Abstract
Purpose
To assess the potential effects of short-term exposure to particulate air pollution during follicular phase on clinical, laboratory, and pregnancy outcomes of women undergoing IVF/ET.
Methods
Retrospective cohort study of 400 first IVF/ET cycles of women exposed to ambient particulate matter during follicular phase. Particulate matter (PM) was categorized into quartiles (Q1: ≤30.48 µg/m3, Q2: 30.49–42.00 µg/m3, Q3: 42.01–56.72 µg/m3, and Q4: >56.72 µg/m3).
Results
Clinical, laboratory, or treatment variables were not affected by follicular phase PM exposure periods. Women exposed to Q4 period during the follicular phase of conception cycles had a higher risk of miscarriage (odds ratio, 5.05; 95% confidence interval: 1.04–25.51) when compared to women exposed to Q1–3 periods.
Conclusion
Our results show an association between brief exposure to high levels of ambient PM during the preconceptional period and early pregnancy loss, although no effect of this exposure on clinical, laboratory, and treatment outcomes was observed.
Keywords: In vitro fertilization, Particulate air pollution, Embryo development, Implantation, Miscarriage
Introduction
In humans the success or failure of the reproductive process is a delicate balance of numerous factors such as age, behavior, heredity, hormonal and nutritional status, patency of the reproductive tract, gamete reserve and production, embryo development and quality, and uterine receptivity acting in combination. The preimplantation period of development represents a critical time during which the embryo is highly susceptible to exogenous insults that can affect future growth and developmental potential, either pre- and/or postnatally [1]. Abnormalities in developmental potential may arise from the unavoidable maternal exposure to environmental toxic agents during the periconceptional period affecting not only reproductive but also pregnancy outcome and/or postnatal life [2, 3].
In the São Paulo metropolitan area, home to approximately 18 million inhabitants, a fleet of 7.5 million diesel-powered vehicles is the major source of the particulate matter (PM) found in ambient air. The automotive emissions are responsible for 98% of carbon monoxide (CO), 97% of polyaromatic hydrocarbons (PAHs), 96% of nitrogen oxides (NOx), 67% of sulfur oxides (SOx), and 40% of the inhaled PM present in the atmosphere of the city [4, 5]. Throughout the day, people living in metropolitan areas are intermittently subject to diesel exhaust particles (DEP), a major contributor to PM pollution. DEP consists of carbon cores with a high surface-to-volume ratio that absorb trace amounts of heavy metals such as iron, copper, chromium, and nickel and a large number of organic compounds including PAHs, nitroaromatic hydrocarbons, heterocyclics, quinones, aldehydes, and aliphatic hydrocarbons [6, 7]. The total mass of DEP is composed of 40% inorganic carbonaceous substance (elemental carbon, EC), 30% organic carbonaceous substances derived from unburned fuel and oil, and 30% sulfate, water, ash and others. Size distribution of DEP ranges from coarse particles (with aerodynamic diameter 2.5–10 µm) to ultrafine particles (with aerodynamic diameter <0.1 µm), which are dominant in the number-size distribution in contrast to the larger particles that dominate the mass-size distribution [8].
Over the past few years an increasing amount of evidence derived from studies focused particularly on male fertility and pregnancy outcome showed the negative effects of environmental contaminants derived from traffic emissions, including ozone (O3), CO, SOx, NOx, benzene, formaldehyde, PAHs, and suspended PM present in urban air on human reproductive health [9–14]. In contrast, few studies were able to demonstrate an association between air pollution and fertility impairment in women, probably due to the multiplicity of factors involved in female reproductive function [15–17]. Additionally, little is known regarding the impact of preconceptional exposure to metro area ambient air on early embryo development [16].
The advent of in vitro fertilization and embryo culture to clinical practice opened an important window to the observation of unique reproductive and developmental events of the preimplantation embryo that occur immediately before implantation. The possibility to closely monitor distinct aspects of gamete interaction and early embryo development in regard to cell division, morphology and quality (regularity, symmetry, fragmentation, multinucleation) turned both animal and human in vitro fertilization and embryo transfer (IVF/ET) into a relevant model for evaluating the preimplantation effects of acute or chronic exposure to ambient air pollution on reproduction. The main environmental contaminants derived from traffic emissions may alter fertility through direct and/or indirect effects on different pathways in cellular processes including mitotic interference, altered cell signaling, altered energy sources, enzyme inhibition, mutation, alterations in gene expression, alterations in DNA and RNA synthesis and functioning, and programmed cell death [18] or by disrupting the endocrine and/or immune systems [19, 20].
Previous experimental studies conducted in our laboratory have provided evidence that both short- and long-term exposure to particulate air pollution has a significant impact on female reproductive function affecting pre- and post-implantation embryonic development in mice. Acute preconception exposure to diesel exhaust particles and chronic exposure to fine PM (diameter ≤2.5 µm, PM2.5) present in ambient air were implicated in the disruption of the segregation pattern of the inner cell mass and trophectoderm cell lineages at the blastocyst stage [21, 22], an important marker of embryo viability and developmental potential [23]. Defective post-implantation embryonic development resulted in an increased number of implantation failures, decreased number of viable fetuses, and higher rates of miscarriage [14, 16]. Recently, a retrospective epidemiological study confirmed the increased risk of early pregnancy loss previously observed in experimental studies in women exposed to particulate air pollution. In their study, Perin et al. [24] provided evidence for an association between brief exposure to high levels of ambient particulate matter during the preconceptional period and early pregnancy loss, regardless of the method of conception (natural or after IVF treatment), and showed a 2.6-fold increase in the risk of miscarriage.
Despite the ubiquitous and unavoidable nature of traffic-related particulate air pollution exposure in metropolitan areas where women who are trying to conceive (naturally or otherwise) are exposed to high concentrations of environmental toxic agents, no studies focused on the effects of this exposure on early embryo development have been reported so far. Therefore, this study was designed to assess the potential effects of short-term exposure to particulate air pollution during the follicular phase on in vitro early embryo development and on treatment and pregnancy outcomes of women undergoing their first IVF/ET cycle.
Materials and methods
Study population
Clinical and laboratory data from 400 patients consecutively undergoing their first ever IVF/ET cycle due to male factor infertility at our center between January 1, 1997 and December 31, 2006 were retrospectively collected from our IVF database. Male infertility was defined according to World Health Organization criteria [25]: oligozoospermia (<20 × 106 sperm/mL), asthenozoospermia (<50% of sperm with grades a/b motility), and/or teratozoospermia (<40% normal morphology). The ovarian response pattern of the study population was analyzed for each follicular phase PM exposure period. Thereafter, we excluded from this group patients with: 1) no ovarian response to gonadotrophins (n = 27); 2) no oocytes recovered at retrieval (n = 12); 3) fertilization failure (n = 5); or embryo cleavage failure (n = 8). Thus, the study cohort consisted of 348 patients who underwent embryo transfer 72 h after oocyte retrieval (day three), 87% of the original total. Before treatment all women underwent a standard protocol of infertility evaluation including basal follicle-stimulating hormone (FSH) and estradiol (E2) concentration measurements on cycle day three, and hysterosalpingography or hysterosonography. Inclusion criteria for women were: age ≤ 45 years, day three FSH < 15 mIU/mL and E2 < 60 pg/mL, and a normal uterine cavity. Patients included in this study had a negative personal and family history of venous thrombotic events, and congenital or karyotypic abnormalities. All patients had normal thyroid function and no previous history of occupational exposure to environmental toxins, chronic diseases, medications, and abuse of alcohol or drugs. The median age of the women was 35 (range, 19–45). The mean duration of infertility and body mass index (BMI) in the study population were 3.5 ± 2.6 years (range: 1–19) and 22.6 ± 3.4 kg/m2 (range: 16.6–40.0), respectively. All couples were non-smokers and lived in the São Paulo metropolitan area. The study was approved by our institutional review board and written consent was provided by all participants.
Ovarian stimulation and oocyte retrieval
All patients underwent standard long protocol down-regulation using a dose of 1 mg of a gonadotrophin-releasing hormone agonist (GnRH-a, Lupron; Abbott, São Paulo, São Paulo, Brazil), which was reduced once recombinant human FSH (rhFSH, Puregon; Organon, São Paulo, São Paulo, Brazil) and/or human menopausal gonadotrophin (hMG, Menogon; Ferring, São Paulo, São Paulo, Brazil) administration was initiated. The dose of gonadotrophin was individualized according to the patient’s age and response. Ovarian response was assessed by serial transvaginal sonography and serum E2 levels. Final oocyte maturation and ovulation were triggered with 10,000 international units (IU) intramuscular injection of human chorionic gonadotrophin (hCG, Choragon; Ferring) when lead follicles reached 18–20 mm in diameter. Peak E2 level was measured on the day of hCG administration. Transvaginal ultrasound-guided oocyte retrieval under sedation was scheduled 35 to 36 h after hCG administration.
Oocyte fertilization, embryo culture and transfer, and luteal phase support
Intracytoplasmic sperm injection (ICSI) and embryo culture were performed in all cycles according to procedures previously described [26, 27] based upon abnormal semen parameters (<5 × 106 sperm/mL total count, <30% progressive motility, and/or <30% normal morphology). ICSI was performed on day zero on all morphologically intact mature (metaphase II; MII) oocytes. Fertilization assessment was carried out 18–20 h (day one) after ICSI. Oocytes presenting two pronuclei (2PN) and two polar bodies were considered normally fertilized. All cleaving embryos were evaluated, on days two (42–45 h) and three (68–72 h) after ICSI, for: a) number, regularity and symmetry of blastomeres; b) fragmentation (score)—1: ≤10% of anucleated fragments, 2: 11–30% of anucleated fragments, 3: >30% of anucleated fragments; and c) presence (at least one blastomere exhibiting >1 nucleus) or absence of multinucleated blastomeres (MNB). The embryo quality score was evaluated as one of five grades according to the criteria described by Veeck in which the lowest score (grade 1) represents the best-quality embryo [28].
Embryos were classified as being of high quality when they had four to six symmetrical blastomeres with ≤10% fragmentation and no multinucleation on day two, and six to eight symmetrical blastomeres with ≤10% fragmentation and no multinucleation on day three. Immediately before transfer, the selected embryos were scored according to quality and fragmentation and the embryo development rate (the sum of the number of blastomeres of each embryo transferred divided by the number of embryos transferred per patient) was calculated. Up to four embryos were transferred into the uterine cavity under transabdominal ultrasound guidance 72 h after oocyte retrieval. Luteal phase support was initiated on the day of oocyte retrieval in the form of intravaginal natural micronized progesterone (Utrogestan, Besins Iscovesco Laboratoires; Paris, France) at a dose of 800 mg daily and maintained at this dosage level until a serum pregnancy test was done two weeks after embryo transfer. If it was positive (βhCG > 25 mIU/mL), transvaginal sonography was performed three weeks later to confirm the clinical pregnancy, evidenced by fetal heart activity, and to establish the number of gestational sacs. Micronized progesterone supplementation was provided for another 6 weeks to those who achieved pregnancy.
Outcome measures
Clinical outcome measurements used were the duration of ovarian stimulation (in days), amount of gonadotrophins used per treatment cycle (total dose in IU), endometrial thickness (in mm) measured by transvaginal ultrasound and peak E2 level (in pg/mL) both determined on the day of hCG administration, and the total number of follicles aspirated on the day of oocyte retrieval (day zero). Laboratory outcome measurements used included the number of oocytes retrieved per patient, the rate of oocytes retrieved on day zero (total number of oocytes retrieved/total number of follicles aspirated), the rate of MII oocytes on day zero (MII oocytes/total number of oocytes retrieved), the fertilization rate on day one (MII oocytes showing 2PN/total number of MII oocytes injected), day two embryo cleavage rate (cleaved embryos on day two/number of zygotes on day one), day two high quality embryo rate (high quality embryos/number of embryos on day two), day three embryo cleavage rate (cleaved embryos on day three/number of zygotes on day one), and day three high quality embryo rate (high quality embryos/number of embryos on day three). The embryo transfer and treatment outcome methods of measurement included the number, embryo development rate, grade of embryos transferred, pregnancy rate, number of implanted embryos, and implantation rate (number of fetal hearts in activity/number of embryos transferred). Biochemical pregnancy was defined as a pregnancy with a positive pregnancy test that became negative prior to sonographic detection of an embryonic sac after 5 weeks of pregnancy. Early clinical pregnancy loss was defined as a miscarriage up to the 12th week of gestation after sonographic detection of fetal heart activity. Therefore, early pregnancy loss was defined as a pregnancy diagnosed with a positive serum βhCG that failed to reach the 12th week of gestation and included both biochemical and clinical pregnancies [29]. Pregnancy outcome measurements were early pregnancy loss, term pregnancy (delivery ≥37 weeks of gestation) and live birth rate. No ectopic pregnancies, second trimester pregnancy losses, or preterm pregnancies were observed in this study.
Ambient air pollution monitoring and study design
Daily records of PM of less than 10 µm in aerodynamic diameter (PM10) concentrations were provided by the São Paulo State Environmental Protection Agency (CETESB) between January 1997 and December 2006 [30]. Exposure measurements during the study period were taken from 14 monitoring stations covering nearly all areas of the city from 1:00 A.M. to 12:00 P.M. For each station, the 24-h average was adopted and the arithmetical average of PM10 across all monitoring stations was considered representative of the citywide exposure status. Due to the high mobility of patients throughout the city during the day, the arithmetical average of PM10 across all monitoring stations rather than the 24-h average from individual monitors close to the patients’ homes was considered representative of the exposure status.
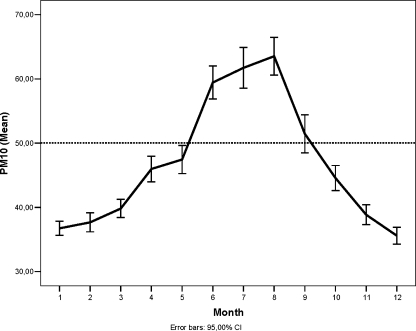
We calculated monthly average concentrations for PM10 throughout the study period (1997–2006) and plotted all available data against time (Fig. 1). The 24-h arithmetical average of PM10 across all monitoring stations was categorized into quartiles (Q1: ≤30.48 µg/m3, Q2: 30.49–42.00 µg/m3, Q3: 42.01–56.72 µg/m3, and Q4: >56.72 µg/m3). The arbitrary PM10 value of 50 µg/m3 was selected based on the Brazilian environmental regulation law (Conselho Nacional do Meio Ambiente [CONAMA] no. 3, 1990) that establishes this value as the maximum annual average of PM10 that should be pursued. The short-term 24-h PM10 average concentration for the 14 days following each patient’s last menstrual period (LMP) was determined as the average follicular phase exposure to represent the exposure of interest. Patients undergoing IVF/ET had the pattern of their ovarian response to superovulation identified and grouped through the K-means cluster analysis method performed on the number of oocytes retrieved and inseminated as poor (centroids: 5.2 ± 2.6 and 3.8 ± 1.9, respectively), normal (centroids: 12.2 ± 2.7 and 9.4 ± 2.3, respectively), or high (centroids: 22.5 ± 4.3 and 17.1 ± 3.9, respectively) responders. Thus, the effects of follicular phase exposure to PM10 levels during Q1 to Q4 periods on in vitro fertilization, embryo development, treatment, and pregnancy outcomes were evaluated.
Fig. 1.
Monthly average and 95% confidence intervals of PM10 concentrations (μg/m3) from 1997 to 2006 in the city of São Paulo, São Paulo, Brazil. The dotted line represents CETESB’s healthbased air quality standard annual mean
Statistical analysis
Correlations among the 24-h average concentrations of PM10 from all monitoring stations were determined with the use of Spearman’s rank-correlation coefficient, adjusted by Bonferroni correction. Descriptive summaries of clinical and laboratory outcomes were expressed as means±standard deviations (SD). PM10 was treated as a categorical variable for the analysis of clinical, laboratory, and treatment outcome dependent variables and as both a continuous and a categorical variable for the pregnancy outcome analysis. PM10 was categorized into quartiles and exposure in the lower quartile was used as the reference category.
Categorical data regarding the four different patterns of ovarian response to superovulation (absent, poor, normal, and high) in each follicular phase PM10 exposure period were evaluated using a chi-square test. Since the outcome of IVF treatment is highly dependent on ovarian response, it was treated as an independent variable. The effects of the ovarian response and follicular phase PM10 exposure period as well as the interactions between them on each dependent variable were evaluated through multivariate analysis of variance (MANOVA). In order to rule out the year of IVF treatment and age as potential sources of bias in the study, they were entered as covariates. Multivariate logistic regression was used to evaluate the effect of ovarian response, follicular phase PM10 exposure period, the year of IVF treatment, and age on treatment outcome. For the pregnancy outcome analysis, multivariate logistic regression was used to evaluate the effect of follicular phase PM10 exposure period on pregnancy outcome using the year of IVF treatment and age as covariates. The effect of follicular phase PM10 exposure either as a continuous or a categorical variable was expressed as an odds ratio (OR) with 95% confidence intervals (CI) and associated p values.
The data were analyzed using the Statistics Package for Social Sciences version 13.0 (SPSS Inc.; Chicago, IL).
Results
Ambient air monitoring
Figure 1 shows the monthly average concentrations of exposure to PM10 from 1997 to 2006 in the city of São Paulo, Brazil. The Q4 period coincided with the winter months (June through August) in Brazil in which average concentrations and confidence intervals for PM10 exceeded CETESB’s arbitrary health-based air quality standard of 50 µg/m3 annual mean. Table 1 shows that the 24-h average concentrations of PM10 from different monitoring stations were highly correlated suggesting that PM10 exposure is relatively uniform across the city of São Paulo.
Table 1.
Spearman’s correlation coefficient among 24-h average PM10 concentrations from different measurement stations throughout the São Paulo (Brazil) metropolitan area (P value, lower-left; correlation coefficient, upper-right)
| Station | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | 0.85 | 0.85 | 0.77 | 0,91 | 0.41 | 0.88 | 0.76 | 0.22 | 0.83 | 0.88 | 0.84 | 0.89 | 0.60 |
| 2 | .000 | – | 0.56 | 0.71 | 0.86 | 0.34 | 0.82 | 0.65 | 0.18 | 0.77 | 0.84 | 0.84 | 0.79 | 0.58 |
| 3 | .000 | .000 | – | 0.46 | 0.84 | 0.64 | 0.72 | 0.61 | 0.20 | 0.67 | 0.75 | 0.70 | 0.66 | 0.75 |
| 4 | .000 | .000 | .003 | – | 0.68 | 0.41 | 0.71 | 0.73 | 0.00 | 0.58 | 0.73 | 0.68 | 0.72 | 0.29 |
| 5 | .000 | .000 | .000 | .000 | – | 0.56 | 0.92 | 0.87 | 0.21 | 0.87 | 0.93 | 0.93 | 0.90 | 0.60 |
| 6 | .000 | .000 | .000 | .000 | .000 | – | 0.70 | 0.70 | 0.22 | 0.54 | 0.70 | 0.57 | 0.53 | 0.47 |
| 7 | .000 | .000 | .000 | .000 | .000 | .000 | – | 0.85 | 0.27 | 0.88 | 0.95 | 0.90 | 0.91 | 0.68 |
| 8 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | – | 0.36 | 0.75 | 0.87 | 0.75 | 0.89 | 0.59 |
| 9 | .000 | .002 | .016 | .954 | .000 | .006 | .000 | .000 | – | 0.19 | 0.27 | 0.25 | 0.30 | 0.35 |
| 10 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .005 | – | 0.89 | 0.87 | 0.85 | 0.99 |
| 11 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | – | 0.94 | 0.91 | 0.76 |
| 12 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | – | 0.84 | 0.67 |
| 13 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | .000 | – | 0.59 |
| 14 | .000 | .000 | .000 | .048 | .000 | .001 | .000 | .000 | .015 | .000 | .000 | .000 | .000 | – |
Clinical and laboratory outcome
Ovarian response to superovulation was absent in 8.9%, 8.4%, 1.8%, and 9.2% of the patients exposed to Q1, Q2, Q3, and Q4 follicular phase PM10 exposure periods, respectively. The number of patients presenting absent, low, moderate or high response to superovulation in the four exposure periods was as follows: in Q1 period, 4, 19, 12, and 10; in Q2 period, 13, 66, 48, and 16; in Q3 period, 2, 46, 43, and 17; and in Q4 period, 8, 33, 26, and 12, respectively, showing that the distribution of the four different patterns of ovarian response was not affected by the follicular phase PM10 exposure period (X2 = 10.950; P = 0.279).
Means (±SD) for all clinical, in vitro embryonic development, and embryo transfer variables according to ovarian response pattern for each follicular phase PM10 exposure period are reported in Table 2.
Table 2.
Effects of follicular phase exposure to particulate matter (PM10) levels during Q1 to Q4 periods on clinical and laboratory parameters, by ovarian response in patients undergoing IVF/ET. For explanation of the embryo development rate (EDR), see text
| Variable | Q1 period (PM10 ≤ 30.48 µg/m3) | Q2 period (PM10: 30.49–42.00 µg/m3) | Q3 period (PM10: 42.01–56.72 µg/m3) | Q4 period (PM10 > 56.72 µg/m3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poor response (N = 19) | Normal response (N = 12) | High response (N = 10) | Poor response (N = 66) | Normal response (N = 48) | High response (N = 16) | Poor response (N = 46) | Normal response (N = 43) | High response (N = 17) | Poor response (N = 33) | Normal response (N = 26) | High response (N = 12) | |
| Clinical variables | ||||||||||||
| Stimulation (days) | 9.2 ± 1.5 | 9.3 ± 1.2 | 10.0 ± 1.2 | 9.9 ± 1.8 | 9.9 ± 1.5 | 9.4 ± 0.8 | 10.1 ± 1.7 | 9.9 ± 1.5 | 10.1 ± 1.1 | 10.0 ± 1.8 | 10.9 ± 2.2 | 10.0 ± 1.1 |
| Gonadotrophins (IU) | 3,168 ± 886 | 2,492 ± 935 | 2,882 ± 973 | 2,946 ± 1,046 | 2,773 ± 1,067 | 2,292 ± 405 | 2,996 ± 1,064 | 2,401 ± 820 | 2,678 ± 690 | 2,864 ± 1,405 | 3,033 ± 1,253 | 2,518 ± 813 |
| Endometrium (mm) | 9.1 ± 1.5 | 9.9 ± 2.3 | 10.2 ± 2.0 | 9.5 ± 2.0 | 10.8 ± 1.8 | 10.7 ± 1.6 | 9.5 ± 2.0 | 11.6 ± 2.3 | 11.6 ± 2.8 | 9.6 ± 2.1 | 10.3 ± 1.9 | 10.7 ± 2.0 |
| Peak E2 (pg/mL) | 831 ± 330 | 2,065 ± 726 | 3,476 ± 2,281 | 1,183 ± 589 | 2,124 ± 794 | 3,353 ± 2,416 | 974 ± 532 | 1,923 ± 790 | 3,105 ± 1,544 | 1,229 ± 749 | 2,004 ± 1,141 | 3,080 ± 447 |
| Laboratory variables | ||||||||||||
| Day 0 | ||||||||||||
| Follicles aspirated | 7.4 ± 2.9 | 19.9 ± 8.0 | 27.9 ± 7.1 | 10.8 ± 6.7 | 18.8 ± 5.8 | 35.6 ± 11.6 | 8.7 ± 4.6 | 17.3 ± 5.4 | 29.7 ± 7.3 | 10.6 ± 4.9 | 17.6 ± 4.8 | 29.3 ± 8.2 |
| Oocytes retrieved | 5.3 ± 1.7 | 13.2 ± 3.0 | 20.8 ± 2.0 | 6.8 ± 2.5 | 13.3 ± 3.4 | 25.2 ± 4.3 | 5.6 ± 2.2 | 12.7 ± 2.9 | 22.5 ± 4.2 | 6.1 ± 2.1 | 13.0 ± 2.5 | 22.3 ± 5.3 |
| Oocyte retrieval rate | 0.76 ± 0.19 | 0.72 ± 0.18 | 0.78 ± 0.15 | 0.72 ± 0.21 | 0.75 ± 0.18 | 0.75 ± 0.17 | 0.71 ± 0.22 | 0.77 ± 0.15 | 0.78 ± 0.10 | 0.65 ± 0.23 | 0.77 ± 0.14 | 0.77 ± 0.09 |
| MII oocytes rate | 0.71 ± 0.32 | 0.82 ± 0.19 | 0.79 ± 0.14 | 0.72 ± 0.24 | 0.72 ± 0.18 | 0.72 ± 0.21 | 0.85 ± 0.19 | 0.80 ± 0.15 | 0.76 ± 0.17 | 0.82 ± 0.20 | 0.79 ± 0.18 | 0.73 ± 0.18 |
| Day 1 | ||||||||||||
| Fertilization rate | 0.81 ± 0.22 | 0.77 ± 0.22 | 0.86 ± 0.09 | 0.83 ± 0.20 | 0.83 ± 0.15 | 0.77 ± 0.23 | 0.85 ± 0.19 | 0.84 ± 0.13 | 0.78 ± 0.22 | 0.92 ± 0.15 | 0.78 ± 0.23 | 0.84 ± 0.13 |
| Day 2 | ||||||||||||
| Cleavage rate | 0.87 ± 0.24 | 0.84 ± 0.14 | 0.88 ± 0.10 | 0.90 ± 0.17 | 0.90 ± 0.14 | 0.90 ± 0.11 | 0.93 ± 0.14 | 0.89 ± 0.17 | 0.92 ± 0.09 | 0.90 ± 0.17 | 0.89 ± 0.18 | 0.90 ± 0.16 |
| Good quality embryos rate | 0.70 ± 0.41 | 0.62 ± 0.20 | 0.66 ± 0.18 | 0.59 ± 0.36 | 0.68 ± 0.24 | 0.60 ± 0.28 | 0.68 ± 0.34 | 0.63 ± 0.27 | 0.57 ± 0.28 | 0.58 ± 0.28 | 0.60 ± 0.25 | 0.57 ± 0.23 |
| Day 3 | ||||||||||||
| Cleavage rate | 0.82 ± 0.21 | 0.73 ± 0.21 | 0.84 ± 0.10 | 0.89 ± 0.18 | 0.83 ± 0.20 | 0.82 ± 0.19 | 0.83 ± 0.24 | 0.84 ± 0.18 | 0.89 ± 0.14 | 0.86 ± 0.21 | 0.87 ± 0.20 | 0.87 ± 0.13 |
| Good quality embryos rate | 0.57 ± 0.40 | 0.48 ± 0.31 | 0.51 ± 0.12 | 0.46 ± 0.36 | 0.57 ± 0.25 | 0.46 ± 0.29 | 0.53 ± 0.38 | 0.53 ± 0.28 | 0.50 ± 0.23 | 0.52 ± 0.28 | 0.45 ± 0.29 | 0.58 ± 0.25 |
| Transfer variables | ||||||||||||
| Embryos transferred | 2.1 ± 1.2 | 2.8 ± 0.7 | 3.4 ± 1.0 | 2.5 ± 1.0 | 3.4 ± 1.3 | 2.8. ± 0.9 | 2.5 ± 1.5 | 3.6 ± 1.0 | 2.8 ± 0.8 | 2.9 ± 1.0 | 3.7 ± 0.9 | 3.7. ± 1.1 |
| EDR | 7.0 ± 1.7 | 7.0 ± 1.0 | 7.8 ± 0.4 | 6.6 ± 1.6 | 7.4 ± 1.0 | 7.3 ± 1.6 | 6.9 ± 1.6 | 7.4 ± 1.3 | 7.7 ± 0.5 | 7.0 ± 1.4 | 7.2 ± 1.1 | 7.9 ± 0.6 |
| Embryo quality score | 3.2 ± 1.2 | 2.9 ± 0.8 | 2.3 ± 0.8 | 2.5 ± 1.0 | 2.0 ± 0.8 | 2.1 ± 1.2 | 2.3 ± 1.0 | 2.2 ± 1.0 | 1.7 ± 0.7 | 2.3 ± 0.9 | 1.9 ± 0.7 | 2.1 ± 1.1 |
| Implanted embryos | 0.4 ± 0.6 | 0.6 ± 0.7 | 0.8 ± 0.6 | 0.7 ± 0.9 | 0.8 ± 0.9 | 0.6 ± 0.9 | 0.8 ± 1.0 | 1.0 ± 1.0 | 0.7 ± 0.9 | 0.6 ± 0.9 | 0.9 ± 1.0 | 1.1 ± 1.2 |
| Implantation rate | 0.25 ± 0.39 | 0.20 ± 0.23 | 0.28 ± 0.23 | 0.27 ± 0.35 | 0.27 ± 0.30 | 0.21 ± 0.27 | 0.29 ± 0.36 | 0.29 ± 0.27 | 0.24 ± 0.28 | 0.21 ± 0.29 | 0.23 ± 0.29 | 0.30 ± 0.32 |
Values are means±standard deviation
A multivariate effect for ovarian response on clinical, laboratory, and embryo transfer variables was found (Pillai’s trace = 0.54; F = 7.45; P = .000; power = 1.000). A significantly lower endometrial thickness and peak E2 level on the day of hCG administration, and oocyte retrieval rate were observed in poor responders. No significant differences were found for any laboratory variables in the univariate analysis. In regard to embryo transfer variables, the analysis showed significantly lower embryo grade and development rate, as well as number of embryos transferred for poor responders. However, no difference was found for the implantation rate when the patients in different groups of ovarian response were compared.
The multivariate analysis adjusting for the year of IVF treatment and patient’s age showed that clinical and laboratory outcomes were not affected by the follicular phase PM10 exposure period (Pillai’s trace = 0.15; F = 1.05; P = 0.381; power = 0.978). Additionally, no significant interaction between follicular phase PM10 exposure period and ovarian response (Pillai’s trace = 0.22; F = 0.76; P = 0.956; power = 0.990) was observed on clinical, laboratory, or embryo transfer variables.
IVF treatment outcome
Treatment outcome according to the follicular phase PM10 exposure period and ovarian response in women undergoing IVF treatment is shown in Table 3. The multivariate logistic regression test showed that clinical pregnancy rates per oocyte retrieval and per embryo transfer were not significantly influenced by the follicular phase PM10 exposure period (Wald Z-value = −0.422; P = 0.673; OR: 0.99; CI: 0.98–1.01), ovarian response (Wald Z-value = 0.824; P = 0.662; OR: 0.99; CI: 0.72–1.13) or the year of IVF treatment (Wald Z-value = 0.580; P = 0.562; OR: 1.03 ; CI: 0.92–1.15). However, age did significantly affect the IVF/ET treatment outcome as evidenced by the reduction in pregnancy rate with the increase of age (Wald Z-value = 3.239; P = .001; OR: 1.08; CI: 1.03–1.13).
Table 3.
Effects of follicular phase exposure to particulate matter (PM10) levels during Q1 to Q4 periods on IVF/ET treatment outcome by ovarian response
| Outcome | Q1 period (PM10 ≤ 30.48 µg/m3) | Q2 period (PM10: 30.49–42.00 µg/m3) | Q3 period (PM10: 42.01–56.72 µg/m3) | Q4 period (PM10 > 56.72 µg/m3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poor response | Normal response | High response | Poor response | Normal response | High response | Poor response | Normal response | High response | Poor response | Normal response | High response | |
| Clinical pregnancy/oocyte retrieval (%) | 7/19 (37%) | 6/12 (50%) | 7/10 (70%) | 30/73 (41%) | 25/51 (49%) | 7/17 (41%) | 23/52 (44%) | 27/43 (63%) | 8/17 (47%) | 15/41 (37%) | 14/26 (53%) | 8/12 (67%) |
| Clinical pregnancy/embryo transfer (%) | 7/19 (37%) | 6/12 (50%) | 7/10 (70%) | 30/66 (46%) | 25/48 (52%) | 7/16 (44%) | 23/46 (50%) | 27/43 (63%) | 8/17 (47%) | 15/33 (46%) | 14/26 (53%) | 8/12 (67%) |
Wald Z-value = 0.414; P = 0.520
Pregnancy outcome
The effect of follicular phase PM10 exposure period on pregnancy outcome in patients who conceived through IVF/ET was evaluated. A total of 189 (54.3%) women achieved pregnancy through IVF treatment and were included in the analysis. For the logistic regression model, an increase in the odds of early pregnancy loss with increasing follicular phase PM10 exposure was observed. The risk of early pregnancy loss increased 5% per unit increase in follicular phase PM10 average (Wald Z-value = 13.48; P = .000; OR: 1.05; CI: 1.02–1.07). However, increasing follicular phase exposure to PM10 had no effect on the live birth rate (Wald Z-value = 0.94; P = 0.332; OR: 1.02; CI: 0.98–1.06).
No significant modification in the risk of biochemical or clinical early pregnancy loss according to baseline exposure (first PM10 quartile) was seen for each interquartile range increase up to the third quartile. The risk of biochemical early pregnancy loss was not increased in the last quartile. Additionally, the multivariate logistic regression test showed that the risk of biochemical early pregnancy loss was not affected by the year of IVF treatment or by age. On the other hand, follicular phase exposure to PM10 average levels observed in the last quartile significantly increased the risk (Wald Z-value = 4.04; P = .045; OR: 5.05; CI: 1.04–24.51) for clinical early pregnancy loss in the expectant population. The multivariate logistic regression test showed that the risk of clinical early pregnancy loss was not influenced by the year of IVF treatment (Wald Z-value = 0.135; P = 0.714; OR: 1.04; CI: 0.84–1.29), but significantly affected by age (Wald Z-value = 12.20; P = .000; OR: 1.20; CI: 1.08–1.33). The probability of having a live birth was not significantly changed by the follicular phase PM10 exposure period. Live birth rates were: Q1 period—41.5%; Q2 period—41.5% (Wald Z-value = .002; P = 0.968; OR: 1.02; CI: 0.48–2.15); Q3 period—46.2% (Wald Z-value = 0.13; P = 0.721; OR: 0.87; CI: 0.39–1.91); and Q4 period—33.8% (Wald Z-value = 1.48; P = 0.224; OR: 1.71; CI: 0.72–4.09). The biochemical and clinical early pregnancy loss rates and the term pregnancy rates for each exposure period are shown in Table 4.
Table 4.
Pregnancy outcome by particulate matter (PM10) exposure period during the follicular phase after IVF conception
| Exposure Period | Early Pregnancy Loss | Term Pregnancy | |||||
|---|---|---|---|---|---|---|---|
| Biochemical Pregnancy | Clinical Pregnancy | ||||||
| N (%) | OR (95% CI) | P-value | N (%) | OR (95% CI) | P-value | N (%) | |
| Q1 (PM10 ≤ 30.48 µg/m3) | 1 (4.8) | – | – | 3 (14.3) | – | – | 17 (80.9) |
| Q2 (PM10: 30.49–42.00 µg/m3) | 1 (1.6) | 0.36 (.02–6.20) | 0.478 | 8 (12.7) | 0.99 (0.22–4.41) | 0.986 | 54 (85.7) |
| Q3 (PM10: 42.01–56.72 µg/m3) | 4 (6.5) | 1.70 (0.16–18.57) | 0.664 | 9 (14.5) | 1.43 (0.31–6.61) | 0.649 | 49 (79.0) |
| Q4 (PM10 > 56.72 µg/m3) | 6 (14.0) | 5.91 (0.53–65.78) | 0.149 | 13 (30.2) | 5.05 (1.04–24.51) | .045 | 24 (55.8) |
OR odds ratio
95% CI 95% confidence intervals
Discussion
In this study we examined the effect of preconceptional short-term exposure to PM10 in a real world situation on clinical, laboratory, treatment, and pregnancy outcomes of couples undergoing their first IVF/ET cycle due to male factor infertility. The results of this study provide evidence that for infertile women living in a large metropolitan area and undergoing IVF/ET treatment, short-term preconceptional exposure to higher concentrations of ambient PM10 does not affect clinical and laboratory outcomes or treatment outcome as reflected by similar pregnancy and implantation rates. However, we found evidence that the exposure to increasing levels of ambient PM10 during the follicular phase of the conception cycle after IVF/ET was associated with an increased risk of clinical early pregnancy loss. This effect was mainly observed after transient follicular phase exposure to ambient PM10 levels slightly above the air quality standard measured as a 24 h running average (50 µg/m3).
When comparing the clinical and laboratory data from women undergoing IVF/ET that were exposed to periods with distinct levels of ambient PM10 during the follicular phase of the treatment, we were unable to find a negative effect of the exposure either on IVF stimulation parameters (stimulation days, ampules of gonadotrophins used, endometrial thickness and peak E2 level on the day of hCG administration, and the total number of follicles aspirated on the day of oocyte retrieval) or on laboratory parameters (number of oocytes retrieved per patient, oocyte retrieval and MII oocyte rates, fertilization, day two embryo cleavage and high quality, and day three embryo cleavage and high quality). Additionally, response to controlled ovarian hyperstimulation, although individually affecting some of these parameters as expected, did not show any interactions (neither positive nor negative) with the exposure to increased levels of ambient PM10 in the IVF clinical and laboratory outcomes, suggesting that no specific subgroup of patients presents differential susceptibility to this exposure. These results suggest that the early embryonic developmental potential, specifically embryo cleavage rate and quality, was not affected by short-term exposure to ambient PM10 during the follicular phase of the treatment, as pointed out in a previous experimental study from our laboratory in which we followed the fertilization and embryonic development in vitro of mice exposed to DEP during ovarian stimulation. That study found that the number of zygotes that reached the blastocyst stage and the blastocyst hatching pattern, both parameters reflecting the embryonic developmental potential, were not affected by short-term exposure to native or acid-extracted DEP [21].
The analysis of embryo transfer variables in this study showed that there were no statistically significant differences between subgroups of patients in each ovarian response pattern exposed to periods with distinct levels of ambient PM10 during the follicular phase of the treatment in regard to the number of embryos transferred, embryo development rate, embryo quality score, and implantation rates. Although there was a significant decrease in the number of embryos transferred, embryo development rate, and embryo quality score (reflected by a higher score value) in poor responders when compared to normal or high responders exposed to periods with distinct levels of ambient PM10 that could be translated into a lower pregnancy potential, it had no impact on treatment outcome as evidenced by similar implantation rates in these subgroups of patients.
The main IVF/ET treatment outcomes measured, clinical pregnancy rate per oocyte retrieval and per embryo transfer, did not reveal a statistically significant difference between patients exposed to progressively increased levels of ambient PM10 during the preconceptional period. We found that no specific subgroup of patients in regard to ovarian response pattern was negatively affected by this exposure, although this observation is limited in some analyses due to the small number of cycles in specific subgroups. A similar observation was found in a previous experimental study that evaluated the effects of chronic exposure to air pollution in a large urban center on the fertility of Balb/c female mice showing that there was no significant difference in the pregnancy rates of mice exposed to clean or polluted air [16].
In the current study we found that early pregnancy loss rates (biochemical and clinical) among patients that conceived after IVF/ET treatment and were exposed to Q1, Q2 and Q3 periods during the follicular phase of their conception cycle were similar (19.0%, 14.3% and 20.9%, respectively) and in accordance with the rates of reported miscarriage (20–39%) that occur in patients undergoing IVF/ICSI [31, 32]. Conversely, the risk of early pregnancy loss in women exposed to high levels of ambient PM10 (Q4 period) during the preconceptional period was significantly higher than that found in the group of women exposed to lower levels of ambient PM10 (Q1–3 periods). However, when only considering the biochemical early pregnancy loss the logistic regression analysis revealed no difference among the four distinct ambient PM10 exposure periods, though a trend toward a higher biochemical pregnancy rate (14% versus 4.1%, Q4 and Q1–3 periods) was observed for women exposed to ambient PM10 found in the last quartile. We also identified a monotonic effect between short-term exposure to ambient PM10 during the preconceptional period and the incidence of early pregnancy loss. For each unit increase in follicular phase PM10 average there was a 5% increase in the risk of early pregnancy loss.
The exact reason for the increased risk of miscarriage observed in women exposed to high levels of ambient PM10 during the preconceptional period is not known. The disruption of the normal segregation pattern of the first two cell lineages, the inner cell mass (ICM) and trophectoderm (TE), at the blastocyst stage as well as the loss of ICM morphological integrity have been documented in an experimental model used by our group for the evaluation of the effects of short-term exposure to DEP on fertilization and embryo development in vitro [21]. The size of first two cell lineages (ICM and TE) as well as their ratio influence future growth, viability, and implantation potential of the blastocyst. A positive relationship between cell number and morphology of the ICM and the rate of embryo implantation and post-implantation developmental potential were evidenced in experimental and clinical studies [1, 23]. Based on our experimental data and on these findings we hypothesize that the lineage specification defect and the loss of ICM integrity may suggest one of the possible pathways through which increased risk of clinical early pregnancy loss is triggered in women exposed to high levels of ambient PM10. The distinction between biochemical and clinical early pregnancy loss in the present study showed that the majority (73%) of the losses occurred after the detection of fetal heart activity, strengthening the hypothesis that embryo viability rather than implantation potential was affected by preconceptional exposure to high levels of ambient PM10.
Alternatively, the increased risk of early pregnancy loss in women exposed to air pollution could be related to maternal changes in the vascular compartment or uterine environment prior to pregnancy as shown by a previous experimental study conducted in our laboratory. Veras et al. [33] showed that ambient levels of PM may impair reproductive health by affecting the maternal side of the placental interface between the mother and the fetus. Pre- and/or gestational exposure of female mice to non-filtered urban air led to reduced volumes, diameters, and surface areas of maternal blood spaces and significant increases in fetal capillary surface area and the total and mass-specific morphometric diffusive conductances for oxygen of the intervascular barrier. The reported changes suggest compromised delivery of maternal blood to the placenta and an increase in the resistance to its flow, impairing embryo development despite parallel attempts on the fetal side to improve transport by passive diffusion.
The current study benefited from the methodological and statistical approaches through which we controlled specific characteristics of the patient population that could bias the observed effects by association of exposure and outcome to confounding variables. Precise knowledge of the LMP of each patient significantly reduced exposure misclassification despite the retrospective nature of the analysis. The high quality of the treatment and outcome data was only possible because they were electronically recorded in a consistent manner during the study period, a sturdy feature of a private practice setting. To properly analyze the complex and interrelated nature of the IVF/ET treatment dependent variables, multiple logistic regression was selected as the statistical method to assess the effects of different ovarian response patterns to gonadotrophins, exposure, patient’s age, and the year of IVF treatment, as well as their interactions, thus reducing the possibility of an observed effect having been caused by chance (type I error).
We do recognize some limitations to our study. Reproductive health is a couple-dependent process and even though we tried to minimize the male factor effect on the IVF/ET treatment outcome by selecting patients in which fertilization was achieved only by ICSI, the sperm DNA damage that may result from the exposure to intermittent air pollution could be also linked to the increased rate of adverse pregnancy outcome [10]. Although this time-series study has some potential limitations, it is not thought to be confounded by other factors implicated in early pregnancy loss such as uterine anatomic abnormalities, thrombophilia, antiphospholipid syndrome, or autoreactive immune processes [34] because these factors were either previously excluded or would show no significant variation across the study period and are not associated with daily air pollution levels. Exposure assessment was limited since we used the ambient PM10 levels derived from a network average across a number of sites in the city, an approach that could introduce some bias by not considering geographic microclimate differences in exposure [35]. The consequence of using ambient PM10 concentrations instead of average personal exposure measure is to underestimate pollution’s effects. However, Zeger et al. [36] showed that differences between individual exposures on a given day and the risk-weighted average of personal exposures are not likely to cause substantial bias in coefficients from time-series studies. The selection of a single pollutant from a complex mixture of compounds present in air pollution to evaluate its effects on IVF/ET treatment and pregnancy outcome may represent another important source of bias since some of the reported effects may be attributed to other pollutants. However, based on the results of our previous studies showing the effects of ambient PM [22] and DEP [21] on fertilization and embryo development in vitro and on cell lineage segregation at the blastocyst stage and of ambient PM on pregnancy outcome in women [24], we focused our study on the evaluation of the possible effects of ambient PM10 on reproductive health. The lack of difference among the four distinct exposure periods in regard to biochemical early pregnancy loss may be due to the limited number of biochemical pregnancies found in this study. Finally, extrapolation of the results to the general population may be limited by our infertile population and the fact that the patients in this study lived in areas of higher socioeconomic status and had the same ethnic origin (Caucasian), two important covariates that may influence and interact with environmental conditions [37, 38]. Indoor sources produce particles of similar composition and toxicity as those found outdoors and may be a major component of error in air pollution studies. However, since our study is limited to the higher socioeconomic strata of the population, the lifestyle (nonsmoking couples) and a healthier household environment (cleaner energy source, better indoor air distribution) could rule out indoor air pollution as a major confounding factor in this study.
Conclusion
To our knowledge, this is the first study of its kind conducted to evaluate the possible effects of preconceptional short-term exposure to ambient PM10 on treatment outcome of infertile women undergoing IVF/ET who live in a large metropolitan area. The results presented here provide evidence for an association between brief exposure to high levels of ambient PM10 during the preconceptional period and early pregnancy loss and show a monotonic effect of ambient PM10 exposure on reproductive outcome. The increase in the risk of early pregnancy loss during winter time has potential public health implications and warrants stronger environmental policies aimed at reducing urban air pollution during this period. Rescheduling the IVF cycle to avoid the months of the year with highest ambient PM10 levels would be a wise approach in order to maximize pregnancy outcome.
Footnotes
Capsule Short-term exposure of women undergoing IVF/ET to particulate air pollution does not affect fertilization or embryo development in vitro but increases the risk of miscarriage.
References
- 1.Fleming T, Kwong W, Porter R, Ursell E, Fesenko I, Wilkins A, et al. The embryo and its future. Biol Reprod. 2004;71:1046–54. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- 2.Tingen C, Stanford JB, Dunson DB. Methodologic and statistical approaches to studying human fertility and environmental exposure. Environ Health Perspect. 2004;112:87–93. doi: 10.1289/ehp.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu G, Umezawa M, Takeda K. Early development origins of adult disease caused by malnutrition and environmental chemical substances. J Health Sci. 2009;55:11–9. doi: 10.1248/jhs.55.11. [DOI] [Google Scholar]
- 4.Onursal B, Gautam S. Air pollutants and their effects. In: Onursal B, Gautam S, editors. Vehicular air pollution experiences from seven Latin American Urban Centers. Washington: The World Bank; 1997. [Google Scholar]
- 5.Silva A, Cardoso M, Meliefste K, Brunekreef B. Use of passive diffusion sampling method for defining NO2 concentrations gradient in São Paulo, Brazil. Environ Health. 2006;5:19–27. doi: 10.1186/1476-069X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerholm R, Almen J, Li H, Rannug J, Egebaeck K, Graegg K. Chemical and biological characterization of particulate-, semivolatile-, and gas-phase-associated compounds in diluted heavy-duty diesel exhausts: a comparison of three different semivolatile-phase samplers. Environ Sci Technol. 1991;25:332–8. doi: 10.1021/es00014a018. [DOI] [Google Scholar]
- 7.Yoshino S, Hayashi H, Taneda S, Takano H, Sagai M, Mori Y. Effect of diesel exhaust particle extracts on induction of oral tolerance in mice. Toxicol Sci. 2002;66:293–7. doi: 10.1093/toxsci/66.2.293. [DOI] [PubMed] [Google Scholar]
- 8.Ono-Ogasawara M, Smith TJ. Diesel exhaust particles in the work environment and their analysis. Ind Health. 2004;42:389–99. doi: 10.2486/indhealth.42.389. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Ha E, Park H, Kim Y, Hong Y, Kim H, et al. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Hum Reprod. 2003;18:638–43. doi: 10.1093/humrep/deg102. [DOI] [PubMed] [Google Scholar]
- 10.Rubes J, Selevan S, Evenson D, Zudova D, Vozdova M, Zudova Z, et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod. 2005;20:2776–83. doi: 10.1093/humrep/dei122. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenfels A, Gomes J, Pieri P, Miraglia S, Hallak J, Saldiva P. Increased levels of air pollution and a decrease in the human and mouse male-to-female ratio in São Paulo, Brazil. Fertil Steril. 2007;87:230–2. doi: 10.1016/j.fertnstert.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Ritz B, Wilhelm M, Hoggatt K, Ghosh J. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007;166:1045–52. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- 13.Hansen C, Barnett A, Pritchard G. The effect of ambient air pollution during early pregnancy on fetal ultrasonic measurements during mid-pregnancy. Environ Health Perspect. 2008;116:362–9. doi: 10.1289/ehp.10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva I, Lichtenfels A, Pereira L, Saldiva P. Effects of ambient levels of air pollution generated by traffic on birth and placental weights in mice. Fertil Steril. 2008;90:1921–4. doi: 10.1016/j.fertnstert.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter D, Shen Y, Nguyen T, Le L, Lininger L. Incidence of endocrine disease among residents of New York areas of concern. Environ Health Perspect. 2001;109(Suppl 6):845–51. doi: 10.2307/3454646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohallem S, Lobo D, Pesquero C, Assunção J, Andre P, Saldiva P, et al. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ Res. 2005;98:196–202. doi: 10.1016/j.envres.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Tomei G, Ciarrocca M, Fortunato B, Capozzella A, Rosati M, Cerratti D, et al. Exposure to traffic pollutants and effects on 17-beta-estradiol (E2) in female workers. Int Arch Occup Environ Health. 2006;80:70–7. doi: 10.1007/s00420-006-0105-8. [DOI] [PubMed] [Google Scholar]
- 18.Faustman E, Gohlke J, Ponce R, Lewandowski T, Seeley M, Whittaker S, et al. Experimental approaches to evaluate mechanisms of developmental toxicity. In: Hood R, et al., editors. Developmental and reproductive toxicology a practical approach. Boca Raton: Taylor & Francis Group, LLC; 2006. pp. 15–60. [Google Scholar]
- 19.Hoyer P. Ovarian toxicity in small pre-antral follicles. In: Hoyer P, editor. Ovarian toxicology. Boca Raton: CRC Press; 2004. pp. 17–39. [Google Scholar]
- 20.Younglai EV, Holloway AC, Foster WG. Environmental and occupational factors affecting fertility and IVF success. Hum Reprod Updat. 2005;11:43–57. doi: 10.1093/humupd/dmh055. [DOI] [PubMed] [Google Scholar]
- 21.Perin P, Maluf M, Januário D, Saldiva P.Effects of short-term exposure of female mice to diesel exhaust particles on in vitro fertilization and embryo development Fertil Steril 200890Suppl 1S206. 10.1016/j.fertnstert.2008.07.48819007632 [DOI] [Google Scholar]
- 22.Maluf M, Perin P, Januário D, Saldiva P. In vitro fertilization, embryo development, and cell lineage segregation after pre- and/or postnatal exposure of female mice to ambient fine particulate matter. Fertil Steril. 2009;92:1725–35. doi: 10.1016/j.fertnstert.2008.08.081. [DOI] [PubMed] [Google Scholar]
- 23.Kovacic B, Vlaisavljevic V, Reljic M, Sajko M. Developmental capacity of different morphological types of day 5 human morulae and blastocysts. Reprod Biomed Online. 2004;8:687–94. doi: 10.1016/S1472-6483(10)61650-1. [DOI] [PubMed] [Google Scholar]
- 24.Perin P, Maluf M, Czeresnia C, Januário D, Saldiva P. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2010;93:301–3. doi: 10.1016/j.fertnstert.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 26.Palermo G, Schlegel P, Colombero L, Zaninovic N, Moy F, Rosenwaks Z. Aggressive sperm immobilization prior to intracytoplasmic sperm injection with immature spermatozoa improves fertilization and pregnancy rates. Hum Reprod. 1996;11:1023–9. doi: 10.1093/oxfordjournals.humrep.a019290. [DOI] [PubMed] [Google Scholar]
- 27.Perin P, Maluf M, Czeresnia C, Sousa P. The effect of recombinant human luteinizing hormone on oocyte/embryo quality and treatment outcome in down-regulated women undergoing in vitro fertilization. Einstein. 2005;3:96–105. [Google Scholar]
- 28.Veeck L. Preembryo grading and degree of cytoplasmic fragmentation. In: Veeck L, editor. An Atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: The Parthenon Publishing Group, Inc.; 1999. pp. 46–51. [Google Scholar]
- 29.Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Steirteghem AC, Devroey P. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Hum Reprod. 2006;21:352–7. doi: 10.1093/humrep/dei348. [DOI] [PubMed] [Google Scholar]
- 30.CETESB. Companhia de Tecnologia de Saneamento Ambiental (São Paulo State Environmental Protection Agency). Poluentes do ar. São Paulo: Secretaria de Estado do Meio Ambiente do Governo do Estado de São Paulo, 2006. Available at: http://wwwcetesbspgovbr/Ar/ar_saudeasp 2006.
- 31.Poikkeus P, Hiilesmaa V, Tiitinen A. Serum hCG 12 days after embryo transfer in predicting pregnancy outcome. Hum Reprod. 2002;17:1901–5. doi: 10.1093/humrep/17.7.1901. [DOI] [PubMed] [Google Scholar]
- 32.Neubourg D, Gerris J, Mangelschots K, Royen E, Vercruyssen M, Elseviers M. Single top quality embryo transfer as a model for prediction of early pregnancy outcome. Hum Reprod. 2004;19:1476–9. doi: 10.1093/humrep/deh283. [DOI] [PubMed] [Google Scholar]
- 33.Veras M, Damaceno-Rodrigues N, Caldini E, Mayhew T, Saldiva P, Dolhnikoff M. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol Reprod. 2008;79:578–84. doi: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- 34.Shetty S, Ghosh K. Anti-phospholipid antibodies and other immunological causes of recurrent foetal loss—a review of literature of various therapeutic protocols. Am J Reprod Immunol. 2009;62:9–24. doi: 10.1111/j.1600-0897.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 35.Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102:182–90. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–26. doi: 10.2307/3454382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morello-Frosch R, Pastor M, Porras C, Sadd J. Environmental justice and regional inequality in Southern California: implications for future research. Environ Health Perspect. 2002;110(suppl 2):149–54. doi: 10.1289/ehp.02110s2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briggs D. Environmental pollution and the global burden of disease. Br Med Bull. 2003;68:1–24. doi: 10.1093/bmb/ldg019. [DOI] [PubMed] [Google Scholar]