Abstract
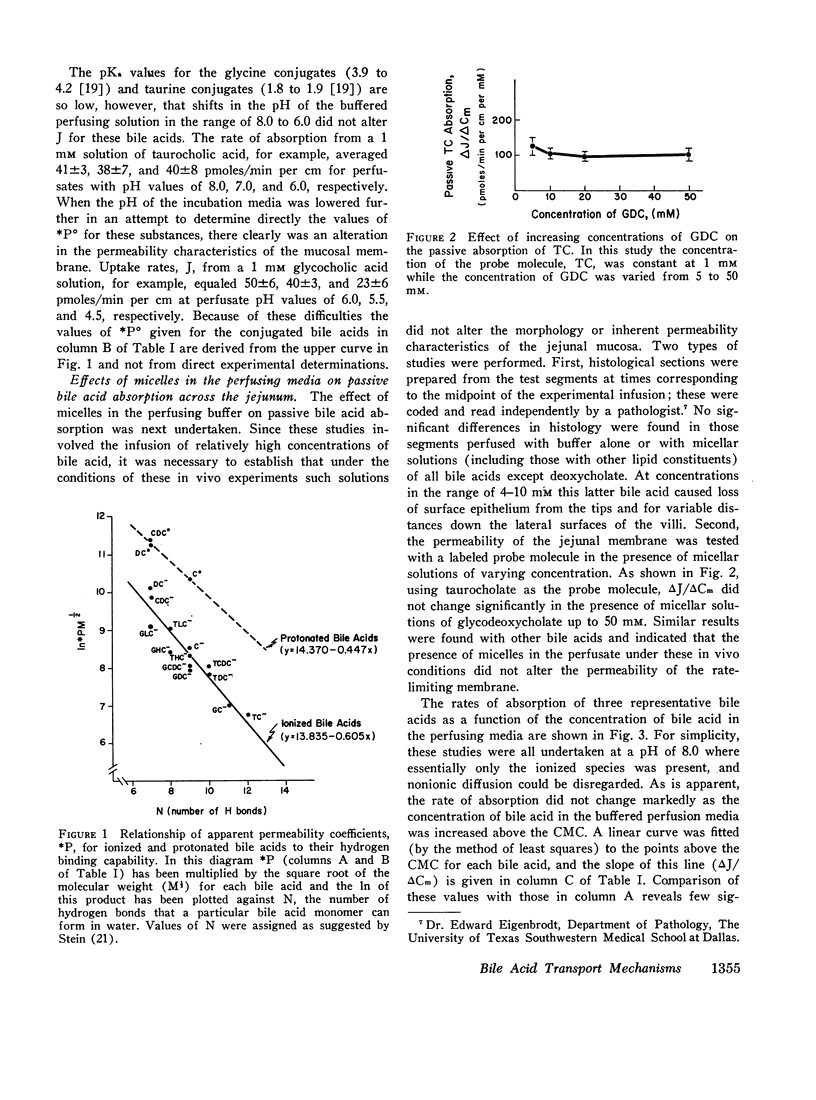
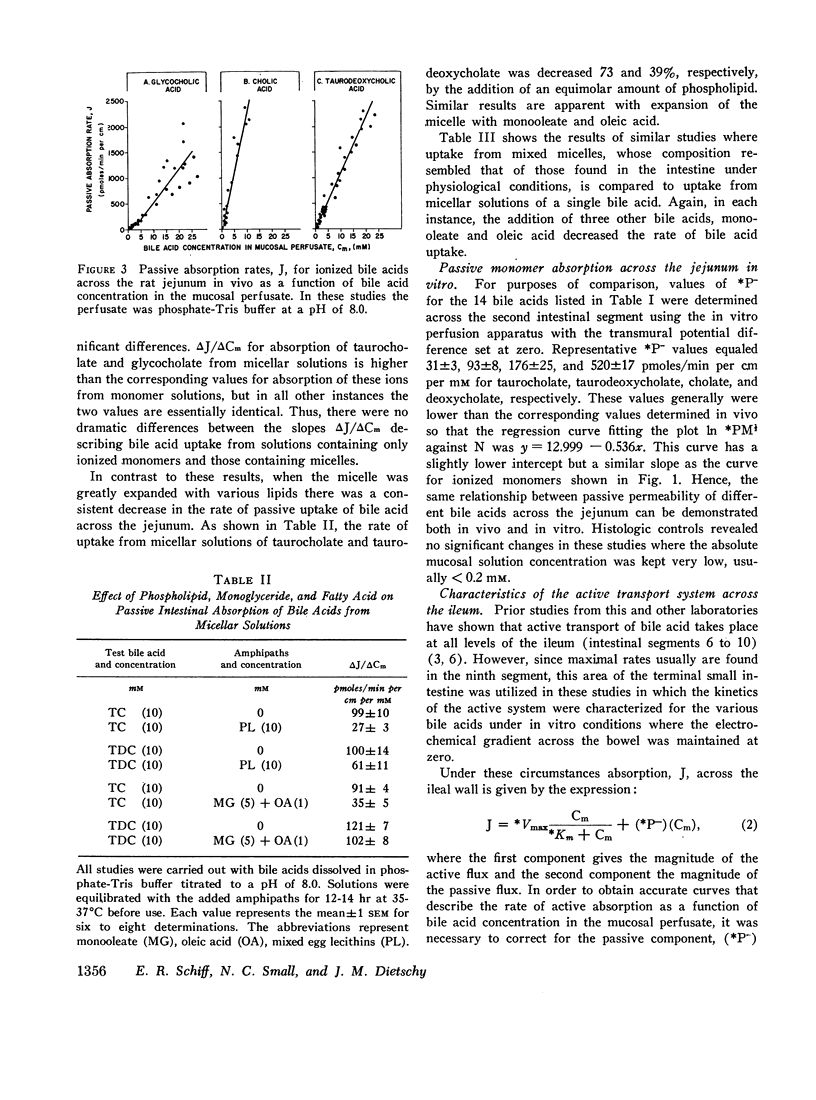
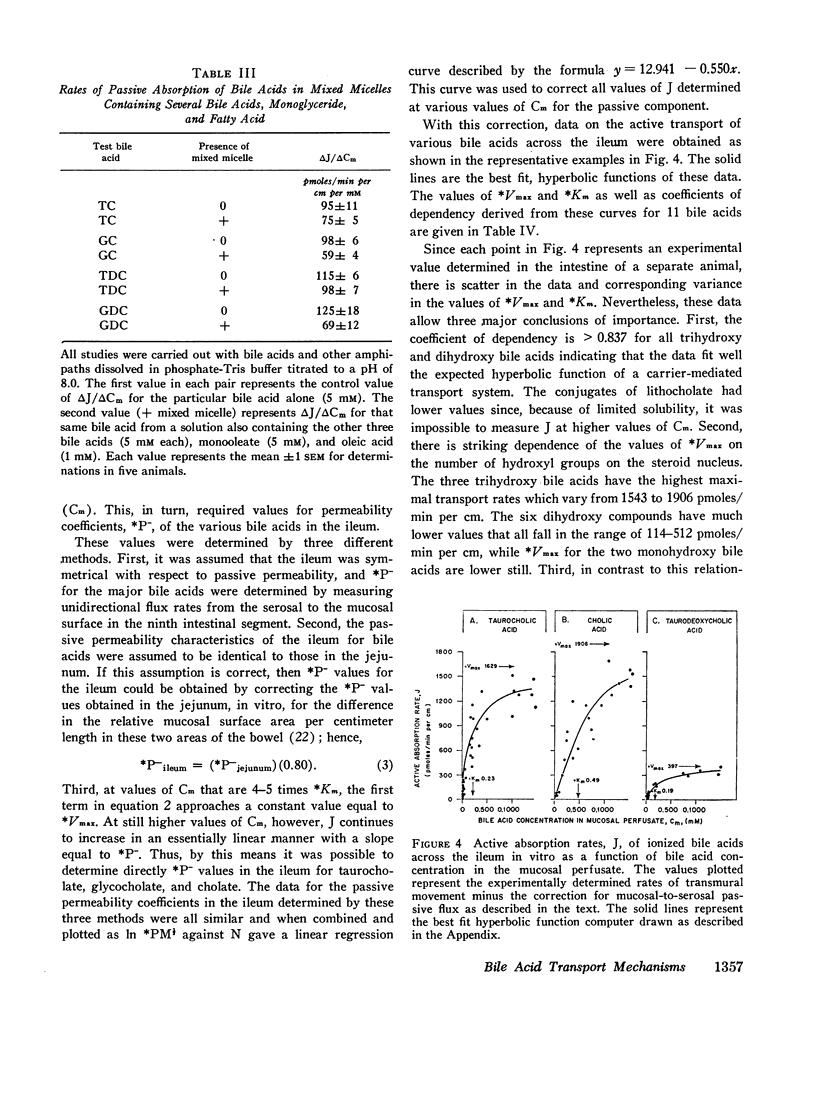
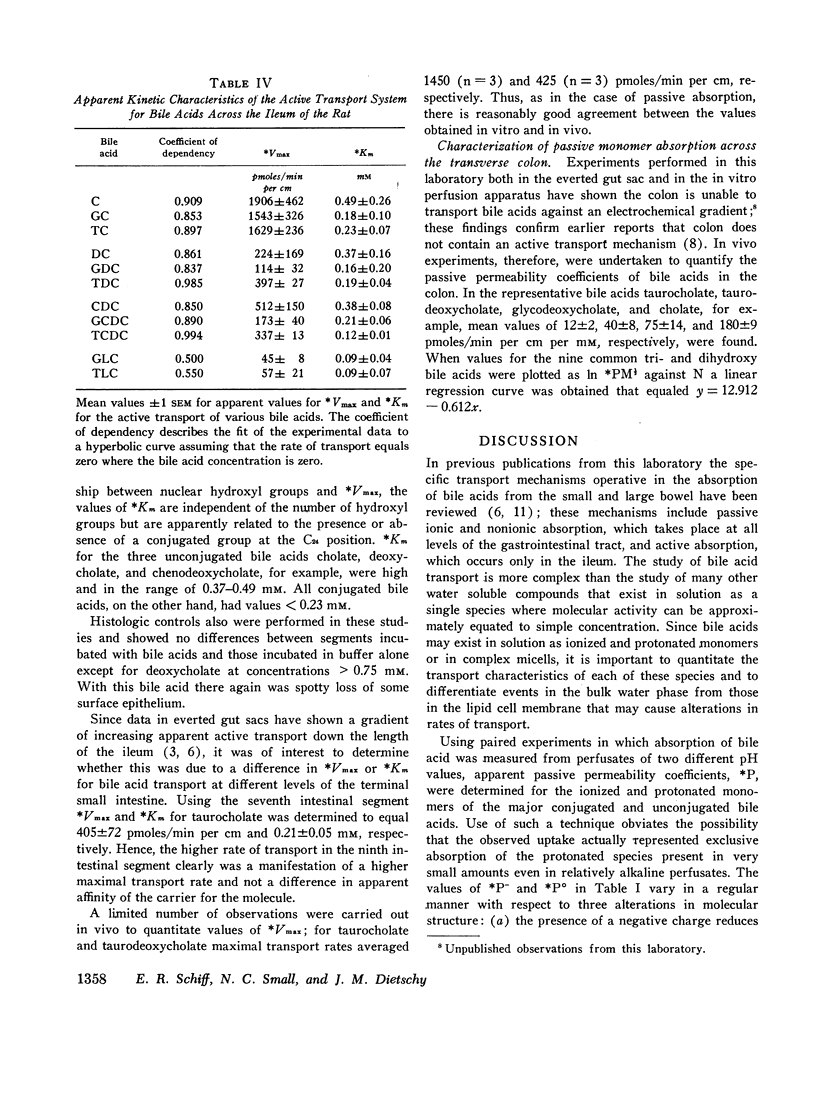
Bile acid uptake occurs via passive diffusion in all regions of the intestine and via active absorption in the ileum. Determination of the passive permeability coefficient for ionized monomers (*P-) demonstrated that permeability decreased by a factor of 3.4, 6.8, and 8.1 for the addition of a hydroxyl, glycine, or taurine group, respectively, to the steroid nucleus. Removal of the negative charge increased permeation by a factor of 4.4; however, permeability coefficients for the protonated monomers showed the same relative decrease with addition of a hydroxyl group. The calculated incremental free energies of solution (δΔFW→1) associated with these additions equaled + 757 (hydroxyl), + 1178 (glycine), and + 1291 (taurine) cal/mole. Passive permeability coefficients for the transverse colon showed the same relative relationships among the various bile acids. After making appropriate corrections for passive permeability across the ileum, apparent values for the maximal transport velocity (*Vmax) and Michaelis constant (*Km) of the active transport system were measured. *Vmax depended upon the number of hydroxyl groups on the steroid nucleus; values for the trihydroxy bile acids were high (1543-1906 pmoles/min per cm) while those for the dihydroxy (114-512 pmoles/min per cm) and monohydroxy (45-57 pmoles/min per cm) acids were lower. In contrast, *Km values were related to whether the bile acid was conjugated; unconjugated bile acids had values ranging from 0.37 to 0.49 mM, while values for the conjugated bile acids were approximately half as high (0.12-0.23 mM).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyne R., Fell B. F., Robb I. The surface area of the intestinal mucosa in the lactating rat. J Physiol. 1966 Apr;183(3):570–575. doi: 10.1113/jphysiol.1966.sp007884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M. Effects of bile salts on intermediate metabolism of the intestinal mucosa. Fed Proc. 1967 Nov-Dec;26(6):1589–1598. [PubMed] [Google Scholar]
- Dietschy J. M. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968 May;9(3):297–309. [PubMed] [Google Scholar]
- Dietschy J. M., Salomon H. S., Siperstein M. D. Bile acid metabolism. I. Studies on the mechanisms of intestinal transport. J Clin Invest. 1966 Jun;45(6):832–846. doi: 10.1172/JCI105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg J. A. New solvent systems for thin-layer chromatography of bile acids. J Lipid Res. 1966 Jul;7(4):579–581. [PubMed] [Google Scholar]
- HOLT P. R. INTESTINAL ABSORPTION OF BILE SALTS IN THE RAT. Am J Physiol. 1964 Jul;207:1–7. doi: 10.1152/ajplegacy.1964.207.1.1. [DOI] [PubMed] [Google Scholar]
- Heaton K. W., Lack L. Ileal bile salt transport: mutual inhibition in an in vivo system. Am J Physiol. 1968 Mar;214(3):585–590. doi: 10.1152/ajplegacy.1968.214.3.585. [DOI] [PubMed] [Google Scholar]
- LACK L., WEINER I. M. In vitro absorption of bile salts by small intestine of rats and guinea pigs. Am J Physiol. 1961 Feb;200:313–317. doi: 10.1152/ajplegacy.1961.200.2.313. [DOI] [PubMed] [Google Scholar]
- Lack L., Weiner I. M. Intestinal bile salt transport: structure-activity relationships and other properties. Am J Physiol. 1966 May;210(5):1142–1152. doi: 10.1152/ajplegacy.1966.210.5.1142. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Schneider R. E., Dobbins W. O. Morphological changes of the small-intestinal mucosa of guinea pig and hamster following incubation in vitro and perfusion in vivo with unconjugated bile salts. Gut. 1970 Jun;11(6):486–492. doi: 10.1136/gut.11.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUNTER L. A., TURNER M. E. The evaluation of Michaelis constants and maximal velocity kinetic studies of enzymic reactions. Enzymologia. 1963 Feb 15;25:225–230. [PubMed] [Google Scholar]
- PARKINSON T. M., OLSON J. A. Inhibitory effects of bile acids on the uptake, metabolism, and transport of water-soluble substances in the small intestine of the rat. Life Sci. 1963 Jun;6:393–398. doi: 10.1016/0024-3205(63)90123-1. [DOI] [PubMed] [Google Scholar]
- PLAYOUST M. R., ISSELBACHER K. J. STUDIES ON THE TRANSPORT AND METABOLISM OF CONJUGATED BILE SALTS BY INTESTINAL MUCOSA. J Clin Invest. 1964 Mar;43:467–476. doi: 10.1172/JCI104932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN M. F. BILE SALT ABSORPTION IN THE IRRADIATED RAT. Am J Physiol. 1965 Jul;209:158–164. doi: 10.1152/ajplegacy.1965.209.1.158. [DOI] [PubMed] [Google Scholar]
- TIDBALL C. S. INTESTINAL AND HEPATIC TRANSPORT OF CHOLATE AND ORGANIC DYES. Am J Physiol. 1964 Jan;206:239–242. doi: 10.1152/ajplegacy.1964.206.1.239. [DOI] [PubMed] [Google Scholar]
- Webling D. D. The site of absorption of taurocholate in chicks, using polyethylene glycol as a reference substance. Aust J Exp Biol Med Sci. 1966 Feb;44(1):101–104. doi: 10.1038/icb.1966.11. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Sallee V. L., Dietschy J. M. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science. 1971 Dec 3;174(4013):1031–1033. doi: 10.1126/science.174.4013.1031. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Patterns of non-electrolyte permeability. Proc R Soc Lond B Biol Sci. 1969 Mar 18;171(1028):227–271. doi: 10.1098/rspb.1969.0021. [DOI] [PubMed] [Google Scholar]



