appeared to be compromised in those cycles with IVM transfer, as indicated by our analysis of implantation and pregnancy rates according to the percentage of IVM embryos transferred. To our knowledge, this oocyte cohort represents the largest to date involving this type and duration of IVM in which embryo quality, implantation potential and pregnancy outcomes were analyzed. Our findings, together with a recent study by de Almeida Ferreira Braga and colleagues involving a similar incubation time-frame, suggest that the clinical contribution of prolonged IVM oocytes is questionable [17].
Early on, IVM from naturally cycling women was appealing as a method for obviating the use of exogenous gonadotropins and their ensuing risks; indeed, IVM of unstimulated oocytes eliminates the need for costly hormonal stimulation, frequent blood sampling, and serial ultrasound imaging, in addition to removing the risks of hyperstimulation syndrome. Such attempts at IVM of immature human oocytes date back to 1965, with the first successful birth reported in 1991 [18, 19]. In the absence of gonadotropin stimulation, however, IVM of immature oocytes remains an inefficient process with fewer oocytes available per cycle, reduced rates of cleavage, and lower pregnancy rates as compared to IVM of immature oocytes retrieved from stimulated cycles, in which maturation is begun in-vivo but completed in-vitro with the oocytes still enclosed within their cumulus masses [7, 20–22]. Rates can be potentially improved by priming with hCG 36 h prior to retrieval, however stimulation with FSH remains most optimal [2]. Based on such observations, we chose to investigate IVM of immature oocytes arising from stimulated cycles.
Various studies have revealed benefit of a brief period (4–6 h) of IVM for immature oocytes from stimulated cycles [2, 3]. This practice allows for production of a maximal number of oocytes to inseminate without significantly impacting laboratory flow or costs, given that re-assessment and subsequent injection of such IVM oocytes can be performed on the same day and shortly after ICSI of IVO-MII-Sib oocytes. Numerous previous publications have commented on this practice [2–4, 11]. Relatively fewer studies, however, have focused on longer periods of maturation, such as that employed here, for those oocytes which fail to extrude their first polar body in that initial window for maturation. Longer duration of IVM culture necessitates additional incubation, a second day of ICSI, and discordant embryo growth between in-vivo and in-vitro matured oocytes, and thus should be analyzed as a separate entity from brief duration (4–6 h) IVM.
In-vivo oocyte meiotic resumption requires intimate paracrine and autocrine signaling between the oocyte and its surrounding granulosa and cumulus cells [23]. ICSI involves the removal of cumulus and corona cells shortly after oocyte retrieval in order to make a determination of nuclear maturity, as it is difficult to identify nuclear maturity under the microscope with the cumulus-oocyte-complex intact [1]. Doing so, however, disrupts signaling pathways between those oocytes discovered to be immature and their surrounding cells. Cumulus cells share an integral role in meiotic arrest via gap-junction mediated signaling and maintenance of oocyte cAMP levels, while also exerting stimulatory effects on nuclear and cytoplasmic maturation, as evidenced by higher rates of maturation, cytoplasmic maturity, and improved cleavage in cumulus-intact oocytes as compared with denuded oocytes subjected to IVM [7, 23]. Cumulus cells have been implicated in reducing reactive oxygen species during oocyte metabolism, and their absence with decreased amino acid intake and anti-oxidant synthesis [24, 25]. In order to analyze the literature regarding IVM of immature oocytes, it is thus critical to note whether cumulus complexes are left intact or are stripped prior to placement in culture media. In this study, stripping of the cumulus cells was necessary to confirm polar body emission and to perform ICSI.
Achievement of nuclear maturation alone, however, does not by itself constitute success of in vitro maturation, and may explain the reduced quality observed in our IVM embryos. Maximal fertilization and lowest incidence of multinucleation occurs 3–6 h subsequent to nuclear maturation, an observation which underscores cytoplasmic maturation as a critical step in achieving complete potential [26]. Cytoplasmic competency, encompassing a wide array of metabolic and structural modifications which allow for normal fertilization, meiotic to mitotic cell cycle progression, and genetic activation, is required to produce embryos with full developmental potential [27–30]. The slower cleavage rates often observed in IVM embryos have been potentially attributed to defective cytoplasmic maturation, either secondary to a loss of appropriate cytoplasmic proteins or due to a loss of synchrony between nuclear and cytoplasmic maturation, as evidenced by deficiencies in microtubule dynamics and chromatin phosphorylation in IVM oocytes [10, 27]. Greater deficiencies in cleavage and genetic competency have been associated with duration of in vitro maturation, with extended duration in culture shown to be associated with spindle vulnerability; the metaphase spindle in aged oocytes no longer is observed on the periphery, but rather moves to the center of the oocyte [31, 32]. Indeed, embryos originating from aged oocytes display a higher likelihood of abnormal cytoskeletal organization [33]. Such deficiencies may very well account for the diminished developmental potential of the IVM embryos in this study.
We did not assess the cytogenetic integrity of the embryos in this study derived from oocytes that were matured in vitro. Cytoplasmic derangements of IVM oocytes and resultant embryos may very well hinge on an altered cytogenetic constitution of such embryos. Immature GV oocytes matured in vitro exhibit a large number of genes expressed at more than a 2-fold higher level than in IVO-MII sibling oocytes, attributed to dysregulation of gene transcription and incorrect temporal utilization of transcripts [34]. Studies have thus demonstrated that IVM oocytes exhibit a higher proportion of complex chromosomal abnormalities as compared with IVO-MII oocytes, consisting of mosaic, nullisomic, and monosomic disruptions of normal chromosomal complements on fluorescent in-situ hybridization analysis of embryos in vitro matured for 24 h [10, 32, 35]. Such observations may account for the poorer reproductive potential for oocytes that have been in vitro matured in the present study, and provide strong evidence to favor embryos arising from in vivo matured oocytes over in vitro matured siblings when making selections for transfer.
Myriad culture media exist with which to perform IVM. In our study, we used SAGE medium (CooperSurgical, Trumbull, CT) for maturation of the immature oocytes, which has some proven efficacy for maturing oocytes retrieved from patients with polycystic ovarian syndrome after brief exposure to hCG in vivo, as well as for maturation of immature oocytes aspirated prior to treatment for ovarian malignancy [36, 37]. However, no consensus exists on the optimal culture medium for in vitro maturation of GV or MI oocytes, nor which, if any, additives are beneficial.
Important distinctions should be drawn between our work and those studies which have preceded it. The study by Shu et al regarding IVM of 518 immature (GV + MI) oocytes from stimulated cycles employed only brief duration (4–6 h) IVM, and moreover involved ICSI of MI oocytes which failed to mature in addition to successfully matured ova [4]. Similarly, De Vos et al. described near-comparable embryo quality with IVM of 2010 MI oocytes as compared with their controls, however limited culture to 4 h [3]. Successful pregnancy and birth were achieved.
In the study by Kim et al, specifically examining IVM of 168 GV stage oocytes from stimulated cycles, cumulus-oocyte complex maturity was determined by identification of a brown spot in the cytoplasm via dish-tilting under a stereoscope, rather than by stripping [9]. Oocytes were left in culture supplemented with human follicular fluid for 44 h after nuclear assessment; assessment made between cumulus-stripped and cumulus-intact oocytes revealed more synchronous oocyte maturation with cumulus cell retention, as well as significantly diminished fertilization in all IVM oocytes as compared with IVO siblings, a finding not corroborated by our analysis [9]. The authors concluded that IVM of stimulated oocytes with subsequent ICSI of successfully matured oocytes ensures a wider range of fertilized oocytes and increases the opportunity for ensuring pregnancy.
In a study comparing maturation and developmental competency of 521 cumulus-free immature oocytes from stimulated ICSI cycles, assessment of maturity was made at 6, 24, and 48 h of IVM culture [2]. Similar blastocyst morphology was noted between in vitro matured and in vivo matured oocytes, although a more rigorous analysis was not performed. The authors concluded that it is important to mature and inseminate immature oocytes from stimulated cycles, because reasonable blastocyst formation rates are obtained. Blastocysts were destroyed on day 5, and thus ultimate reproductive implications were left unassessed.
It is important to note that the present study involved both day 2 and day 3 embryo transfer. We felt it was reasonable, however, to compare reproductive outcomes for embryos arising from in vitro and in vivo matured oocytes, as comparable live-birth rates have been demonstrated between the two [38]. Nevertheless, we performed a match-pair analysis to comment on day 2 development of embryos arising from IVM oocytes, as no meaningful comparison could be drawn by comparing day 3 development of sibling embryos [39].
Based on the present findings showing compromised development and absent implantation potential of IVM embryos, we made the programmatic decision to abandon the routine practice of extended duration IVM for patients with high rates of immaturity in previous cycles, and employ the practice only as a “heroic” measure in cycles marked by extenuating circumstances. Moreover, our counseling now reflects the reduced quality of embryos derived from such practices, and embryo selection protocols de-prioritize IVM embryos when in vivo matured oocytes are available.
Our study was not without limitations. It is possible that we did not allow for adequate time for maturation of GV oocytes, as some studies have demonstrated a 30–32 h time requirement to reach MII, albeit with different incubation media than that used in this study [9]. Such a duration, however, was infeasible based on the logistic framework of our laboratory. Whereas the incubation may have been too brief for GV oocytes which did not achieve maturity, by the same token it may have been too long for MI oocytes that did achieve maturity, as the 24 h period of maturation may have exposed IVM matured MI oocytes to a longer-than-necessary incubation period. Given the logistics of our lab, however, it was infeasible to assess maturity at multiple time-points.
Additionally, it is important to note that the same sperm samples were used for both first and second day ICSI. Rates of fertilization, however, were equal between first and second day ICSI (in vivo matured versus in vitro), and thus we presume using older sperm on second day injections had little deleterious effect. Lastly, our criteria for inclusion was not limited to a strict percentage of immature oocytes retrieved, but rather was, for the time period of this study, a clinical decision made between the embryology laboratory and clinician. It is thus difficult to assess from our sample whether patients with the highest degree of immaturity suffer from worse oocyte competency. As is the case with any such study design, our findings are retrospective in nature, however we believe represent compelling evidence against the routine implementation of such practices.
Conclusion
In this study, we examined the quality and developmental potential of embryos from stimulated cycles which were derived from oocytes retrieved in the immature state and then subjected to 24 h of in vitro maturation followed by second day ICSI. The present results indicate that such oocytes give rise to embryos with compromised developmental characteristics and very low, if not absent, implantation potential, with a greater detrimental effect regarding cell number, symmetry, and fragmentation observed in GV as opposed to MI oocytes. We therefore do not recommend the routine use of extended duration IVM of immature, cumulus-stripped oocytes. Moreover, such oocytes, as evidenced by the abnormal embryos they produce, have little utility for studying normal mechanisms underpinning meiotic maturation in the human, and should thus be used with caution for scientific research aimed at studying such processes.
Footnotes
Capsule Twenty-four hour in vitro maturation of immature cumulus-stripped oocytes from stimulated cycles results in embryos with compromised development and reduced implantation potential.
References
- 1.Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103–20. doi: 10.1093/humupd/4.2.103. [DOI] [PubMed] [Google Scholar]
- 2.Chian RC, Tan SL. Maturational and developmental competence of cumulus-free immature human oocytes derived from stimulated and intracytoplasmic sperm injection cycles. Reprod Biomed Online. 2002;5:125–32. doi: 10.1016/S1472-6483(10)61614-8. [DOI] [PubMed] [Google Scholar]
- 3.Vos A, Velde H, Joris H, Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14:1859–63. doi: 10.1093/humrep/14.7.1859. [DOI] [PubMed] [Google Scholar]
- 4.Shu Y, Gebhardt J, Watt J, Lyon J, Dasig D, Behr B. Fertilization, embryo development, and clinical outcome of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Fertil Steril. 2007;87:1022–7. doi: 10.1016/j.fertnstert.2006.08.110. [DOI] [PubMed] [Google Scholar]
- 5.Ge HS, Huang XF, Zhang W, Zhao JZ, Lin JJ, Zhou W. Exposure to human chorionic gonadotropin during in vitro maturation does not improve the maturation rate and developmental potential of immature oocytes from patients with polycystic ovary syndrome. Fertil Steril. 2008;89:98–103. doi: 10.1016/j.fertnstert.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Chian RC, Buckett WM, Too LL, Tan SL. Pregnancies resulting from in vitro matured oocytes retrieved from patients with polycystic ovary syndrome after priming with human chorionic gonadotropin. Fertil Steril. 1999;72:639–42. doi: 10.1016/S0015-0282(99)00323-4. [DOI] [PubMed] [Google Scholar]
- 7.Goud PT, Goud AP, Qian C, Laverge H, Elst J, Sutter P, et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–44. doi: 10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Lu G, Qian Y, Mao Y, Ding W. Pregnancies and births achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil Steril. 2003;80:447–9. doi: 10.1016/S0015-0282(03)00665-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim BK, Lee SC, Kim KJ, Han CH, Kim JH. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil Steril. 2000;74:1153–8. doi: 10.1016/S0015-0282(00)01617-4. [DOI] [PubMed] [Google Scholar]
- 10.Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86:629–35. doi: 10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 11.Strassburger D, Friedler S, Raziel A, Kasterstein E, Schachter M, Ron-El R. The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19:1587–90. doi: 10.1093/humrep/deh236. [DOI] [PubMed] [Google Scholar]
- 12.Racowsky C, Combelles CM, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod Biomed Online. 2003;6:323–31. doi: 10.1016/S1472-6483(10)61852-4. [DOI] [PubMed] [Google Scholar]
- 13.Racowsky C, Ohno-Machado L, Kim J, Biggers JD. Is there an advantage in scoring early embryos on more than one day? Hum Reprod. 2009;24:2104–13. doi: 10.1093/humrep/dep198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edirisinghe WR, Junk SM, Matson PL, Yovich JL. Birth from cryopreserved embryos following in-vitro maturation of oocytes and intracytoplasmic sperm injection. Hum Reprod. 1997;12:1056–8. doi: 10.1093/humrep/12.5.1056. [DOI] [PubMed] [Google Scholar]
- 15.Tucker MJ, Wright G, Morton PC, Massey JB. Birth after cryopreservation of immature oocytes with subsequent in vitro maturation. Fertil Steril. 1998;70:578–9. doi: 10.1016/S0015-0282(98)00205-2. [DOI] [PubMed] [Google Scholar]
- 16.Vanhoutte L, Sutter P, Elst J, Dhont M. Clinical benefit of metaphase I oocytes. Reprod Biol Endocrinol. 2005;3:71. doi: 10.1186/1477-7827-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Almeida Ferreira Braga DP, de Cassia Savio Figueira R, Ferreira RC, Pasqualotto FF, Iaconelli A, Jr., Borges E, Jr. Contribution of in-vitro maturation in ovarian stimulation cycles of poor-responder patients. Reprod Biomed Online;20:335–40. [DOI] [PubMed]
- 18.Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109–13. doi: 10.1016/s0015-0282(16)54068-0. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–51. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 20.Russell JB, Knezevich KM, Fabian KF, Dickson JA. Unstimulated immature oocyte retrieval: early versus midfollicular endometrial priming. Fertil Steril. 1997;67:616–20. doi: 10.1016/S0015-0282(97)81354-4. [DOI] [PubMed] [Google Scholar]
- 21.Barnes FL, Crombie A, Gardner DK, Kausche A, Lacham-Kaplan O, Suikkari AM, et al. Blastocyst development and birth after in-vitro maturation of human primary oocytes, intracytoplasmic sperm injection and assisted hatching. Hum Reprod. 1995;10:3243–7. doi: 10.1093/oxfordjournals.humrep.a135896. [DOI] [PubMed] [Google Scholar]
- 22.Barnes FL, Kausche A, Tiglias J, Wood C, Wilton L, Trounson A. Production of embryos from in vitro-matured primary human oocytes. Fertil Steril. 1996;65:1151–6. doi: 10.1016/s0015-0282(16)58330-7. [DOI] [PubMed] [Google Scholar]
- 23.Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009;8:2741–7. doi: 10.4161/cc.8.17.9471. [DOI] [PubMed] [Google Scholar]
- 24.Mattioli M, Galeati G, Seren E. Effect of follicle somatic cells during pig oocyte maturation on egg penetrability and male pronucleus formation. Gamete Res. 1988;20:177–83. doi: 10.1002/mrd.1120200208. [DOI] [PubMed] [Google Scholar]
- 25.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–10. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- 26.Balakier H, Sojecki A, Motamedi G, Librach C. Time-dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum Reprod. 2004;19:982–7. doi: 10.1093/humrep/deh158. [DOI] [PubMed] [Google Scholar]
- 27.Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–16. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- 28.Heikinheimo O, Gibbons WE. The molecular mechanisms of oocyte maturation and early embryonic development are unveiling new insights into reproductive medicine. Mol Hum Reprod. 1998;4:745–56. doi: 10.1093/molehr/4.8.745. [DOI] [PubMed] [Google Scholar]
- 29.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 30.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–9. doi: 10.1071/RD9960485. [DOI] [PubMed] [Google Scholar]
- 31.Mrazek M, Fulka J., Jr Failure of oocyte maturation: possible mechanisms for oocyte maturation arrest. Hum Reprod. 2003;18:2249–52. doi: 10.1093/humrep/deg434. [DOI] [PubMed] [Google Scholar]
- 32.Strassburger D, Goldstein A, Friedler S, Raziel A, Kasterstein E, Mashevich M, et al. The cytogenetic constitution of embryos derived from immature (metaphase I) oocytes obtained after ovarian hyperstimulation. Fertil Steril 2009. [DOI] [PubMed]
- 33.Racowsky C, Kaufman ML. Nuclear degeneration and meiotic aberrations observed in human oocytes matured in vitro: analysis by light microscopy. Fertil Steril. 1992;58:750–5. doi: 10.1016/s0015-0282(16)55323-0. [DOI] [PubMed] [Google Scholar]
- 34.Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, et al. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23:1138–44. doi: 10.1093/humrep/den085. [DOI] [PubMed] [Google Scholar]
- 35.Emery BR, Wilcox AL, Aoki VW, Peterson CM, Carrell DT. In vitro oocyte maturation and subsequent delayed fertilization is associated with increased embryo aneuploidy. Fertil Steril. 2005;84:1027–9. doi: 10.1016/j.fertnstert.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Son WJ, Chung J, Cui S, Dean N, Tan S, Chian R. Comparison of embryology and clinical outcome between IVM cycles with and without mature oocytes collected following HCG priming. Fertil Steril. 2006;86:S394–5. doi: 10.1016/j.fertnstert.2006.07.1087. [DOI] [Google Scholar]
- 37.Huang JY, Buckett WM, Gilbert L, Tan SL, Chian RC. Retrieval of immature oocytes followed by in vitro maturation and vitrification: a case report on a new strategy of fertility preservation in women with borderline ovarian malignancy. Gynecol Oncol. 2007;105:542–4. doi: 10.1016/j.ygyno.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Oatway C, Gunby J, Daya S. Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection. Cochrane Database Syst Rev 2004;CD004378. [DOI] [PubMed]
- 39.Laverge H, Sutter P, Elst J, Dhont M. A prospective, randomized study comparing day 2 and day 3 embryo transfer in human IVF. Hum Reprod. 2001;16:476–80. doi: 10.1093/humrep/16.3.476. [DOI] [PubMed] [Google Scholar]