Abstract
Purposes
To investigate the frequencies of AZF microdeletions and chromosomal abnormalities in infertile men from Northeastern China. Moreover, to compare the prevalence of these abnormalities with other countries and regions in the world.
Methods
305 infertile men were enrolled. A complete semen analysis and reproductive hormones were measured according to standard methods. Multiplex polymerase chain reaction (PCR) amplification using nine specific sequence-tagged sites (STS) were used to detect AZF microdeletions. Karyotype analyses were performed on peripheral blood lymphocytes with standard G-banding.
Results
Of the 305 infertile men, 28 (9.2%) had AZF microdeletions and 26 (8.5%) had chromosomal abnormalities. The most frequent microdeletions were in the AZFc+d, followed by AZFc, AZFb+c+d and AZFa. A total of 19 patients (82.6%) had Klinefelter′s syndrome (47, XXY) in the azoospermic group.
Conclusions
The freqencies of AZF microdeletions and chromosomal abnormalities in infertile men from Northeastern China were comparable with infertile men from other countries and regions. However, there was a slightly higher prevalence rate of AZF microdeletions in oligozoospermic patients than reported in previous studies.
Keywords: Azoospermia, Oligozoospermia, Oligo-asteno-teratozoospermia syndrome, AZF microdeletion, Chromosomal abnormality
Introduction
Infertility, which is best defined as the inability to conceive or produce an offspring after one year of unprotected intercouse, is affecting about 15% of all couples attempting to generate a pregnancy [1, 2]. Moreover, infertility is showing a tendency to increase and approximately 40–50% of the infertile cases can be attributed to male factors [3].
As early as 1976, Tiepolo et al. reported six azoospermic patients with a long arm deletion of the Y chromosome and postulated that genes or gene families were located in the distal Yq11 region, defined as the AZF [4, 5]. Four subregions of the AZF region (AZFa, AZFb, AZFc and AZFd) were associated with spermatogenesis. Microdeletions of the four subregions result in spermatogenic arrest, and are related to azoospermia and oligozoospermia at different phases of spermatogenesis [1]. Microdeletion of AZFa is relevant to complete Sertoli cell-only syndrome (SCOS) and azoospermia, while microdeletion of AZFb or AZFc results in a variable clinical and histological phenotype ranging from SCOS to oligozoospermia [6].
Furthermore, the Y chromosome is remarkable for its high level of structural variabilities, including deletions, duplications, and inversions [7]. And Y chromosome polymorphism, especially a wide range in length, was reported to be related to reproductive dysfunction [8].
It is remarkable that AZF region microdeletions and chromosomal abnormalities have become clinically important since assisted reproductive techniques (ART), such as intracytoplasmic sperm injection (ICSI), have been introduced to clinical treatments successfully [1, 2, 9, 10]. However, AZF microdeletion and chromosomal abnormality have potential chances to be transmitted readily from infertile fathers to their offspring by ICSI/IVF. Thus, it is essential for infertile male patients to obtain genetic counseling and reliable screening for genetic defects before ICSI/IVF is performed.
In the present study, it is aimed to investigate the frequencies of AZF microdeletions and chromosomal abnormalities in infertile men from Northeastern China. And, to compare the prevalence of these abnormalities with other countries and regions in the world.
Materials and methods
Patients
From November 2006 to September 2009, 305 infertile men were recruited from infertile male patients attending the First Affiliated Hospital and Cell Biology Department, Norman Bethune College of Jilin University. A detailed medical history was taken for every patient. These patients were also interviewed about their medical histories, family backgrounds, reproductive problems, and possible consanguinities. And a physical examination was conducted in all cases, in order to identify anatomical problems. Semen samples were obtained after a 7 days period of sex-inhibition. The urethral fluid and semen were tested for microbial infections, such as mycoplasma infection. In addition, semen analysis was performed according to the World Health Organization Guidelines [11]. All patients underwent semen analysis at least twice before a diagnosis of azoospermia, oligozoospermia and oligo-asteno-teratozoospermia (OAT) syndrome [12]. Blood samples were stored for hormonal evaluations, molecular studies, and cytogenetic evaluations. And sixty fertile men and women were used as controls.
Hormonal analysis
Reproductive hormone levels, including prolactin (PRL), luteinizing hormone (LH), plasma follicle-stimulating hormone (FSH), testosterone (T) and estradiol (E2), were measured for all infertile men. Normal reference ranges for men were: PRL 70–480 μIU/ml, LH 0.4–6.8 mIU/ml, FSH 0.8–15 mIU/ml, T 2.6–13.2 ng/ml, E2 0–70 pg/ml.
Molecular analysis
DNA isolation
Genomic DNA was isolated from peripheral blood lymphocytes by the Tiangen blood DNA extraction mini kit (Beijing Tiangen Biotech Co., Ltd, China).
Selection of primers
The screening for AZF microdeletions was performed by multiplex PCR. A series of 9 specific STSs, mapped in the AZF region, and human zinc-finger protein-encoding genes (ZFX/ZFY) located on the X and Y chromosomes were selected. The ZFX/ZFY, acting as an internal control primers, were selected for molecular genetic analysis of microdeletion [13, 14]. These specific STSs included SY84 and SY86 for AZFa, SY27, SY134, and SY143 for AZFb, SY157, SY254, and SY255 for AZFc, and SY152 for AZFd.
Multiplex PCR amplification was performed in a 10 μl reaction system, containing 200 ng of genomic DNA, 1.5 mmol/L Mg2+, 800 μmol/L deoxyribonucleotide triphosphates (dNTP), 10 pmol/L of each primer and 2 U Taq polymerase. Thermocycling (Veriti Thermal Cycler 96-well, Alpha-SE, Applied Biosystems, USA) consisted of an initial denaturation of 6 min at 94°C, followed by 35 cycles of 40 s at 94°C, 45 s at 55°C and 60 s at 72°C, with a final extension step at 72°C for 6 min. Sixty fertile men and women were used as positive and negative controls to ensure performance of the amplification reaction. Moreover, blank controls were also applied to make sure the samples were not contaminated in the processing operations. The products were finally stored at 4°C before electrophoretic detection.
PCR products (8 μl) mixed with 6× loading buffers (1–2 μl) were separated on 1.5% agarose gel (LP0028A, OXOID, UK) containing ethidium bromide (0.5 μg/ml) at 100 volts for 30 mins. The samples, which were detected to have deletions of STSs, were further confirmed by another repeated test as above.
Cytogenetic analysis
In 305 infertile patients, karyotype analysis was performed on peripheral blood lymphocytes by chromosome G-banding. Briefly, peripheral blood lymphocytes with RPMI Medium 1640 (GIBCO, Invitrogen Corporation, USA), phytohaemagglutinin (PHA) (Shanghai Yihua Medical Technology Co., Ltd, China), and fetal bovine serum (Beijing Dingguo Biotechnology, China) were cultured, followed by being treated with colcemi after a 72 h incubation period. G-banding of metaphase chromosomes was performed by hypotension, fixation, trypsinization, Giemsa staining, etc. At least 20 metaphase spreads were routinely analyzed from each subject. The chromosomal abnormalities were described according to the criteria established by the International System for Human Cytogenetic Nomenclature [15].
Statistical analysis
Statistical analysis was carried out using SPSS 11.5 (Chicago, IL, USA). Students “t-test” was used as appropriate. Statistical significance was assessed at P < 0.05 and all reported P values were two sided.
Results
Clinical findings
The average age of the patients was 26 years (range 19–40 years). There were 219 patients (219/305 or 71.8%) with azoospermia (where no sperm was found in the ejaculate even after centrifugation), 74 patients (74/305 or 24.3%) with oligozoospermia (<20 × 106 spermatozoa/mL), and 12 patients (12/305 or 3.9%) with oligo-asteno-teratozoospermia syndrome (sperm count <20 × 106 mL, motility <40% and normal forms <40%) among these 305 male patients. And 23 patients with severe oligozoospermia and and seven patients with idiopathic oligozoospermia were found in the oligospermia group. Unilateral or bilateral cryptorchidism was present (two were unilateral and one was bilateral) in three azoospermic patients and one patient with unilateral cryptorchidism had oligo-asteno-teratozoospermia. The frequencies of unilateral or bilateral varicocele in infertile men with azoospermia (20 were unilateral and one was bilateral) and oligozoospermia (16 were unilateral and two were bilateral) were 9.6% (21/219) and 24.3% (18/74), repectively. Sertoli cell-only syndrome (SCOS) was present in one azoospermic patient. Two of 74 oligozoospermic men (2.7%) had a history of mycoplasma infection. Furthermore, 114 infertile patients had a past or present smoking history with a frequency of 37.4% (114/305), including 73 azoospermic men, 36 oligozoospermic men, and 5 oligo-asteno-teratozoospermic men. Exposure history, such as toxic exposure or medication-related factors, did not occurred in these infertile men.
Hormonal analysis
The levels of LH and FSH in the azoospermic men (7.3 ± 4.6 and 12.1 ± 5.7 mIU/ml, respectively) were significantly higher than those in the oligozoospermic men (4.7 ± 2.9 and 8.7 ± 3.9 mIU/ml, respectively, P < 0.05) and oligo-asteno-teratozoospermic men (3.5 ± 1.3 and 6.3 ± 3.7 mIU/ml, respectively, P < 0.05). However, the levels of T in the patients with azoospermia (3.02 ± 1.6 ng/ml) were significantly lower than those in the patients with oligozoospermia (4.7 ± 2.5 ng/ml, P < 0.05) and oligo-asteno-teratozoospermia (4.9 ± 1.8 ng/ml, P < 0.05). There was no significant difference in the mean levels of PRL, E2 in these three groups (Table 1).
Table 1.
Hormone levels in male patients with azoospermia, oligozoospermia and oligo-asteno-teratozoospermia syndrome (OAT syndrome). Values are mean ± SD, if applicable. *, P < 0.05, significant difference compared with azoospermic group
| Group | Number | PRL (μIU/ml) | LH (mIU/ml) | FSH (mIU/ml) | T (ng/ml) | E2 (pg/ml) |
|---|---|---|---|---|---|---|
| Azoospermia | 219 | 366.8 ± 212.6 | 7.3 ± 4.6 | 12.1 ± 5.7 | 3.02 ± 1.6 | 66.37 ± 29.8 |
| Oligozoospermia | 74 | 327.7 ± 192.8 | 4.7 ± 2.9* | 8.7 ± 3.9* | 4.7 ± 2.5* | 61.59 ± 23.2 |
| OAT syndrome | 12 | 380.8 ± 182.3 | 3.5 ± 1.3* | 6.3 ± 3.7* | 4.9 ± 1.8* | 63.3 ± 23.4 |
Microdeletion analysis in the AZF region
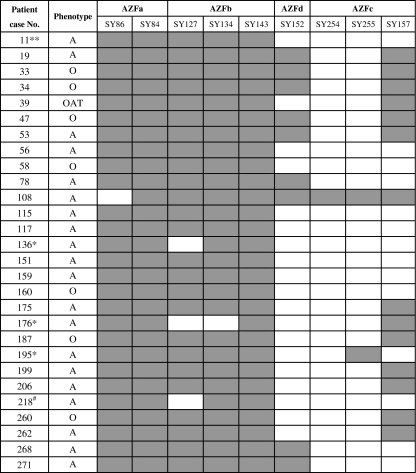
In the present study, screening for AZF microdeletions was carried out in the 305 infertile patients as well as 60 fertile male and female controls. Of 305 infertile cases, AZF region microdeletions on the Y chromosome were detected in 28 infetile male patients with a frenquency of 9.2%. AZF microdeletions were present in 20 patients (9.1% or 20/219) with azoospermia, seven patients (9.5% or 7/74) with oligozoospermia and one patient (8.3% or 1/12) with oligo-asteno-teratozoospermia syndrome. The most frequent microdeletions were in the AZFc+d, followed by AZFc, AZFb+c+d and AZFa (Table 2). All infetile men with complete AZFc and AZFb+c+d deletions had azoospermia (Fig. 1). Of 28 cases with AZF microdeletions, the loss of SY254 (96.4% or 27/28) and SY255 (92.9% or 26/28) were commonest, followed by SY152 (67.9% or 19/28) and SY157 (50% or 14/28). Notably, the skipping microdeletions were found in the four azoospermic patients (No.136, 176, 195, 218), three (No.136, 176, 218) with AZFb+c+d deletions and one (No.195) with AZFc+d deletions. No microdeletions was found in the fertile controls.
Table 2.
The microdeletion of AZF region on the Y chromosome in infertile male patients. OAT, oligo-asteno-teratozoospermia
| AZF region | Azoospermia (n = 219) | Oligozoospermia (n = 74) | OAT syndrome (n = 12) | Total (n = 305) | Deletion rate (%) |
|---|---|---|---|---|---|
| AZFa | 1 | 1 | 0.3 | ||
| AZFc | 6 | 3 | 9 | 3.0 | |
| AZFc+d | 10 | 4 | 1 | 15 | 4.9 |
| AZFb+c+d | 3 | 3 | 1.0 | ||
| Total | 20 | 7 | 1 | 28 | 9.2 |
Fig. 1.
A schematic diagram of AZF microdeletion in 28 infertile men. A, azoospermia; O, oligozoospermia; OAT, oligo-asteno-teratozoospermia syndrome. The solid and open boxes indicate the presence of genes and gene deletions, respectively. *the patients with skipping microdeletions, ** the patient with an abnormal karyotype, # the patient with skipping microdeletions and an abnormal karyotype
Karyotype analysis
Chromosomal abnormalities were found in 26 (23 azoospermic and 3 oligozoospermic men) out of 305 infertile male patients, corresponding to a frequency of 8.5%. A total of 19 infertile patients (19/23 = 82.6%) had Klinefelter′s syndrome (47, XXY) in the azoospermic group. Two azoospermic cases (No.11, 213) had abnormal karyotypes, accompanied by AZFc+d and AZFb+c+d microdeletion, respectively (Table 3). Of 26 cases with chromosomal abnormalities, four azoospermic and oligozoospermic patients (No.11, 218, 254, 280) had chromosome mosaicism, corresponding to a frequency of 15.4%. Y chromosomal polymorphism in length (46, XY, Y < 21) was present in one azoospermic and one oligozoospermic man (No.192, 213). One man (No.211) with oligozoospermia had translocations involving chromosomes Y and 4 (46, XY, t[Y; 4]). No abnormal karyotypes were found in the oligo-asteno-teratozoospermic group or in the fertile controls.
Table 3.
Abnormal karyotypes, clinical findings and AZF microdeletions in azoospermic and oligozoospermic patients, apart from the azoospermic patients with Klinefelter′s syndrome (47, XXY)
| Karyotype | Case No. | Age (year) | Infertile duration (year) | Smoking (+/−) | Left/right testis volume (ml) | AZF microdeletion |
|---|---|---|---|---|---|---|
| Azoospermia (n = 4) | ||||||
| 46, XX/47, XX, +del(Yq) | 11 | 29 | 7 | + | 2&2 | sY157/254/255 |
| 46, XY/46, XX | 117 | 40 | 13 | + | 6&6 | None |
| 46, XY, Y < 21 | 218 | 37 | 3 | – | 15&15 | sY127/152/157/254/255 |
| 46, XX/47,XXY | 280 | 26 | 2 | + | 8&10 | None |
| Oligozoospermia (n = 3) | ||||||
| 46,XY,Y < 21 | 192 | 28 | 3 | – | 12&10 | None |
| 46, XY, t(Y; 4) | 211 | 33 | 6 | + | 12&12 | None |
| 46, XY/47, XXY | 254 | 24 | 1 | + | 15&15 | None |
Discussion
In this study, the frequency of AZF microdeletions was 9.2% in infertile men from Northeastern China. This is consistent with the frequencies reported by other centers, which range from 3% to 55% [1, 16, 17]. The prevalence of AZF microdeletion in the patients with azoospermia, oligozoospermia and oligo-asteno-teratozoospermia syndrome was 9.1%, 9.5% and 8.3%, respectively. It has been reported that the prevalence rates of AZF microdeletion are in the range of 4.25% to 23% and 0.1% to 8.5% in patients with non-obstructive azoospermia and oligozoospermia [18–22]. Thus, our data revealed that there was a slightly higher prevalence of AZF microdeletions in oligozoospermic patients than previous studies. It is widely accepted that patient selection criteria, methodological aspects, ethnic variances, genetic background, and environmental influences can account for such a wide variation in the reported frequencies of AZF microdeletion [2, 18].
The DAZ (deleted in azoospermia) gene family, mapped in the AZFc region, had been regarded as the most likely candidate genes for spermatogenesis deficiency. In our stuty, the SY254 and SY255 for DAZ gene family presented a higher prevalence of deletions than other STSs. One possible explanation is the existence of repetitive sequences of DAZ and an increase or decrease of the repetitive gene clusters may result in male reproductive dysfunction [2].
The prevalence of abnormal karyotypes in this study was 8.5%. This is in agreement with the previously reported rangs of 2.2–14.3% for infertile men [18, 23, 24]. The most common chromosomal abnormality was Klinefelter′s syndrome (47, XXY), which accounted for 73.1% of all chromosomal abnormalities. Four patients with chromosome mosaicism were found among the patients with chromosomal abnormalities.
The genes associated with germ cell development have been mapped to the long arm of the Y chromosome. Thus, Y chromosome polymorphism, especially in length, might have genetic effects on reproductive function. In the present study, Y chromosomal polymorphism in length was present in two patients (No.192 was oligozoospermic and No.213 was azoospermic).
The levels of FSH and LH in infertile men with azoospermia were significantly higher than those in the infertile men with oligozoospermia and oligo-asteno-teratozoospermia. This is consistent with a large study by Balkan et al. [1]. However, the levels of T in the patients with azoospermia were significantly lower than those in the oligozoospermic and oligo-asteno-teratozoospermic patients.
Although the practical application of multiplex PCR remains many problems such as the specificity, sensitivity and throughput of the method, it is the most frequently used method for the detection of AZF microdeletions. In this study, nine STSs, including six STSs strongly recommended by European Molecular Genetics Quality Network (EMQN) and European Academy of Andrology (EAA), were used in the detection. In the current study, SY254 and SY255 for the DAZ gene family were found to be hot points of microdeletion. This is in agreement with a previous study by Forestats et al. [25]. Simoni et al. have developed a minimum detection system (SY84 and SY86 for AZFa, SY127 and SY134 for AZFb, SY254 and SY255 for AZFc) which has been considered as a relatively reliable method to identify 90% of AZF microdeletions and been widely applied by many laboratories [14, 26].
It is remarkable that more and more infertile men are beginning to choose the ART, such as ICSI/IVF. Nevertheless, AZF microdeletions and chromosomal abnormalities have potential chances to be transmitted readily from infertile fathers to their offspring by ICSI. The frequency of chromosomal abnormalities transmitted from infertile fathers to their children is in the range of 9.4–33% [27, 28]. Thus, it is essential for infertile male patients to undertake genetic counseling and reliable screening for genetic defects before ISCI/IVF.
In summary, the frequencies of AZF microdeletions and chromosomal abnormalities in infertile men from Northeastern China were comparable with infertile men from other countries and regions in the world. However, there was a slightly higher prevalence rate of AZF microdeletion in oligozoospermic patients than reported in previous studies. Therefore, in order to get a better treatment plan, it is essential for infertile men from Northeastern China to undertake genetic counseling and reliable screening for AZF microdeletions and chromosomal abnormalities.
Acknowledgements
We thank all the patients and donors of DNA samples. We are grateful to all staff of the Andrology Laboratory for their excellent work. We also thank Professor Frederick William Orr for his English-language assistance and critical review. This work was kindly supported by the funds of the Office of Science and Technology project in Jilin Province (NO. 200705371).
Footnotes
Capsule It is essential for infertile men from Northeastern China to take genetic counseling and reliable screening for AZF microdeletions and chromosomal abnormalities.
References
- 1.Balkan M, Tekes S, Gedik A. Cytogenetic and Y chromosome microdeletion screening studies in infertile males with Oligozoospermia and Azoospermia in Southeast Turkey. J Assist Reprod Genet. 2008;25:559–65. doi: 10.1007/s10815-008-9272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu YJ, Liu SY, Wang H, Wei P, Ding XP. The prevalence of azoospermia factor microdeletion on the Y chromosome of Chinese infertile men detected by multi-analyte suspension array technology. Asian J Androl. 2008;10(6):873–81. doi: 10.1111/j.1745-7262.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Towards more objectivity in diagnosis and management of male fertility. Int J Androl. 1987;7(Suppl):1–53. [Google Scholar]
- 4.Tiepolo L, Zuffardi O. Location of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34:119–24. doi: 10.1007/BF00278879. [DOI] [PubMed] [Google Scholar]
- 5.Fu JJ, Li LY, Lu GX. Relationship between microd eletion on Y chromosome and patients with idiopathic azoospermia and severe oligozoospermia in the Chinese. Chin Med J. 2002;115(1):72–5. [PubMed] [Google Scholar]
- 6.Zhou CA, Yang Y, Zhang SZ, Zhang W, Lin L. Chromosomal abnormality and Y chromosome microdeletion in Chinese patients with Azoospermia or severe Oligozoospermia. Yi Chuan Xue Bao. 2006;33(2):111–6. doi: 10.1016/s0379-4172(06)60029-2. [DOI] [PubMed] [Google Scholar]
- 7.Patricia B, Georgina RB, Emma JP, Ghada AO, Evelyne H. Dynamic nature of the proximal AZFc region of the human Y chromosome: multiplex independent deletion and duplication events revealed by microsatellite analysis. Hum Mutat. 2008;29(10):1171–80. doi: 10.1002/humu.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SB FU. Medical genetics. In: Bu XB, editor. Chromosomal disease. 2. Beijing: Peking University Medical Press; 2009. pp. 63–88. [Google Scholar]
- 9.Devroey P, Fauser BC, Diedrich K. Approaches to improve the diagnosis and management of infertility. Hum Reprod Update. 2009;15(4):391–408. doi: 10.1093/humupd/dmp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong D, Liu YL, Zheng Z, Tian YF, Li Z. An overview on ethical issues about sperm donation. Asian J Androl. 2009;11:645–52. doi: 10.1038/aja.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 12.Francavilla F, Sciarretta F, Sorgentone S, Necozione S, Santucci R, Barbonetti A, et al. Intrauterine insemination with or without mild ovarian stimulation in couples with male subfertility due to oligo/astheno- and/or teratozoospermia or antisperm antibodies: a prospective cross-over trial. Fertil Steril. 2009;92(3):1009–11. doi: 10.1016/j.fertnstert.2009.01.112. [DOI] [PubMed] [Google Scholar]
- 13.Dada R, Gupta NP, Kucheria K, et al. Molecular screening for Yq microdeletion in men with idiopathic oligozoospermia and azoospermia. J Biosci. 2003;28(2):163–8. doi: 10.1007/BF02706215. [DOI] [PubMed] [Google Scholar]
- 14.Simoni M, Bakker E, Eurlings MC, et al. Laboratory guidelines for molecular diagnosis of Y-chromosomal microdeletions. Int J Androl. 1999;22(5):292–9. doi: 10.1046/j.1365-2605.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitelman F, editor. ISCN (1995). An international system for human cytogenetic nomenclature. Basel: Karger; 1995.
- 16.SaoPedro SL, Fraietta R, Spaine D, Porto CS, Srougi M, Cedenho AP, et al. Prevalence of Y chromosome deletions in a Brazilian population of nonobstructive azoospermic and severily oligozoospermic men. Braz J Med Biol Res. 2003;36:787–93. doi: 10.1590/S0100-879X2003000600015. [DOI] [PubMed] [Google Scholar]
- 17.Osterlund C, Segersteen E, Arver S, Pousette A. Low number of Y-chromosome deletions in infertile azoospermic men at a Swedish andrology center. Int J Androl. 2000;23(4):225–9. doi: 10.1046/j.1365-2605.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 18.Vutyavanich T, Piromlertamorn W, Sirirungsi W, Sirisukkasem S. Frequency of Y chromosome microdeletions and chromosomal abnormalities in infertile Thai men with oligozoospermia and azoospermia. Asian J Androl. 2007;9:68–75. doi: 10.1111/j.1745-7262.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 19.Sargin CF, Berker-Karauzum S, Manguoglu E, Erdogru T, Karaveli S, Gulkesen KH, et al. AZF microdeletions on the Y chromosome of infertile men from Turkey. Ann Génét. 2004;47:61–8. doi: 10.1016/j.anngen.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Tse JY, Yeung WS, Ng EH, Cheng LN, Zhu HB, Teng XM, et al. A comparative study of Y chromosome microdeletions in infertile males from two Chinese populations. J Assist Reprod Genet. 2002;19:376–83. doi: 10.1023/A:1016346421177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Lien Y, Ko T, Ho H, Yang Y, Chang H. Genetic screening of karyotypes and azoospermic factors for infertile men who are candidates for ICSI. Arch Androl. 2003;49:423–7. doi: 10.1080/0145010390219700. [DOI] [PubMed] [Google Scholar]
- 22.Aho M, Harkonen K, Suikkari A, Juvonen V, Anttila L, Lahdetie J. Y-chromosomal microdeletions among infertile Finnish men. Acta Obstet Gynecol Scand. 2001;80:652–6. doi: 10.1080/j.1600-0412.2001.800711.x. [DOI] [PubMed] [Google Scholar]
- 23.Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996;11:1–24. doi: 10.1093/humrep/11.suppl_4.1. [DOI] [PubMed] [Google Scholar]
- 24.Vicdan A, Vicdan K, Gunalp S, Kence A, Akarsu C, Isik AZ, et al. Genetic aspects of human male infertility: the frequency of chromosomal abnormalities and Y chromosome microdeletions in severe male factor infertility. Eur J Obstet Gynecol Reprod Biol. 2004;117:49–54. doi: 10.1016/j.ejogrb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Forestats C, Moro E, Garolla A, Onisto M, Ferlin A. Y chromosome microdeletions in cryptorchidism and idiopathic infertility. J Clin Endocrinol Metab. 1999;84:3660–5. doi: 10.1210/jc.84.10.3660. [DOI] [PubMed] [Google Scholar]
- 26.Aknin-Seifer IE, Touraine RL. A simple low cost and non-invasive method for screening Y-chromosome microdeletions in infertile men. Hum Reprod. 2003;18:257–61. doi: 10.1093/humrep/deg067. [DOI] [PubMed] [Google Scholar]
- 27.Kent-First MG, Kol S, Muallem A, et al. The incidence and possible relevance of Y-linked microdeletions in babiesborn after intracytoplasmic sperm injection and their infertile fathers. Mol Hum Reprod. 1996;2:943–50. doi: 10.1093/molehr/2.12.943. [DOI] [PubMed] [Google Scholar]
- 28.Liebaers I, Bonduelle M, Assche E, Devroey P, Steirteghem A. Sex chromosome abnormalities after intracytoplasmic sperm injection. Lancet. 1995;346:1095. doi: 10.1016/S0140-6736(95)91768-3. [DOI] [PubMed] [Google Scholar]