Abstract
Introduction
According french legislation, sperm freezing/thawing procedures are used to prevent ART contaminations in couple with HIV-1 infected men. We determined sperm nuclear fragmentation rate before and after selection and freezing/thawing in HIV-1 14 patients.
Methods
Two groups of patients were studied: 20 control patients with normal sperm (group 1) and without viral infection and 20 fertile treated HIV-1 patients (group 2). DNA fragmentation was evaluated using terminal uridine nick end labeling, before and after gradient selection, and after cryopreservation and thawing procedures.
Results
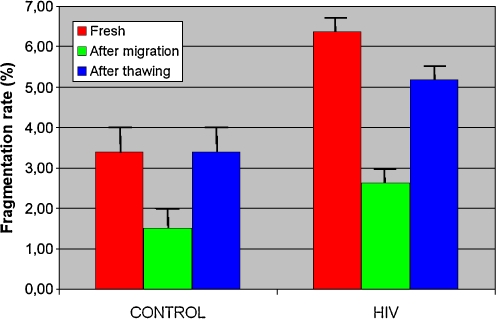
DNA fragmentation rates in fresh semen were increased in HIV patients (6.38% vs 3.39%) (p < 0.05) compared with control patients. After sperm migration, fragmentation rates were significantly lower (p < 0.0001) in the two groups compared with fresh sperm rates. After freezing/thawing, values were similar to those of fresh semen with an increased rate (p < 0.01) for HIV-1 patients, with respectively 3.40% and 5.18% rates in control and infected patients. HIV-1-infected patients treated by antiretroviral therapy showed a significant increase in sperm DNA fragmentation in fresh sperm and also after freezing/thawing procedures, but these two fragmentation rates were not significantly different.
Conclusion
So, freezing/thawing procedures do not seem to impair sperm DNA and preserve probability of conception for couples with HIV-1 infected men.
Keywords: HIV-1, Freezing, Thawing, DNA fragmentation, Sperm motility
Introduction
According to French legislation, sperm contaminated with human immunodeficiency virus type 1 (HIV-1) must be frozen after migration on a density gradient, in order to check the absence of HIV-1 viral RNA, in either seminal plasma or sperm fraction or these combined, by reverse-transcriptase polymerase chain reaction (RT-PCR) [1–3]. In most reports, density gradient centrifugation has been shown to considerably reduce viral load in the migrated sperm fraction of HIV-1-infected patients [4–8]. This method was proposed to prevent infection of spouse and fetus.
After thawing, impairment of sperm progressive motility and vitality [8, 9] is observed and this phenomenon is accentuated in oligozoospermia [9, 10] whether sperm is selected beforehand by migration on a density gradient or by swim-up. Sperm cryopreservation seems to impair sperm DNA integrity, particularly when sperm parameters are altered [9, 11–14]. Furthermore, patients infected with chronic viral diseases like HIV-1 can be exposed to reactive oxygen species, as released in all patients suffering inflammatory reactions [15]. These patients might have reduced defenses against oxidative stress [15–20]. On the other hand, a pre-existing reduction of progressive motility was evident in sperm of patients treated by reverse transcriptase nucleoside inhibitors [21, 22]. This deterioration could be linked to drugs which damage mitochondrial DNA of sperm tails [22]. Moreover, antiretroviral treatments, in particular reverse transcriptase nucleoside inhibitors, could increase nuclear sperm DNA alterations and sensitize sperm to the freezing/thawing technique [23].
The aim of this study was to evaluate nuclear fragmentation rates in sperm before and after selection with density gradient centrifugation and freezing/thawing, in HIV-1-infected men and in a control group.
Materials and methods
Patients
Forty patients consulting for assisted reproductive techniques (ARTs) at the Bichat/Claude Bernard Hospital were included in the study and divided into two groups: a control group of uninfected patients and a group of HIV-1-infected patients. Patient characteristics and sperm parameters (total sperm count, progressive motility, vitality and typical forms), defined according to the World Health Organization (WHO) and the DAVID classification, are described in Table 1. Prior to the study, the patient’s informed consent was obtained. Sperm was bacteria-free before cryopreservation in all patients, and the leukocyte count was under the WHO threshold (<1 million/ml).
Table 1.
Characteristics of the population
| Groups | Control (group 1) (n = 20) | HIV-1 (group 2) (n = 20) |
|---|---|---|
| Characteristics | ||
| Age (years) | 38.07 ± 5.93 | 41.44 ± 5.80 |
| Previous paternity (n=) | 12 (46.1%) | 10 (47.6%) |
| Toxic exposure | 11 (53.00%) | 13 (66.00%) |
| Total sperm count (106) | 417.24 ± 62.75 | 289.73 ± 56.09 |
| Motility (a + b) (%) | 43.00 ± 2.46 | 35.57 ± 3.72 |
| Vitality (%) | 73.15 ± 2.80 | 65.76 ± 2.69 |
| Typical forms (%) | 42.87 ± 3.68 | 32.33 ± 2.41 |
Statistical value:
Motility: -Group 2 versus 1: N.S.
Typical forms: -Group 2 versus 1: N.S.
Total sperm count: -Group 2 versus 1: NS
Group 1 Control group, 20 patients of mean age 38.07 ± 5.93 years who were not infected by HIV-1 (tested within the last year) and whose normal sperm (according to WHO criteria) was to be used for vitro fertilization for their partners infertility. 53% of patients had a history of smoking (about 10 cigarettes/day) and/or alcohol abuse.
Group 2 20 HIV-1-infected fertile patients of mean age 41.44 ± 5.80 years treated with antiretroviral therapy (two or three drugs for more than 6 months), with a viral load undetectable by RT-PCR, and plasma CD4+ T lymphocyte count measured by flow cytometry above 200/mm3 (mean 645 ± 72/mm3), according to French protocol of cryopreservation for HIV-1 patients. These patients did not present any clinical sign of their disease and ARTs were indicated to prevent viral infection of their partner. 66% of patients had a history of smoking (about 10 cigarettes/day) and/or alcohol abuse. Sperm samples for HIV-1-infected patients were collected after at least 70 days of spermatogenesis (after two cycles of spermatogenesis).For both groups, alcohol abuse was stopped at least 2 years before inclusion and sperm was bacteria-free.
Sperm preparation for freezing/thawing and DNA fragmentation analysis
A first fresh sperm fraction was centrifuged (1,500 rpm for 5 min). The pellet was washed twice with distilled water, fixed in acetic Carnoy and stored at −20°C for DNA fragmentation analysis. The second sperm fraction was treated by a density Puresperm® gradient (45%, and 90%). Progressive motility, vitality as well as a sperm count were estimated in the final sperm fraction. Half of the final fraction obtained was fixed by acetic Carnoy (ethanol 3 volumes: acetic acid 1 volume) and stored at −20°C for DNA nuclear fragmentation analysis.
The second part of the final sperm fraction pellet was mixed with Sperm-Freeze (Médicult)®, transferred into 0.3 ml high-safety straws (Cryo-Bio-System®) and frozen (freeze control CL 2200®, Cryologic). After storage (at least 24 h), straws were then thawed at ambient temperature over 10 min. Thawed pellets were resuspended in IVFm® and fixed in acetic Carnoy after progressive motility and vitality estimation. DNA nuclear fragmentation was then estimated.
Viral load (HIV-1) was determined in fresh seminal plasma of infected patients by RT-PCR.
Nuclear sperm DNA fragmentation analysis
Sperm nuclear DNA fragmentation rate was evaluated by the terminal uridine nick end labeling (TUNEL) in situ technique (In Situ Cell Death Detection Kit, Roche®). Suspension (5 µl) was spread over a degreased Superfrost® glass slide and, after air drying, immersed in a 10% SDS and 0.1% citrate solution for 15 min, rinsed twice for 3 min in PBS, and air dried. A hybridization mixture was dropped onto the sample, a coverslip applied, and incubation followed for 2 h at 37°C. Slides were then rinsed twice for 3 min and counterstained with DAPI (Vysis-Abott®). An optical fluorescence microscope (Olympus® BX60/BX 41) equipped with DAPI (blue) and FITC (green) filters was used. Only spermatozoa with DAPI counterstaining were considered (spermatozoa not counterstained with DAPI being considered necrotic). Four hundred spermatozoa were observed for each sample.
Statistical analysis
Global comparisons of rates (or of change in rates) between the two groups and in same groups between fresh, migrated sperm and freezing/thawing procedure were performed using the nonparametric Kruskal-Wallis test. When this test was significant, pairwise comparisons were tested according to the Tukey HSD procedure. Statistical analyses were done with the R statistical language R Development Core Team (2005). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL: http://www.R-project.org.
Results
Fresh sperm motility and typical forms were statistically not different in groups 1 and 2 with, respectively, 43.00 ± 2.46% and 42.87 ± 3.68% for group 1 and 35.57 ± 3.72% and 32.33 ± 2.41% for group 2. Mean total sperm count fell above the WHO threshold in these two groups.
Sperm nuclear fragmentation rate was increased (p < 0.05) in group 2 (HIV-1-infected patients) compared with the control group (respectively 6.38 ± 1.36% and 3.39 ± 0.43%).
In group 2, no correlation was found between the CD4+ T lymphocyte count and sperm nuclear fragmentation rates. No correlation was found between treatment duration and fresh sperm fragmentation rates. In the whole population, fragmentation rates were similar for patients with and without toxic (smoking and alcohol) exposure (3.35% versus 3.44%). Furthermore, there was no correlation between sperm DNA fragmentation rate and sperm characteristics: total sperm count, progressive motility or vitality.
Fragmentation rates after migration and freezing/thawing are shown in Fig. 1.
Fig. 1.
DNA fragmentation rates in two groups at different stages of the technique. Statistical value: Group 1: -p < 0.0001: Fresh sperm/after migration. -p < 0.0001: After migration/after thawing. -p=; NS: Fresh sperm/after thawing. Group 2: -p < 0.005: Fresh sperm/after migration. -p < 0.005: After migration/after thawing. -p=NS: Fresh sperm/after thawing
After migration on a density gradient, nuclear DNA fragmentation was similar in the two groups, respectively 1.50% and 2.62% for groups 1 and 2. These rates were significantly decreased (p < 0.05) compared with those of initial fresh semen.
After thawing, nuclear DNA fragmentation was similar to that observed in fresh semen, respectively, 3.40% and, 5.18% in groups 1 and 2.
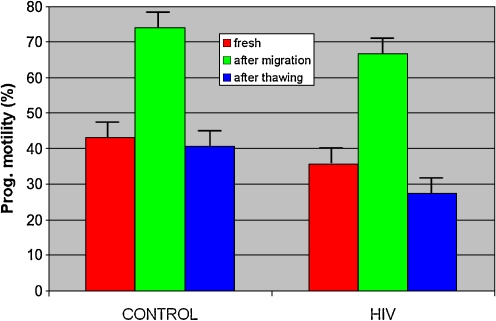
Progressive motility (Fig. 2) and vitality in groups 1 and 2 were significantly improved after migration (p < 0.05) with, respectively, 74.00 ± 16.28% and 75.87 ± 12.23% for group 1 and 66.67 ± 17.91% and 72.60 ± 15.36% for group 2, and decreased after freezing/thawing with, respectively, 40.57 ± 16.23% and 40.93 ± 15.72% for group 1 and 27.44 ± 16.11% and 36.92 ± 13.52% for group 2.
Fig. 2.
Progressive motility in the two groups at different stages of the technique. Statistical values: Group 1: -Motility: Fresh sperm/after migration: p < 0.0001. -Motility: After migration/after thawing.: p < 0.0001. -Motility: Fresh sperm/after thawing.: p=N.S. Group 2: -Motility: Fresh sperm/after migration: p < 0.0005. -Motility: After migration/after thawing.: p < 0.0005. -Motility: Fresh sperm/after thawing.: p=N.S.
Discussion
We used the in situ TUNEL technique to analyze DNA fragmentation rates in freezing/thawing of sperm with or without viral contamination. Cell count was determined on 400 cells as described previously [24–27] and interpretation criteria were similar to those described by Barosso [24]. This technique, used in cases of severe oligospermia, is sensitive, specific, and reproducible [22], but only explores the final stage of apoptosis and probably underestimates the real DNA damage in each cell.
Fragmentation rate and viral infection
The sperm DNA fragmentation rates in HIV-infected patients (6.38%) were statistically increased compared with control (3.39%) (p < 0.05). These results agree with these found by only one author [28], to the best of our knowledge, who used the same slide technique to analyze sperm DNA fragmentation in HIV-1 infected patients. In this previous study, sperm DNA fragmentation rates were higher than ours, but no details on sperm parameters were indicated. These data could be explained by several hypotheses: (1) direct effects of HIV-1 viruses on sperm nuclear structure, (2) indirect effects of HIV-1 by cytokine secretion, (3) direct drugs effects, (4) decrease in CD4+ T lymphocyte count, (5) toxic habits.
HIV-1 viruses induce free radical production (reactive oxygen species) [15], and reduce defenses against oxidative stress, [15–20, 29]. Moreover, some RNA retroviruses have been shown to impair spermatogenesis. First, the porcine reproductive and respiratory syndrome virus (arteriviridae) directly impairs spermatogenesis and DNA of sperm nuclei, as shown in pigs [30]. This retrovirus is functionally similar to human retroviruses, like HIV-1. The contamination route is similar, through the mucosa or blood [31]. Virus proliferation can occur in tissue macrophages of the genital tract, or in lymph nodes and pass through the blood to the genital tract [30, 32]. Secondly, simian immunodeficiency virus could also be responsible for direct effects on the testis [31, 34], but these data are controversial, and some authors think that retroviruses could affect germinal cells by interaction with the alternative HIV receptor GalCer [35] and could induce apoptosis of germinal cells via seminal epithelium desquamation [30] or directly by cytokine secretion [30, 33].
Such retroviruses can be localized in the prostate, seminal vesicle, epididymis, and testis and are characterized by secretion of interleukins like Il10, InFγ, TNF α, and Il1 β. These cytokines could be produced locally by leukocytes and play a role in regulation of immune response in retroviral infections, but could induce an inflammatory environment in all male genital tracts. [33]. Furthermore, some other authors think that infected macrophages/lymphocytes could interact with Leydig cells, be responsible for a decrease in testosterone level by inhibition of steroidogenesis or dysfunction of the pituitary axis [36], and could induce degeneration of the seminal epithelium. In our study, sperm was bacteria-free in both groups before cryopreservation, and the leukocyte count was under the WHO threshold (<1 million/ml).
Sperm characteristics were similar in our two groups, as has been previously demonstrated in treated patients and untreated patients [37]. But it has been shown that antiviral drugs reduce sperm progressive motility, especially when reverse transcriptase nucleoside inhibitors are used [21, 22]. Deterioration could be explained by drug-induced damage in mitochondrial DNA in the sperm tail [22]. All patients in our study were treated for more than 6 months and antiretroviral therapy was maintained during sperm cryopreservation to lower the viral load in the seminal plasma to undetectable levels. To explain DNA fragmentation rate in HIV-1 patients, we can hypothesize that antiretroviral treatments, in particular reverse transcriptase nucleoside inhibitors, modify the sperm nucleus and increase sperm nuclear apoptosis, as they modify fetal heterochromatin when used alone or in combination with other drugs [23].
A recent study [38] showed that the number of CD4+ T lymphocytes is positively correlated with slow progressive motility (component ‘b’) in ejaculated sperm, and we can hypothesize that nuclear DNA sperm may be altered too. In this study, minimal CD4+ T lymphocyte counts were lower than 26/mm3 and thus, at this stage of the disease, frequency of opportunistic local or general infections can be increased [39, 40] and indirectly correlated with increasing oxidative stress caused by inflammation. A decrease in sperm count was also observed when CD4+ T lymphocyte counts were lower than 200/mm3 [38]. However, in our study, CD4+ T lymphocyte counts were higher than 200/mm3 in all HIV-1-infected patients, with a frequency of opportunistic infections similar to that in the control population. This could explain the absence of correlation between plasma CD4+ T lymphocyte counts and sperm apoptosis rates in our study.
Even if we cannot exclude an impact of the history of toxic exposure and infertility on sperm DNA fragmentation rate, we can notice that HIV-1 patients had normal sperm characteristics according to WHO criteria and that toxic habit frequency was not statistically different in our population compared with the control group. Finally, fragmentation rate were similar for patients with or without toxic exposure (3.35% versus 3.44%, NS).
Finally, according to our results and the type of population, we can hypothesize that both direct and indirect HIV-1 effects can alter sperm nuclear DNA, but some antiretroviral drugs could also damage it.
Effects of freezing and thawing on sperm fragmentation rates
As previously reported [10, 11, 24, 25, 41, 42], migration on a density gradient improved progressive motility, vitality and percentage of typical forms, because sperm migration gradient eliminates sperm with no or low motility or with atypical forms. We also observed a decrease of fragmentation rate after migration, whatever the viral status of the patients. This is probably explained by the efficient selection of spermatozoa by this technique.
As previously reported [11], the freezing/thawing of spermatozoa significantly decreased total sperm count, progressive motility and vitality, and increased nuclear fragmentation rate in comparison with the migrated fraction. This could be explained by the fact that the freezing/thawing technique weakens some spermatozoa and activates apoptosis. Taken together, migration and freezing/thawing techniques do not modify nuclear fragmentation rate in all groups. However, initial sperm characteristics are important. Some authors [43, 44] report that sperm DNA was altered in infertile patients and in cases of oligospermia [11, 45, 46]. A reduction of mitochondrial membrane potential analyzed by rhodamine (R123) was also observed after thawing in this category of patient [45]. Other studies showed that among control patients, sperm apoptosis rates increased only moderately after thawing [47].
No deleterious effects on sperm DNA fragmentation were seen in HIV-1 patients after freezing/thawing. We can suggest that HIV-1 infection is compatible with good ART results. In England, an 18.8% pregnancy rate was observed per intrauterine insemination (IUI) cycle [48], with migrated but not frozen sperm. This resembles the results of French ART centers which currently practice I.UI with cryopreserved sperm from donors (mean 15%–20% pregnancy rate per I.U.I. cycle) [49]. Similarly, the European network CREAThE (Centres for REproductive Assistance Techniques in HIV in Europe) [50] records 15% pregnancies per cycle (3,359 cycles in 2002). Sperm profiles of the HIV-1-infected patients are generally normal.
In this study, therefore, we show that sperm nuclear DNA fragmentation is increased in fresh sperm of HIV-1 patients treated by antiretroviral therapy compared with the control group, but not modified by the combined migration and freezing/thawing procedures. A direct deleterious effect of HIV-1 virus, or of antiretroviral therapy, could explain the increase in nuclear DNA damage in fresh sperm of HIV-1 patients. On the other hand, we can hypothesize that the deleterious effect of freezing/thawing procedures is balanced by the selection effect of migration. This hypothesis should be confirmed by other studies with an enhanced effective group. Sperm DNA fragmentation with freezing/thawing techniques in cases of HCV infection would also be interesting to analyze.
Footnotes
Capsule No impact of freezing/thawing methods on sperm DNA integrity in HIV-1 infected patients.
References
- 1.Bourlet T, et al. Detection of GB virus C/hepatitis G virus in semen and saliva of HIV type-1 infected men. Clin Microbiol Infect. 2002;8(6):352–7. doi: 10.1046/j.1469-0691.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 2.Bourlet T, et al. Multicenter quality control for the detection of hepatitis C virus RNA in seminal plasma specimens. J Clin Microbiol. 2003;41(2):789–93. doi: 10.1128/JCM.41.2.789-793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquier C, et al. Intermittent detection of hepatitis C virus (HCV) in semen from men with human immunodeficiency virus type 1 (HIV-1) and HCV. J Med Virol. 2003;69(3):344–9. doi: 10.1002/jmv.10295. [DOI] [PubMed] [Google Scholar]
- 4.Pasquier C, et al. Sperm washing and virus nucleic acid detection to reduce HIV and hepatitis C virus transmission in serodiscordant couples wishing to have children. AIDS. 2000;14(14):2093–9. doi: 10.1097/00002030-200009290-00004. [DOI] [PubMed] [Google Scholar]
- 5.Meseguer M, et al. Comparison of polymerase chain reaction-dependent methods for determining the presence of human immunodeficiency virus and hepatitis C virus in washed sperm. Fertil Steril. 2002;78(6):1199–202. doi: 10.1016/S0015-0282(02)04275-9. [DOI] [PubMed] [Google Scholar]
- 6.Baker HW, et al. Use of assisted reproductive technology to reduce the risk of transmission of HIV in discordant couples wishing to have their own children where the male partner is seropositive with an undetectable viral load. J Med Ethics. 2003;29(6):315–20. doi: 10.1136/jme.29.6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sifer C, et al. Risks in medically-assisted procreation in case of positivity for HIV, hepatitis C virus or hepatitis B virus. The French law at the end of 2001. Gynécol Obstét Fertil. 2003;31(5):410–21. doi: 10.1016/S1297-9589(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 8.Fiore JR, et al. The efficiency of sperm washing in removing human immunodeficiency virus type 1 varies according to the seminal viral load. Fertil Steril. 2005;84(1):232–4. doi: 10.1016/j.fertnstert.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 9.Centola GM, Raubertas RF, Mattox JH. Cryopreservation of human semen. Comparison of cryopreservatives, sources of variability, and prediction of post-thaw survival. J Androl. 1992;13(3):283–8. [PubMed] [Google Scholar]
- 10.Donnelly ET, et al. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod. 2001;16(6):1191–9. doi: 10.1093/humrep/16.6.1191. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76(5):892–900. doi: 10.1016/S0015-0282(01)02834-5. [DOI] [PubMed] [Google Scholar]
- 12.Host E, et al. DNA strand breaks in human spermatozoa: a possible factor, to be considered in couples suffering from unexplained infertility. Acta Obstet Gynecol Scand. 1999;78(7):622–5. doi: 10.1080/j.1600-0412.1999.780710.x. [DOI] [PubMed] [Google Scholar]
- 13.Host E, et al. DNA strand breaks in human sperm cells: a comparison between men with normal and oligozoospermic sperm samples. Acta Obstet Gynecol Scand. 1999;78(4):336–9. doi: 10.1080/j.1600-0412.1999.780412.x. [DOI] [PubMed] [Google Scholar]
- 14.Sion B, et al. Annexin V binding to plasma membrane predicts the quality of human cryopreserved spermatozoa. Int J Androl. 2004;27(2):108–14. doi: 10.1046/j.1365-2605.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 15.Baker HW, et al. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril. 1996;65(2):411–9. doi: 10.1016/s0015-0282(16)58109-6. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong JS, et al. Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic Biol Med. 1999;26(7–8):869–80. doi: 10.1016/S0891-5849(98)00275-5. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, et al. Induction of OGG1 gene expression by HIV-1 Tat. J Biol Chem. 2005;280(29):26701–13. doi: 10.1074/jbc.M503313200. [DOI] [PubMed] [Google Scholar]
- 18.Pocernich, C.B., et al. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Brain Res Rev, 2005. [DOI] [PubMed]
- 19.Pocernich CB, et al. Proteomic analysis of oxidatively modified proteins induced by the mitochondrial toxin 3-nitropropionic acid in human astrocytes expressing the HIV protein tat. Brain Res Mol Brain Res. 2005;133(2):299–306. doi: 10.1016/j.molbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Price TO, et al. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045(1–2):57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Carr A, et al. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14(3):F25–32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 22.Sergerie M, et al. Impact of reverse transcriptase inhibitors on sperm mitochondrial and genomic DNA in assisted reproduction techniques. Gynécol Obstét Fertil. 2004;32(10):841–9. doi: 10.1016/j.gyobfe.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Senda S, et al. Altered heterochromatin organization after perinatal exposure to zidovudine. Antivir Ther. 2007;12(2):179–87. [PubMed] [Google Scholar]
- 24.Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15(6):1338–44. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- 25.Muratori M, et al. Functional and ultrastructural features of DNA-fragmented human sperm. J Androl. 2000;21(6):903–12. [PubMed] [Google Scholar]
- 26.Zini A, et al. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75(4):674–7. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 27.Benchaib M, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18(5):1023–8. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 28.Muciaccia B, et al. HIV-1 viral DNA is present in ejaculated abnormal spermatozoa of seropositive subjects. Hum Reprod. 2007;22(11):2868–78. doi: 10.1093/humrep/dem288. [DOI] [PubMed] [Google Scholar]
- 29.Kovalski NN, Lamirande E, Gagnon C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: protective effect of seminal plasma and scavengers. Fertil Steril. 1992;58(4):809–16. [PubMed] [Google Scholar]
- 30.Sur JH, et al. Porcine reproductive and respiratory syndrome virus replicates in testicular germ cells, alters spermatogenesis, and induces germ cell death by apoptosis. J Virol. 1997;71(12):9170–9. doi: 10.1128/jvi.71.12.9170-9179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller CJ. Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques. J Reprod Immunol. 1998;41(1–2):331–9. doi: 10.1016/S0165-0378(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 32.Christopher-Hennings J, et al. Identification of porcine reproductive and respiratory syndrome virus in semen and tissues from vasectomized and nonvasectomized boars. Vet Pathol. 1998;35(4):260–7. doi: 10.1177/030098589803500404. [DOI] [PubMed] [Google Scholar]
- 33.Tortorec A, et al. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One. 2008;3(3):e1792. doi: 10.1371/journal.pone.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arimi MM, et al. Evidence for expression of endogenous retroviral sequences on primate reproductive tissues and detection of cross-reactive ERVS antigens in the baboon ovary: a review. East Afr Med J. 2006;83(2):106–12. doi: 10.4314/eamj.v83i2.9397. [DOI] [PubMed] [Google Scholar]
- 35.Brogi A, et al. Human sperm and spermatogonia express a galactoglycerolipid which interacts with gp120. J Submicrosc Cytol Pathol. 1995;27(4):565–71. [PubMed] [Google Scholar]
- 36.Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65(2):208–31. doi: 10.1128/MMBR.65.2.208-231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrido N, et al. Assisted reproduction in HIV and HCV infected men of serodiscordant couples. Arch Androl. 2004;50(2):105–11. [PubMed] [Google Scholar]
- 38.Garrido N, et al. Semen characteristics in human immunodeficiency virus (HIV)- and hepatitis C (HCV)-seropositive males: predictors of the success of viral removal after sperm washing. Hum Reprod. 2005;20(4):1028–34. doi: 10.1093/humrep/deh699. [DOI] [PubMed] [Google Scholar]
- 39.Lasheeb AS, et al. Semen characteristics in HIV-1 positive men and the effect of semen washing. Genitourin Med. 1997;73(4):303–5. doi: 10.1136/sti.73.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dulioust E, et al. Semen alterations in HIV-1 infected men. Hum Reprod. 2002;17(8):2112–8. doi: 10.1093/humrep/17.8.2112. [DOI] [PubMed] [Google Scholar]
- 41.Twigg J, et al. Iatrogenic DNA damage induced in human spermatozoa during sperm preparation: protective significance of seminal plasma. Mol Hum Reprod. 1998;4(5):439–45. doi: 10.1093/molehr/4.5.439. [DOI] [PubMed] [Google Scholar]
- 42.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 43.Duru NK, et al. Cryopreservation-thawing of fractionated human spermatozoa and plasma membrane translocation of phosphatidylserine. Fertil Steril. 2001;75(2):263–8. doi: 10.1016/S0015-0282(00)01694-0. [DOI] [PubMed] [Google Scholar]
- 44.Duru NK, et al. Cryopreservation-Thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalization and not DNA fragmentation. J Androl. 2001;22(4):646–51. [PubMed] [Google Scholar]
- 45.Royere D, et al. Cryopreservation of spermatozoa: a 1996 review. Hum Reprod Update. 1996;2(6):553–9. doi: 10.1093/humupd/2.6.553. [DOI] [PubMed] [Google Scholar]
- 46.Saritha KR, Bongso A. Comparative evaluation of fresh and washed human sperm cryopreserved in vapor and liquid phases of liquid nitrogen. J Androl. 2001;22(5):857–62. [PubMed] [Google Scholar]
- 47.Paasch U, et al. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol Reprod. 2004;71(6):1828–37. doi: 10.1095/biolreprod.103.025627. [DOI] [PubMed] [Google Scholar]
- 48.Nicopoullos JD, et al. The effect of human immunodeficiency virus on sperm parameters and the outcome of intrauterine insemination following sperm washing. Hum Reprod. 2004;19(10):2289–97. doi: 10.1093/humrep/deh426. [DOI] [PubMed] [Google Scholar]
- 49.Lannou D. Artificial insemination with donor frozen sperm. Gynécol Obstét Fertil. 2004;32(10):894–7. doi: 10.1016/j.gyobfe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Bujan L, et al. Safety and efficacy of sperm washing in HIV-1-serodiscordant couples where the male is infected: results from the European CREAThE network. AIDS. 2007;21(14):1909–14. doi: 10.1097/QAD.0b013e3282703879. [DOI] [PubMed] [Google Scholar]