Abstract
Purpose
Controlled ovarian hyperstimulation has been shown to advance endometrial maturation and adversely affects implantation in ART. It has been reported that there is a better embryo-endometrium synchrony in frozen-thawed embryo transfer cycles than fresh embryo transfer cycles. The objective of this study was to compare ongoing pregnancy rates between fresh ET and FET cycles.
Methods
In an open prospective, controlled study, the patients who were classified as high responders, were randomized to either fresh ET or FET. The embryos in FET group were cryopreserved with vitrification by Cryotop method.
Results
A total of 374 patients were included, 187 of which were randomized to FET and 187 to fresh ET. There were 39% (n = 73) ongoing pregnancy in FET group compared with 27.8% (n = 52) in fresh ET group[odds ratio = 1.66;95% confidence interval = 1.07–2.56; p = 0.02].
Conclusions
FETs can be performed instead of fresh ETs to improve the outcome of ART in highly selected patients.
Keywords: Endometrial receptivity, Fresh embryo transfer, Frozen-thawed embryo transfer, Ongoing pregnancy, Vitrification
Introduction
Despite many advances in ART, pregnancy rates remain low [1]. Implantation is the “rate-limiting step” in the success of in vitro fertilization (IVF) cycles [2]. Controlled ovarian hyperstimulation (COH) adversely affects implantation following IVF-ET [3]. Periovulatory endometrial characteristics in stimulated cycles are considerably different compared with the natural cycles, and periovulatory secretory transformation is consistently advanced [4]. It has been apparent that if secretory endometrial advancement in the early luteal phase is more than 3 days, implantation does not occur in human [5]. An embryo-endometrium asynchrony in COH cycles impairs implantation and it has been suggested that the asynchrony problem in fresh cycles can be solved by cryopreservation of all embryos and transferring them subsequently in optimal conditions. The endometrial development in frozen-thawed cycles can be controlled more precisely than in the cycles of COH with gonadotropins [6], therefore there is less asynchrony between endometrium and embryos in frozen-thawed embryo transfer (FET) cycles and such patients would have an increased chance of pregnancy.
Recently, in many reproductive centers, embryos are cryopreserved using vitrification. Vitrification is an ultra-rapid method of cryopreservation whereby the embryo is transitioned from 37°C to −196°C in <1 s, resulting in extremely fast rates of cooling. High concentrations of cryoprotectants together with rapid cooling rates are essential to cryopreserve embryos in a vitrified, glass-like state [7].
Vitrification has several advantages . The main benefits include the lack of ice crystal formation, made possible through increased speed of temperature conduction, reducing associated chilling injuries, therefore, vitrification is associated with less cellular trauma than slow freezing and it has been reported that the cryosurvival rate with vitrification is ∼95% [8]. Additionally, a practical advantage is the speed of the process and there is no need for expensive equipments [9–11]. Therefore, vitrification is considered as the method of choice for human embryo cryopreservation.
We designed this study to evaluate the effect of performing FETs instead of fresh embryo transfers (ETs) on ongoing pregnancy rates in IVF/intracytoplasmic sperm injection (ICSI) cycles. Clinical data suggest that cryopreservation of all embryos by vitrification and transferring them subsequently may be an effective strategy to enhance outcomes in assisted reproduction technology (ART). It was our aim to determine whether we could apply this new strategy to clinical practice.
Materias and methods
Study design and participants
This study was a prospective randomized controlled trial to assess the hypothesis that replacement of fresh ET by FET would enhance outcomes of ART cycles. The study was performed at university-based and a private assisted reproduction center between February 1, 2007 and February 1, 2009, including 374 patients who were candidates for IVF/ICSI and who were classified as high responders. Because of ethical concerns we chose high responders. This study was approved by the ethics committee of Research and Clinical Center for Infertility, Shahid Sadoughi Univesity of Medical Science. All couples were required to sign a written informed consent after the provision of complete information to them.
Inclusion criteria
Patients who had ≥15 follicles with a mean diameter ≥12 mm per ovary at the end of the follicular phase of COH, and/or E2 levels on the day of hCG administration >3,000 pg/mL, and/or >15 retrieved oocytes.
Patients who had at least two top-quality embryos appropriate for cryopreservation. Top-quality embryos were defined as day 2 embryos having four or more evenly sized and equally shaped blastomeres, with <20% fragmentation and no multinucleation [12].
Exclusion criteria
Patients who were ≥38 years old, patients with serum day 3 FSH levels ≥10, patients who did not undergo their first assisted reproduction treatment cycles, patiens who were coasted more than 2 days, patients with any symptoms and signs of ovarian hyperstimulation syndrome (OHSS) on the day of ET, and patients who did not have top-quality embryos appropriate for cryopreservation.
Randomization
Eligible women were randomized to either group in a ratio of 1:1 by means of computer-generated random numbers on the day of ET. Selection into the groups and randomization were performed by a nurse not involved in the study by using a series of consecutively numbered sealed opaque envelopes. Both the patients and the clinicians were aware of the allocated arm.
Interventions
All patients in the initial cohort were treated with long protocol for ovarian stimulation. For pituitary down-regulation, patients were treated with daily administration of 0.5 mg buserelin (suprefact, Aventis, Frankfurt, Germany) from day 21 of menstrual cycle. Buserelin was reduced to 0.25 mg daily when ovaries were quiescent on ultrasound, and COH was initiated with recombinant FSH (Gonal F, Serono, Aubnne, Switzerland) 150 IU/day on day 2 of withdrawal bleeding. Serial ultrasound examinations and evaluation of serum E2 levels were used to assess ovarian response, and then gonadotropin dose adjustments were done as required. Human chorionic gonadotropin (pregnyl, Organon, Oss, the Netherlands) 10,000 IU was administered when at least two follicles reached a mean diameter of 18 mm.
Oocyte retrieval was performed 34–36 h after hCG administration and conventional insemination or ICSI was performed as clinically appropriate.
In 187 patients allocated to fresh ET group, ET were performed on day 2. Embryos were transferred under ultrasound guidance, with a C.C.D. embryo transfer catheter (Laboratory C.C.D., Paris, France). Luteal support with progesterone in oil (Progesterone, Aburaihan Co., Tehran, Iran) 100 mg daily IM was started on the day of oocyte retrieval and continued until the documentation of fetal heart activity on ultrasound.
In 187 patients allocated to FET group, cryopreservation of all embryos were undertaken with vitrification by Cryotop method on day 2 and after 2 months, embryos were transferred.
The protocol for the Cryotop vitrification of embryos was described previously [13, 14].
After a two-step loading with equilibration solution containing 7.5% (v/v) ethylene glycol and 7.5% (v/v) dimethyl sulfoxide, and vitrification solution containing 15% (v/v) ethylene glycol, 15% (v/v) dimethyl sulfoxide and 0.5 mol/L sucrose, embryos were loaded with a narrow glass capillary onto the Cryotop in a volume of <0.1 µL . After loading, almost all the solution was removed to leave only a thin layer covering the embryos, and the sample was quickly immersed into liquid nitrogen (LN). Subsequently, the plastic cap was pulled over the film part of the Cryotop, and the sample was stored under LN. At warming, the protective cap was removed from the Cryotop while it was still submerged in LN and the Cryotop was immersed directly into a 37°C medium containing sucrose. The embryos were then sequentially incubated in diluents solution before further in vitro culture for transfer.
Each embryo was carefully evaluated twice, immediately after thawing for the number of surviving blastomeres and a second evaluation was performed 18 h later prior to transfer in order to assess the resumption of mitosis and the total number of blastomeres. Embryos were considered to have survived if >50% of the blastomeres were intact, and showing at least one blastomere divided by 18 h of post-thaw culture (Balaban et al., 2008). Embryos were classified as either fully intact (100% cells survived), partially damaged (≥50% cells survived) or degenerated (<50% cells survived) after thawing. Only intact and partially damaged embryos were transferred. Thawed embryos were graded using the same criteria as in the fresh cycles [15].
Patients were prepared for ET with oral E2 to attain endometrial thickness ≥8 mm and triple line pattern on ultrasound scans. At that time, patients were given 100 mg of IM progesterone in oil daily and ET was preformed 3 days later under abdominal ultrasound guidance as described earlier. Oral E2 and progesterone were continued until documentation of fetal heart activity by ultrasonography.
Outcome measures
Primary outcome measures were the ongoing pregnancy rates and the secondary outcome measures were implantation, clinical pregnancy and multiple pregnancy rates. Ongoing pregnancy was defined as pregnancy proceeding beyond the 12th gestational week. Implantation rate reflected the number of gestational sacs seen per embryo transferred. Clinical pregnancy was considered as the presence of a gestational sac with fetal heart activity, as assessed by ultrasound at 7 weeks of gestation. Multiple pregnancy was defined as a gestation with more than one fetus.
Statistical analysis
Sample size calculation
Ongoing pregnancy rate was the primary outcome measure. Based on previous clinical study results that an effective strategy in ART cycles leads to absolute difference of 10–15% in ongoing pregnancy rate, the study was designed to have sufficient power to detect an absolute difference of 14% in the ongoing pregnancy rate. It was calculated that 187 subjects in each group would be an adequate number to achieve an 80% power of detection of difference at a significant level (alpha) of 0.05. It should be noted that the difference of 14% was arbitrarily defined in order to complete the study in two years.
Statistical tests
All patients randomized, were included in the analyses of the primary efficacy end point (intention-to-treat analysis). Data were expressed as mean ± SD unless otherwise stated. The normality of distribution of variables was tested by using the Kolmogorov-Smirnov test. Independent sample t-test was used for continuous variables which were normally distributed and Mann-Whitney U test for data not normally distributed. Chi-squared test with Yates correction or Fisher exact test were used for qualitative variables as appropriate. P < 0 .05 was considered statistically significant. A logit model based on generalized estimating equation methods was applied to determine the odds ratio and its associated p value and confidence interval when comparing outcomes between study and control groups. The Statistical Package for Social Science (SPSS, version 15.0 for windows; SPSS Inc., Chicago. IL) was used for data analysis.
Results
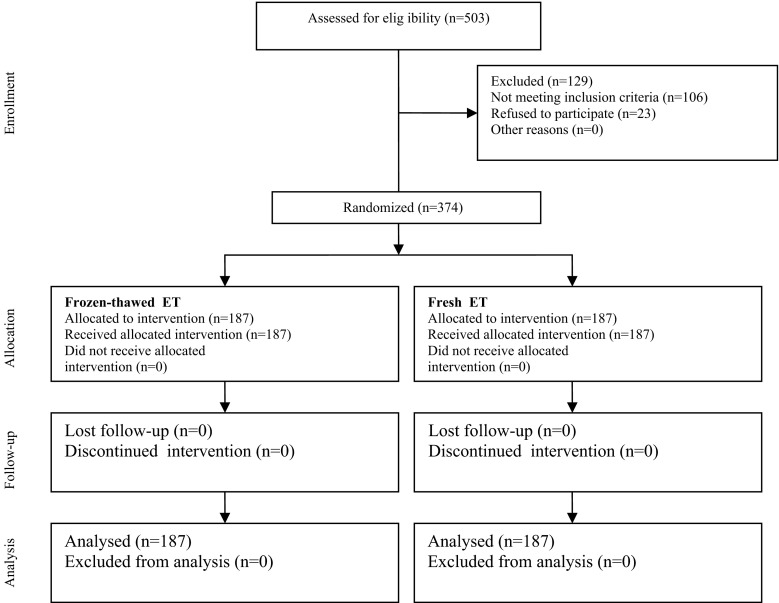
The results were reported in accordance with the CONSORT statement. Of 503 women eligible to the study, 129 were excluded, and finally 374 patients were enrolled and randomized. There were not any women lost to follow up or otherwise dropping out from the study post-randomization. However, four women in FET group did not have top-quality embryos after thawing, but they were included in the final analysis in accordance with the intention-to-treat method. The CONSORT statement flow diagram is presented in Fig. 1.
Fig. 1.
Recruitment follow-up and drop outs over the course of the study
There were no significant differences regarding demographic characteristics between the groups (Table 1).
Table 1.
Patients characteristics
| Parameter (Unit) | FET group | Fresh ET group | P value |
|---|---|---|---|
| Number of patients | 187 | 187 | |
| Age (years)ª | 27.3 ± 4.4 | 28.1 ± 3.5 | 0.07 |
| BMI (Kg/m²)ª | 25.5 ± 3.4 | 26.0 ± 3 | 0.09 |
| Day 3 FSH (IU/mL)ª | 4.5 ± 1.2 | 4.7 ± 1.3 | 0.07 |
| Duration of infertility (years)ª | 4.8 ± 2.1 | 4.4 ± 2.7 | 0.07 |
| Cause of infertility | |||
| Male factor (%) | 36.9 | 34.2 | 0.66 |
| Ovulatory factor (%) | 17.1 | 20.8 | 0.42 |
| Tubal factor (%) | 18.7 | 19.3 | 1.00 |
| Endometriosis (%) | 9.6 | 10.7 | 0.86 |
| Unexplained (%) | 17.6 | 15 | 0.57 |
ªValues are mean ± SD
The cycle characteristics and outcome of vitrification and warming procedures are demonstrated in Table 2. The mean number of retrieved oocytes, E2 levels on hCG day, gonadotropin dose, percentage of ICSI performance, fertilization rate, mean number of embryos transferred, and mean number of top-quality embryos transferred were similar in the two groups. Assisted hatching performance was significantly different in two groups. The cryosurvival rate was 96.02%, and the percentage of embryos with all blastomeres intact after warming was 75.3%.
Table 2.
Cycle characteristics
| Parameter (Unit) | FET group | Fresh ET group | P value |
|---|---|---|---|
| Number of patients | 187 | 187 | |
| Gonadotropin dose | 15.68 ± 1.3 | 15.66 ± 1.4 | 0.88 |
| (No. of 75 IU ampules) | |||
| E2 day of hCG (pg/mL)ª | 3128.5 ± 701 | 3140.1 ± 806 | 0.88 |
| No. of oocytes retrievedª | 15.3 ± 2.5 | 15.8 ± 2.2 | 0.056 |
| ICSI performance (%) | 49.7 | 47.1 | 0.67 |
| Assisted hatching (%) | 62 | 4.8 | 0.00 |
| Fertilization rate (%) | 72.9 | 72.7 | 0.56 |
| No. of embryos vitrifiedª | 7.4 ± 2.8 | 5 ± 2.1 | 0.00 |
| No. of embryos warmedª | 3.2 ± 1.4 | – | – |
| Cryosurvival n (%) | 1328 /1383 (96.02) | – | – |
| No. of embryos with 100% | |||
| Blastomere survival n (%) | 1000/1328 (75.3) | – | – |
| No. of embryos transferredª | 2.16 ± 0.36 | 2.22 ± 0.41 | 0.11 |
| No. of top-quality embryos transferredª | 1.84 ± 0.36 | 1.90 ± 0.29 | 0.08 |
| Implantation rate n (%) | 100/404 (24.7) | 72/416 (17.5) | 0.02 |
ªValues are mean ± SD
Cycle outcome characteristics are shown in Table 3. Ongoing pregnancy, clinical pregnancy, and implantation rate were significantly higher in FET group. A trend towards a higher multiple pregnancy rate, which did not achieve statistical significance, was also noted in FET cycles.
Table 3.
Outcome of cycle
| Parameter (Unit) | FET group | Fresh ET group | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Clinical pregnancy rate, n (%) | 78/187 (41.7) | 58/187 (31) | 1.59 (1.04–2.43) | 0.03 |
| Ongoing pregnancy rate, n (%) | 73/187 (39) | 52/187 (27.8) | 1.66 (1.07–2.56) | 0.02 |
| Multiple pregnancy rate, n (%) | 19/73 (26) | 8/52 (15.4) | 1.93 (0.77–4.84) | 0.158 |
There were 73 (39%) ongoing pregnancies in the FET group, and 52 (27.8%) in the fresh TE group (OR = 1.66; 95% CI = 1.07–2.56; p = 0.02). There were 78 (41.7%) clinical pregnancies in the FET group, and 58 (31%) in the fresh TE group (OR = 1.59; 95% CI = 1.04–2.43; p = 0.03). Multiple pregnancy rates were 26% (19/73) in the FET group and 15.4% (8/52) in the fresh ET group (OR = 1.93; 95% CI = 0.77–4.84; P = 0.158). Implantation rates were 100/404 (24.7%) and 72/416 (17.5%) in the FET and fresh ET groups, respectively (p < 0.05).
Discussion
In the present study, we found that the implantation, clinical, and ongoing pregnancy rates were significantly higher in FET group. A trend towards higher multiple pregnancy rate was also noted in FET cycles. Two previous studies have compared cryopreservation of all embryos with either human albumin or fresh ET and in the former, higher pregnancy rate was found in FET group [16] and in the latter no difference was found [17].
Different implantation rates in two groups may reflect different endometrial receptivity and higher synchronization between embryo and endometrial development in FET cycles.
Advanced endometrial development especially in stroma of endometrium after ovulation induction with hMG/hCG for IVF has been reported in many studies [18, 19]. High E2 levels in proliferative phase in COH cycles cause up-regulation of progesterone receptors in endometrium [20], furthermore, high serum E2 and/or progesterone affect the gene expression profiles of human endometrium, therefore, endometrial receptivity may be altered [21]. The comparison of gene expression from the same patients between natural and stimulated cycles revealed endometrial profile associated with either a moderately altered receptivity in most cases (86%) or a strongly altered receptivity during the COS protocol in a few cases (14%). These data suggest that either the duration or FSH dose in gonadotropins treatment under COH cycles leads to the transcriptional activation of the other genes which are not involved in physiological endometrium receptivity. In addition major differences in biological functions known to be involved in the implantation process such as the TGFβ signaling pathway, the complement and coagulation cascades and the leukocyte transendothelial migration were observed between the natural and stimulated cycles. Haouzi et al. demonstrated that gonadotropin treatment in COH cycles led to disruption of the transcriptional activation of genes involved in normal endometrial receptivity, and they suggested that when the receptiveness of the endometrium was seriously compromised by the COH, fresh ET should be cancelled [22].
Embryo and endometrial asynchrony is a limiting factor in IVF success in fresh cycles [2]. Embryo and endometrial development synchronization can be attained better by timing progesterone administration in FET cycles. Shapiro et al. found that large blastocyst diameter, early blastulation, and low preovulatory serum progesterone were dominant predictors of clinical pregnancy in fresh autologous cycles. They suggested that embryo-endometrium asynchrony was a dominant mechanism in cycle failure and recommended when all these three variables were suboptimal, the embryos should be cryopreserved for later use under more optimal conditions [23].
Therefore, the hypothesis of cryopreservation of all embryos and transferring them subsequently, may enhance endometrial receptivity and implantation rate and outcome of ART cycles in some patients. Thus, it provides many clinical benefits, including the increasing of cumulative pregnancy rates and reducing the risk of OHSS. Modifying the transfer strategy also allows the number of replaced embryos to be reduced, thereby diminishing the rate of multiple pregnancies. Ultimately, by improvement of the success rate/ET we can use mild stimulation protocols which might decrease patient discomfort, emotional ditress and cost.
In the current study, embryo morphology was similar in both groups before cryopreservation. In spite of similar morphology, all embryos did not tolerate cryopreservation equally. It has been reported that embryos that have further cleaved during the post-thaw period have the significantly higher chance of implantation and a large number of uncleaved frozen-thawed embryos have chromosomal aberrations [24]. Therefore, the other explanation for increasing pregnancy rates in FET group might be that embryos with higher implantation potential survived after thawing and these embryos improved outcomes.
There are two major concerns for our study. First is,the toxic effects of high concentration of cryoprotectant agents in vitrification may affect adversely the embryos [25, 26]. However, it has been proven that embryo survival and subsequent embryo development are significantly high following vitrification [8]. In addition, we performed cryopreservation of all embryos only in patients who had adequate number of good quality embryos appropriate for freezing and we recommend this strategy only in patients who have sufficient embryos appropriate for cryopreservation. Further studies are needed to maximize cryosurvival rate in vitrification. We hope that with advancement in this era we can use this strategy in all IVF/ICSI cycles.
The second concern is that vitrification is a novel method and the controversy regarding the overall safety of this method in ART is ongoing. However, Takahashi et al. compared the perinatal outcome of 413 cryoloop vitrified-warmed blastocyst transfers with that of 602 fresh blastocyst transfers. No significant differences were reported in the mean gestational age, birth weight, preterm birth rate or congenital birth defect rate [27].
Recently, Rama Raju et al. compared and evaluated the neonatal outcomes in infants born after vitrified day 3 ETs with that of fresh day 3 ETs. The preliminary study showed that neonatal outcomes in vitrified FETs were comparable with fresh ET cycles [28].
The possibility of viral contamination has been suggested following spiking of LN storage with high viral titers [29]. Publication by Kyuwa et al. indicates that cross-contamination is unlikely [30]. Furthermore, Cryotop method, an advanced version of the minimal volume approaches helps to eliminate potential dangers of disease transmission in vitrification technique [31, 32].
In conclusion, the strategy of cryopreservation of all embryos and transferring them subsequently in optimal conditions in highly selected patients increases the synchrony between embryo and endometrial development, and therefore, improves the ongoing pregnancy rate and outcome of ART cycles. Much work remains to be done in optimizing this strategy in different cycle characteristics and evaluating it in all ART cycles.
Vitrification is the preferred method for cryopreservation of embryos with high survival rates and low rates of cooling injury. Follow up investigations should be performed to ensure the safety of vitrification.
Acknowledgements
The authors are grateful to the nursing and embryology staff of the Yazd Research and Clinical Center for Infertility and Madar Hospital for their assistance.
Footnotes
Capsule
The implantation and ongoing pregnancy rates are higher in frozen-thawed embryo transfer using vitrification than fresh embryo transfer cycles.
Clinicaltrials.gov Trials registration number NCT00823121.
This article has been retracted at the request of the Editor and the ASRM Publications Committee, based on the results of an investigation which found serious methodological flaws in the study.
The retraction note to this article can be found online at 10.1007/s10815-013-0084-0.
Contributor Information
Abbas Aflatoonian, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: abbas_aflatoonian@yahoo.com.
Homa Oskouian, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: homaoskouian@gmail.com.
Shahnaz Ahmadi, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: AHMADISHAHNAZ2005@yahoo.com.
Leila Oskouian, Phone: +98-351-8247085, FAX: +98-351-8247087, Email: leilaoskouian0695@gmail.com.
References
- 1.Donaghy M, Lessey BA. Uterine receptivity: alternations associated with benign gynecologic disease. Semin Reprod Med. 2007;25:461–475. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- 2.Paulson RJ, Sauer MV, Lobo RA. Factors affecting implantation after human in vitro fertilization: a hypothesis. Am J Obstet Gynecol. 1990;163:2020–2023. doi: 10.1016/0002-9378(90)90790-E. [DOI] [PubMed] [Google Scholar]
- 3.Check JH, Choe JK, Katsoff D, Summers-Chase D, Wilson C. Controlled ovarian hyperstimulation adversely affects implantation following in vitro fertilization-embryo transfer. J Assist Reprod Genet. 1999;16:416–420. doi: 10.1023/A:1020565408018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devroey P, Bourgian C, Macklon N, Fauser B. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone-antagonist and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025–1029. doi: 10.1016/S0015-0282(02)03323-X. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro B, Daneshmand S, Garner F, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect difference in embryo-endometrium synchrony. Fertil Steril. 2008;89:20–26. doi: 10.1016/j.fertnstert.2006.08.092. [DOI] [PubMed] [Google Scholar]
- 7.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65:236–244. doi: 10.1016/j.theriogenology.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Balaban B, Urrman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–1982. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- 9.Kuleshova LL, Lopata A. Vitrification can be more favorable than slow cooling. Fertil Steril. 2002;78:449–454. doi: 10.1016/S0015-0282(02)03305-8. [DOI] [PubMed] [Google Scholar]
- 10.Liebermann J, Nawroth F, Isachenko V, Isachenko E, Rahimi G, Tucker MJ. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67:1671–1680. doi: 10.1095/biolreprod.102.006833. [DOI] [PubMed] [Google Scholar]
- 11.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12:779–796. doi: 10.1016/S1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 12.Van Royen E, Mangelschots K, De Neubourg D, Valkenburg M, Van de Meerssche M, Ryckaert G, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14:2345–2349. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 13.Kuwayama M, Vaita G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 14.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos, the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Van der Elst J, Van den Abbeel E, Vitrier S, Camus M, Devroey P, Van Steirteghem A. Selective transfer of cryopreserved human embryos with further cleavage after thawing increases delivery and implantation rates. Hum Reprod. 1997;12:1513–1521. doi: 10.1093/humrep/12.7.1513. [DOI] [PubMed] [Google Scholar]
- 16.Shaker AG, Zosmer A, Dean N, Bekir SJ, Jacobs HS, Tan S. Comparison of intravenous albumin and transfer of fresh embryos with cryopreservation of all embryos for subsequent transfer in prevention of ovarian hyperstimulation syndrome. Fertil Steril. 1996;65:992–996. doi: 10.1016/s0015-0282(16)58275-2. [DOI] [PubMed] [Google Scholar]
- 17.Ferraretti AP, Gianaroli L, Magli C, Selman HA, Feliciani E. Elective cryopreservation of all embryos of all pronucleate embryos in woman at risk of ovarian hyperstimulation syndrome efficiency and safety. Hum Reprod. 1999;14:1457–1460. doi: 10.1093/humrep/14.6.1457. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JE, Acosta AA, Hsiu JG, Jones HW., Jr Advanced endometrial maturation after ovulation induction with human menopausal gonadotropin/human chorionic gonadotropin for in vitro fertilization. Fertil Steril. 1984;41:31–35. doi: 10.1016/s0015-0282(16)47536-9. [DOI] [PubMed] [Google Scholar]
- 19.Acosta AA, Elberger L, Borghi M, Calamera JC, Chemes H, Doncel GF, et al. Endometrial dating and determination of the window of implantation in healthy fertile women. Fertil Steril. 2000;73:788–798. doi: 10.1016/S0015-0282(99)00605-6. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Lee K, Ng E, Yeung W, Ho P. Gene expression profiling of human peri-implantation endometrial between natural and stimulated cycles. Fertil Steril. 2008;90:2152–2164. doi: 10.1016/j.fertnstert.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Haouzi D, Assou S, Mahmoud K, Tondeur S, Rème T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–45. [DOI] [PMC free article] [PubMed]
- 23.Shapiro B, Daneshmand S, Garner F, Aguirre M, Thomas S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil Steril. 2008;90:302–309. doi: 10.1016/j.fertnstert.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 24.Guerif F, Bidault R, Cadoret V, Couet M, Lansac J, Royere D. Parameters guiding selection of best embryos for transfer after cryopreservation: a reappraisal. Hum Reprod. 2002;17:1321–1326. doi: 10.1093/humrep/17.5.1321. [DOI] [PubMed] [Google Scholar]
- 25.Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2007;368:39–57. doi: 10.1007/978-1-59745-362-2_3. [DOI] [PubMed] [Google Scholar]
- 26.Yavin S, Aroyo A, Roth Z, Arav A. Embryo cryopreservation in the presence of low concentration of vitrification solution with sealed pulled straws in liquid nitrogen slush. Hum Reprod. 2009;24:797–804. [DOI] [PubMed]
- 27.Takahashi K, Mukaida T, Goto T, Oka C. Perinatal outcome of blastocyst transfer with vitrification using cryoloop: a 4-year follow-up study. Fertil Steril. 2005;84:88–92. doi: 10.1016/j.fertnstert.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Rama Raju GA, Jaya Prakash G, Murali Krishna K, Madan K. Neonatal outcome after vitrified day 3 embryo transfers: a preliminary study. Fertil Steril. 2009;92:143–8. [DOI] [PubMed]
- 29.Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology. 2000;40:110–116. doi: 10.1006/cryo.1999.2227. [DOI] [PubMed] [Google Scholar]
- 30.Kyuwa S, Nishikawa T, Kaneko T, Nakashima T, Kawano K, Nakamura N, et al. Experimental evaluation of cross-contamination between cryotubes containing mouse 2-cell embryos and murine pathogens in liquid nitrogen tanks. Exp Anim. 2003;52:67–70. doi: 10.1538/expanim.52.67. [DOI] [PubMed] [Google Scholar]
- 31.Isachenko V, Montag M, Isachenko E, Vander Ven H. Universal aseptic technology of vitrification of human oocytes and embryos (VitAsep): test on the mouse biopsied pronuclear oocytes. Fertil Steril. 2005;84(Supp):S400. doi: 10.1016/j.fertnstert.2005.07.1045. [DOI] [Google Scholar]
- 32.Larman MG, Sheehan CB, Gardner DK. Vitrification of mouse pronuclear oocytes with no direct liquid nitrogen contact. Reprod Biomed Online. 2006;12:66–69. doi: 10.1016/S1472-6483(10)60982-0. [DOI] [PubMed] [Google Scholar]