Abstract
Purpose
To determine if diminished ovarian reserve (measured by maternal antimullerian hormone (AMH) levels), is associated with fetal aneuploidy (determined by prenatal karyotype).
Methods
This case-control study included 213 women with singleton pregnancies who underwent both serum aneuploidy screening and invasive prenatal diagnosis. 18 patients carrying an aneuploid fetus served as cases and the remaining 195 women with a euploid fetus were controls. Serum AMH was measured using two assays: AMHbc (Beckman-Coulter) and AMHdsl (Diagnostic Systems Laboratories). Karyotypes were determined by chorionic villus sampling or amniocentesis.
Results
AMHbc levels did not differ between women with an aneuploid fetus and women with a euploid fetus (p = 0.46) and did not predict aneuploidy (ROC Area = 0.57). Additionally, AMHbc values declined significantly with advancing gestational age.
Conclusions
Maternal AMH does not appear to be a marker of fetal aneuploidy in ongoing pregnancies. Contrary to previous reports, we found a significant decline in maternal AMH levels with advancing gestational age.
Keywords: Antimullerian hormone, Fetal aneuploidy, Ovarian reserve, Pregnancy
Introduction
Multiple diagnostic strategies, including follicle stimulating hormone (FSH), estradiol, and inhibin-B measured in the first few days of the menstrual cycle, have been used in an effort to identify women with diminished ovarian reserve [1, 2]. When these tests are abnormal, they often predict a poor response to infertility treatment. Anti-mullerian hormone (AMH), a homodimeric glycoprotein growth factor within the transforming growth factor-beta family, is synthesized by human granulosa cells in the ovary and has exhibited significant promise as a potential marker of ovarian reserve [2–5]. AMH appears to offer several advantages over other traditional markers of ovarian reserve. Studies suggest that AMH is constant throughout the menstrual cycle and is not influenced by gonadotropin-releasing hormone (GnRH) agonists, pregnancy, or oral contraceptive pills [3, 6–9]. As might be expected with a marker of ovarian reserve, serum AMH levels decline with age [10, 11].
From a clinical standpoint, it is unclear if diminished ovarian reserve, particularly in younger women, is also a proxy for egg quality. If such an association were true, then diminished ovarian reserve could impact a woman’s risk for fetal aneuploidy. Maternal nondisjunction during oogenesis leads to chromosomal abnormalities in the conceptus and is a significant cause of embryonic and fetal loss [12, 13]. The frequency of these oocyte-related chromosomal abnormalities increases with advancing maternal age [14–19]. This increase in aneuploid oocytes parallels the diminishing ovarian reserve, decreased fertility, and increased miscarriage rates that older women exhibit [20–22]. Questions remain, however, regarding a direct relationship between fetal aneuploidy and diminished ovarian reserve, independent of maternal age. In other words, does diminished egg quantity correlate with poor egg quality?
In some women, the ovarian aging process does not always correlate with chronologic age. Consequently, ovaries may be depleted of their oocytes at a rate faster than that which would be predicted by age alone. In these women who undergo accelerated ovarian aging, it is plausible that oocyte quality is also compromised. Our objective was to test the hypothesis that low maternal AMH levels (an indication of ovarian aging) are associated with an increased risk of fetal aneuploidy, independent of maternal age.
Materials and methods
Study design
This case-control study included all pregnant patients with singleton pregnancies who underwent both maternal serum screening between 11 and 24 weeks of gestation and subsequent invasive prenatal diagnosis between 2004 and 2007 at the University of North Carolina (UNC). During this time period, women were offered first or second trimester serum screening. First trimester screening included: pregnancy-associated plasma protein A (PAPP-A) and beta-human chorionic gonadotropin (beta-hCG). Second trimester screening included: maternal serum alpha-fetoprotein (MSAFP), hCG, unconjugated estriol, and inhibin-A. The results were immediately available to the patient and the ordering practitioner. The unused residual portion of serum was stored at −80°C for research purposes. Invasive prenatal diagnosis was performed via amniocentesis or chorionic villus sampling (CVS) at the discretion of the patient and her healthcare providers. Prenatal karyotypes were performed through the UNC Cytogenetics Laboratory.
Subjects were identified through the Prenatal Diagnosis Program at UNC. To be included in our study, a subject must have: (1) undergone serum screening for fetal aneuploidy and CVS or amniocentesis at the UNC Prenatal Diagnosis Center between 2004 and 2007, (2) have sufficient stored serum available, (3) have available cytogenetic results from CVS or amniocentesis, and (4) have a singleton pregnancy. There were no exclusions based on maternal age. Within the study population, cases and controls were identified according to the results of their fetal karyotypes. Those patients with normal fetal karyotypes were considered controls, and those with abnormal (aneuploid) karyotypes were considered cases. A retrospective chart review was performed on all cases and controls to collect demographic, clinical, laboratory, and karyotypic data. Institutional Review Board approval was obtained for this research at the University of North Carolina at Chapel Hill.
AMH assays
Each patient’s serum AMH level, serving as a marker of ovarian reserve, was measured as the exposure of interest. Serum stored in small aliquots at −80°C was thawed once and used for the assays. AMH stability during long-term freezer storage has previously been demonstrated [10]. Serum AMH was quantified in duplicate using two different assays: AMHbc (Immunotech, Beckman-Coulter) and AMHdsl (Diagnostic Systems Laboratories). Both assays are standard dual monoclonal antibody sandwich enzyme immunoassays which are specific for AMH and do not exhibit any significant cross-reactivity with related molecules. AMHbc’s lower limit of sensitivity was 0.7 pM. Intra-assay and interassay coefficients of variation for AMHbc were 4% and 9%, respectively. AMHdsl’s lower limit of sensitivity was 0.017 ng/mL. Intra-assay and interassay coefficients of variation for AMHdsl were 3% and 5%, respectively. AMHbc and AMHdsl levels were significantly correlated (R = 0.68, p < 0.001).
Statistical analysis
Our power analysis was determined by the anticipated prevalence of fetal aneuploidy in our sample and the mean AMH levels in the normal and abnormal groups. Assuming AMH levels would differ, we used means in infertile women who did not conceive (1.1 ± 1.6 ng/mL) and means in infertile women who did conceive (2.4 ± 2.9 ng/mL) as described in the Hazout et al. study to calculate sample size [2]. We assumed an aneuploidy prevalence of 10% in this high-risk population of women who had undergone both serum screening and invasive prenatal diagnosis based on our pre-study analysis. We needed 160 patients in the euploid group and 16 patients in the aneuploid group to have 80% power to detect a 1.3 ng/mL difference between the two groups at a significance level of 0.05.
Commercial statistical software (STATA 10.0; College Station, TX) was used for the statistical analysis. Nonparametric and parametric tests were performed as appropriate for the entire study population and according to fetal karyotype status (cases versus controls). Baseline characteristics including demographic, clinical, and laboratory features, were compared between cases and controls with either a t test for continuous data or chi2 test for categorical data. A normal distribution of AMH values was achieved by square root transformation. Logistic regression analysis was used to adjust for maternal age, gestational age at blood draw, maternal weight, and smoking. A two-sided P value less than 0.05 was considered significant.
In a sensitivity analysis, the above analysis was repeated and only fetal trisomies were considered abnormal. Subsequent AMH means were compared using non-parametric tests and logistic regression analysis with square root-transformed AMH used to adjust for confounders.
Results
Maternal demographics in the overall study cohort (N = 213) revealed a mean age of 32.9 ± 6.9 years, 61% were parous, 71% were privately insured, 85% were non-smokers and the mean weight was 156.1 ± 39.6 pounds. Four patients had a history of fetal aneuploidy in the past. 66% of patients had an abnormal serum screen for aneuploidy. The mean AMHbc level for the entire cohort was 15.0 ± 13.2 pM, and the mean AMHdsl level was 1.67 ± 1.30 ng/mL. A total of eighteen patients with an aneuploid fetus were identified as cases, and the remaining 195 women with a euploid fetus were selected as controls. The following aneuploidies were found in the study group: trisomy 21 (N = 10), trisomy 18 (N = 3), and various other chromosomal rearrangements/inversions (N = 5). Demographic characteristics of the cases and controls are summarized in Table 1.
Table 1.
Demographic characteristics
| Characteristic | Controls (N = 195) | Cases (N = 18) | P-value |
|---|---|---|---|
| Maternal age (years) | 33.0 (±6.9) | 31.4 (7.3) | 0.348 |
| Gestational age (weeks)a | 14.3 (±2.65) | 15.1 (3.13) | 0.277 |
| Race/Ethnicity | 0.072 | ||
| White | 114 (59%) | 5 (28%) | |
| Black | 34 (17%) | 5 (28%) | |
| Hispanic | 18 (9%) | 4 (22%) | |
| Other | 29 (15%) | 4 (22%) | |
| Maternal weight (pounds) | 155.2 (±37.7) | 165.3 (56.7) | 0.300 |
| Primagravida | 45 (23%) | 1 (6%) | 0.084 |
| Nulliparous | 79 (41%) | 4 (22%) | 0.128 |
| Current Smoker | 30 (16%) | 2 (11%) | 0.622 |
| Uninsured | 15 (8%) | 2 (11%) | 0.865 |
| Abnormal serum screen | 124 (65%) | 15 (83%) | 0.120 |
Results are presented as mean (±standard deviation) or n (%)
aGestational age at the time of serum screening
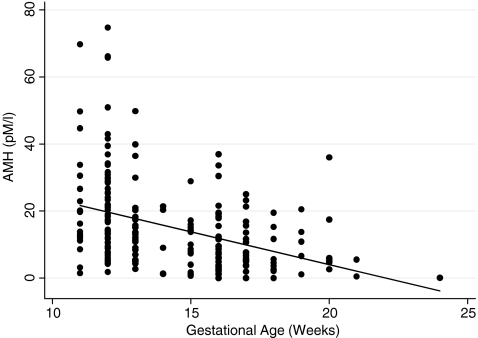
To determine if we should adjust for gestational age, a bivariate analysis was conducted to determine the relationship between AMH and gestational age. AMHbc values declined significantly with advancing gestational age (p < 0.001) (Fig. 1). AMHbc values were 20.2 ± 14.9 pM in the first trimester and 9.7 ± 8.4 pM in the second trimester. This trend was not seen with AMHdsl, however (p = 0.46).
Fig. 1.
AMHbc values declined significantly with advancing gestational age (p < 0.001)
In terms of our primary question, mean AMHbc levels did not differ significantly between women with a euploid fetus (15.2 ± 13.3 pM) and an aneuploid fetus (13.0 ± 12.6 pM, p = 0.46), and AMHbc levels did not predict aneuploidy (ROCArea = 0.57). Similarly, there was no detectable difference in AMHdsl values in euploid (1.66 ± 1.31 ng/mL) and aneuploid pregnancies (1.73 ± 1.25 p = 0.81). Mean AMHbc values did not differ between women with positive maternal serum screens and women with negative screens (15.6 ± 14.2 pM vs. 14.4 ± 11.0 pM, p = 0.61). In multivariate analysis, adjusting for maternal age, maternal weight, gestational age, and smoking did not alter the results between AMH and fetal aneuploidy.
When only trisomy pregnancies were considered as aneuploid, mean AMHbc levels did not differ significantly between women with a euploid fetus (15.0 ± 13.2 pM) and an aneuploid fetus (14.2 ± 14.2 pM, p = 0.55), and AMHbc levels did not predict aneuploidy (ROCArea = 0.55). Similarly, there was no detectable difference in AMHdsl values in euploid (1.68 ± 1.31 ng/mL) and aneuploid pregnancies (1.48 ± 1.23, p = 0.60). In multivariate analysis with fetal trisomy as the outcome, adjusting for maternal age, maternal weight, gestational age, and smoking did not alter the results.
Discussion
In this study, maternal AMH was not a marker of fetal aneuploidy in ongoing pregnancies. Multiple investigators have explored the possible link between diminished ovarian reserve and fetal aneuploidy [14, 23–31]. The results of these studies are mixed, but several of them suggest an association between elevated basal FSH levels and fetal aneuploidy that might not be predicted by age alone [23, 24, 26, 30]. Because elevated FSH levels are often a late sign of diminished ovarian reserve, we hypothesized that an earlier, more sensitive marker of diminished ovarian reserve might be more clearly associated with fetal aneuploidy [10, 11].
Several studies have already highlighted AMH’s potential as an early marker of ovarian reserve [2, 32–34]. To our knowledge, there is only one study that has examined AMH’s potential as predictor of fetal aneuploidy [31]. This study did not find an association between maternal AMH levels and Down syndrome. It is unclear to us, however, whether their cases were identified through invasive prenatal testing or by use of a Down syndrome birth registry that only included children born with Down syndrome and would not have taken into account the pregnancies that were terminated prematurely once a diagnosis of Down syndrome was made. Additionally, this study focused on Down syndrome alone and did not explore any association between low AMH levels and other types of fetal aneuploidy.
Our case-control study identified aneuploid fetuses that were diagnosed during pregnancy. Consequently, we were able to investigate many pregnancies that were ultimately electively terminated. In addition, we included all abnormal karyotypes, instead of limiting our sample to Down syndrome alone. Finally, our results were confirmed with two different AMH assays, thus eliminating the possibility that our choice of assay influenced our results. Despite some differences in study design, our findings were consistent with previous work and lend further strength to the conclusion that there is no association between low AMH levels and fetal aneuploidy. In addition, a recent study explored the relationship between AMH levels and embryo quality [35]. Embryo quality was assessed by both an embryo morphology score and preimplantation genetic screening. This study found that AMH levels did not correlate with either embryo morphology or embryo aneuploidy and concluded that there is not a direct relationship between egg quantity and embryo quality.
While AMH levels may not correlate with egg quality, it is also possible that women with the lowest AMH levels experience early pregnancy loss or do not conceive at all. In our study, the mean AMH levels for both cases and controls were well within the normal range and may reflect the fact that all of our patients had conceived and sustained a pregnancy to at least eleven weeks. Our findings suggest that once an ongoing pregnancy has been established, there is no difference in maternal AMH levels between aneuploid and euploid pregnancies. Consequently, AMH is unlikely to serve as a useful serum marker for fetal aneuploidy.
As previously discussed, AMH appears to offer several potential advantages over other tests of ovarian reserve. One of the touted benefits of AMH is its stability in the non-pregnant, pregnant, and postpartum states [8, 36]. During pregnancy, maternal AMH appears to be derived solely from maternal follicles, without any fetal contribution, thus implying that AMH is unlikely to cross or be produced by the placenta [36]. However, we found a significant decline (over 50% decline from the first to second trimester) in maternal AMHbc values with advancing gestational age. Similarly, a recent longitudinal study (using the DSL assay) found that circulating AMH levels decline during the second and third trimesters of pregnancy [37]. Our cross-sectional findings, in conjunction with the recent longitudinal study, suggest that AMH values are more dynamic in pregnancy than previously thought.
The first study of AMH in pregnancy, a cross-sectional study including fewer than 30 patients in each group (nonpregnant, first, second, and third trimester, and early postpartum) found no significant differences in AMH levels among their groups using the Immunotech assay [8]. Our findings using the same assay differed considerably. It is possible that geographic differences in weight gain during pregnancy may account for the discrepancy between the two studies. Obese women appear to have lower AMH levels than non-obese women [37, 38]. Significant weight gain or changes in intravascular volume during pregnancy may, in part, account for the decline in AMH levels that we observed. Adjusting for maternal age and weight, however, did not weaken the association between advancing gestational age and declining AMHbc values.
Despite the dramatic decline in AMHbc levels with advancing gestational age, AMHdsl values did not follow the same pattern. The range for the AMHbc assay in our pregnant cohort (0–74.65 pM) was much broader than that of the AMHdsl assay (0–6.32 ng/mL), which may in part explain this inconsistency. One of the only other studies to directly compare the two assays also found a linear relationship between the assays, but found that they differed in prognostic value [39]. AMHbc assay values could be used to distinguish between pregnant and non-pregnant groups, while the AMHdsl could not.
While our results do not suggest that there is an association between low AMH values and aneuploidy, we cannot eliminate the possibility of a Type II error. Based on our initial power analysis, we needed 160 patients in the euploid group and 16 patients in the aneuploid group to have 80% power to detect a 1.3 ng/mL difference in AMHbc means between the two groups at a significance level of 0.05. With these assumptions, our actual cohort should have had 85% power to detect a difference in AMH values between the cases and controls. Because the actual AMHbc means for the cases and controls were much more similar than we had anticipated, we would have needed over 260 patients in the aneuploid group and over 2,600 patients in the normal group in order to have 80% power to demonstrate that the means were actually different at a significance level of 0.05. While it is possible that our sample size was underpowered, our findings are consistent with other available literature and do not even show a trend to suggest that there is a direct association between maternal AMH and embryonic or fetal aneuploidy.
Conclusions
In summary, maternal AMH does not appear to be a marker of fetal aneuploidy in ongoing pregnancies. A possible association between low AMH levels and early pregnancy loss warrants further investigation, however. The relationship between egg quantity and egg quality in younger women remains elusive, and alternative measures of oocyte quality are clearly necessary. In addition, AMHbc values declined with advancing gestational age in our cohort, thus challenging the notion that AMH levels do not change during pregnancy. Larger cohort studies are needed to further characterize the dynamic changes in AMH values both before and during pregnancy.
Acknowledgements
We thank Beckman Coulter for donating the Immunotech AMH assay kits used for this project.
Funding 1. University of North Carolina Medical Alumni Endowment Fund Grant2. University of North Carolina Women’s Reproductive HealthResearch (WRHR) Program, 5K12HD050113 (NICHD)
Conflicts of interest None
Footnotes
IRB approval: Protocol approved by the Institutional Review Board at the University of North Carolina at Chapel Hill
Capsule
Maternal antimullerian hormone is not a marker of fetal aneuploidy. Contrary to previous reports, antimullerian hormone levels in pregnancy decline significantly with advancing gestational age.
References
- 1.Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve—should we perform tests of ovarian reserve routinely? Hum Reprod. 2006;21(11):2729–35. doi: 10.1093/humrep/del188. [DOI] [PubMed] [Google Scholar]
- 2.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82(5):1323–9. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–46. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Josso N, Picard JY, Rey R, Clemente N. Testicular anti-Mullerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev. 2006;3(4):347–58. [PubMed] [Google Scholar]
- 5.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2008 Nov 29. [DOI] [PubMed]
- 6.Hehenkamp WJ, Looman CW, Themmen AP, Jong FH, Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 7.Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Mullerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003;18(2):328–32. doi: 10.1093/humrep/deg043. [DOI] [PubMed] [Google Scholar]
- 8.Marca A, Giulini S, Orvieto R, Leo V, Volpe A. Anti-Mullerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20(6):1569–72. doi: 10.1093/humrep/deh819. [DOI] [PubMed] [Google Scholar]
- 9.Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134(2):196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Vet A, Laven JS, Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–62. doi: 10.1016/S0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 11.Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Mattei JF, Mattei MG, Ayme S, Giraud F. Origin of the extra chromosome in trisomy 21. Hum Genet. 1979;46(1):107–10. doi: 10.1007/BF00278908. [DOI] [PubMed] [Google Scholar]
- 13.Boue J, Bou A, Lazar P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology. 1975;12(1):11–26. doi: 10.1002/tera.1420120103. [DOI] [PubMed] [Google Scholar]
- 14.Thum MY, Abdalla HI, Taylor D. Relationship between women’s age and basal follicle-stimulating hormone levels with aneuploidy risk in in vitro fertilization treatment. Fertil Steril. 2007 Oct 20. [DOI] [PubMed]
- 15.Angell R. First-meiotic-division nondisjunction in human oocytes. Am J Hum Genet. 1997;61(1):23–32. doi: 10.1086/513890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angell R. Mechanism of chromosome nondisjunction in human oocytes. Prog Clin Biol Res. 1995;393:13–26. [PubMed] [Google Scholar]
- 17.Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112(2):195–203. doi: 10.1007/s00439-002-0852-x. [DOI] [PubMed] [Google Scholar]
- 18.Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6, 733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online. 2003;6(1):54–9. doi: 10.1016/S1472-6483(10)62055-X. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji K, Nakano R. Chromosome studies of embryos from induced abortions in pregnant women age 35 and over. Obstet Gynecol. 1978;52(5):542–4. [PubMed] [Google Scholar]
- 20.Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 21.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70(1):11–7. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 22.Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233(4771):1389–94. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 23.Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB. Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril. 1999;71(4):715–8. doi: 10.1016/S0015-0282(98)00525-1. [DOI] [PubMed] [Google Scholar]
- 24.Montfrans JM, Dorland M, Oosterhuis GJ, Vugt JM, Rekers-Mombarg LT, Lambalk CB. Increased concentrations of follicle-stimulating hormone in mothers of children with Down’s syndrome. Lancet. 1999;353(9167):1853–4. doi: 10.1016/S0140-6736(99)00936-8. [DOI] [PubMed] [Google Scholar]
- 25.Freeman SB, Yang Q, Allran K, Taft LF, Sherman SL. Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am J Hum Genet. 2000;66(5):1680–3. doi: 10.1086/302907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montfrans JM, Hooff MH, Martens F, Lambalk CB. Basal FSH, estradiol and inhibin B concentrations in women with a previous Down’s syndrome affected pregnancy. Hum Reprod. 2002;17(1):44–7. doi: 10.1093/humrep/17.1.44. [DOI] [PubMed] [Google Scholar]
- 27.Kline J, Kinney A, Reuss ML, Kelly A, Levin B, Ferin M, et al. Trisomic pregnancy and the oocyte pool. Hum Reprod. 2004;19(7):1633–43. doi: 10.1093/humrep/deh310. [DOI] [PubMed] [Google Scholar]
- 28.Massie JA, Burney RO, Milki AA, Westphal LM, Lathi RB. Basal follicle-stimulating hormone as a predictor of fetal aneuploidy. Fertil Steril. 2008;90(6):2351–5. doi: 10.1016/j.fertnstert.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Weghofer A, Barad D, Li J, Gleicher N. Aneuploidy rates in embryos from women with prematurely declining ovarian function: a pilot study. Fertil Steril. 2007;88(1):90–4. doi: 10.1016/j.fertnstert.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 30.Montfrans JM, Lambalk CB, Hooff MH, Vugt JM. Are elevated FSH concentrations in the pre-conceptional period a risk factor for Down’s syndrome pregnancies? Hum Reprod. 2001;16(6):1270–3. doi: 10.1093/humrep/16.6.1270. [DOI] [PubMed] [Google Scholar]
- 31.Seifer DB, MacLaughlin DT, Cuckle HS. Serum mullerian-inhibiting substance in Down’s syndrome pregnancies. Hum Reprod. 2007;22(4):1017–20. doi: 10.1093/humrep/del497. [DOI] [PubMed] [Google Scholar]
- 32.Penarrubia J, Fabregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—gonadotropin treatment. Hum Reprod. 2005;20(4):915–22. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 33.Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20(11):3178–83. doi: 10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- 34.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022–6. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 35.Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, et al. Anti-Mullerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online. 2008;16(5):664–70. doi: 10.1016/S1472-6483(10)60480-4. [DOI] [PubMed] [Google Scholar]
- 36.Lutterodt M, Byskov AG, Skouby SO, Tabor A, Yding Andersen C. Anti-Mullerian hormone in pregnant women in relation to other hormones, fetal sex and in circulation of second trimester fetuses. Reprod Biomed Online. 2009;18(5):694–9. doi: 10.1016/S1472-6483(10)60016-8. [DOI] [PubMed] [Google Scholar]
- 37.Nelson SM, Stewart F, Fleming R, Freeman DJ. Longitudinal assessment of antimullerian hormone during pregnancy-relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril. 2009 Sep 30. [DOI] [PubMed]
- 38.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87(1):101–6. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 39.Freour T, Mirallie S, Bach-Ngohou K, Denis M, Barriere P, Masson D. Measurement of serum anti-Mullerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375(1–2):162–4. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]