Abstract
Objective
To determine: 1) if periodontal treatment in pregnant women before 21 weeks of gestation alters levels of inflammatory mediators in serum; and 2) if changes in these mediators are associated with birth outcomes.
Methods
823 pregnant women with periodontitis were randomly assigned to receive scaling and root planing before 21 weeks of gestation or after delivery. Serum obtained between 13 weeks and 16 weeks 6 days (study baseline) and 29–32 weeks gestation was analyzed for C-reactive protein, prostaglandin E2, matrix metalloproteinase-9, fibrinogen, endotoxin, interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α. Cox regression, multiple linear regression, t-tests, chi-square and Fisher’s exact tests were used to examine associations between the biomarkers, periodontal treatment, and gestational age at delivery and birthweight.
Results
796 women had baseline serum data; 620 had baseline and follow-up serum and birth data. Periodontal treatment did not significantly alter the level of any biomarker (P>0.05). Neither baseline levels nor change from baseline in any biomarker was significantly associated with preterm birth or infant birthweight (P>0.05). In treatment subjects, change in endotoxin was negatively associated with change in probing depth (P<0.05).
Conclusions
Non-surgical mechanical periodontal treatment in pregnant women delivered before 21 weeks of gestation did not reduce systemic (serum) markers of inflammation. In pregnant women with periodontitis, levels of these markers at 13–17 weeks and 29–32 weeks gestation were not associated with risk for preterm birth or with infant birthweight.
Keywords: Pregnancy, preterm birth, periodontitis, inflammation, cytokines
INTRODUCTION
Preterm birth is a growing problem worldwide, and has been an intractable one in the US over the last two decades. Nearly 13% of all births in the US occur before 37 weeks of gestation1, and preterm birth is the leading cause of perinatal morbidity and mortality, with costs estimated at $26 billion a year.2
Maternal infection and inflammation have long been thought to contribute to poor pregnancy outcomes. Over 50 years ago, investigators demonstrated that administration of Shigella and Salmonella endotoxin was capable of inducing abortions in monkeys.3 Porphyromonas gingivalis lipopolysaccharide and heat-killed whole bacterium are also known to cause fetal resorption and growth restriction in rodents.4, 5 While the associations between preterm birth and infections such as chorioamnionitis, bacterial vaginosis, and urinary tract infections are well-established, there also is evidence that “distant” infections, including periodontal disease, may also increase a woman’s risk for preterm birth. 3, 6
Collectively, case-control and cohort studies indicate that women with periodontitis are about two to three times more likely than healthy women to experience preterm birth or deliver a low birthweight infant. 7, 8 Whether periodontitis is causally associated with adverse pregnancy outcomes or is merely a marker for other risk factors or behaviors, however, continues to be debated.9 Periodontitis and adverse pregnancy outcomes may be linked through a chronic, systemic inflammatory challenge to the mother and fetus in response to pathogens in the mother’s oral cavity. Alternatively, these pathogens may affect the uterus more directly through repeated bacteremias with periodontitis-associated microbial species. Cytokines, which are capable of eliciting the acute phase response, are a component of this inflammatory response and are classified as: 1) pro-inflammatory, which initiate or enhance a cascade of events (e.g., tumor necrosis factor-α, interleukin (IL)-1); 2) IL-6-like (e.g., IL-6, IL-11), which propagate many of the systemic manifestations of acute phase responses; or 3) anti-inflammatory, which downregulate the acute phase response (e.g,. IL-10, transforming growth factor-β).10, 11
Because of the associations between infection, inflammation and preterm birth, researchers have attempted to identify inflammatory biomarkers that predict preterm labor and delivery. Depending on the timing and source of the sample (maternal serum, amniotic fluid, or vaginal swabs), numerous classical markers of inflammation, including white blood cell counts, C-reactive protein, IL-1β, IL-6, IL-8 and alkaline phosphatase, have been variably associated with preterm labor and/or delivery.12, 13 In general, the utility of these markers to predict preterm birth is improved when combined with cervical length measures.
Elsewhere we reported that scaling and root planing in pregnant women with periodontitis, delivered before 21 weeks of gestation, was not associated with improvements in preterm birth or birthweight.14 This paper examines the effect of periodontal therapy on selected serum biomarkers and the relationship between these markers and birth outcomes in these same pregnant women.
MATERIALS & METHODS
The design of the interventional study has been described in more detail elsewhere.14 Briefly, 823 pregnant women with periodontitis were enrolled between March 2003 and June 2005 at four clinical centers. Women were enrolled between 13 weeks and 16 weeks 6 days of gestation and randomly assigned to receive scaling and root planing before 21 weeks gestation, followed by monthly periodontal maintenance (treatment group), or to receive scaling and root planing after delivery (control group). Women were ineligible if they had multiple fetuses, required antibiotic prophylaxis prior to dental treatment, had a medical condition that precluded elective dental treatment, had extensive tooth decay, or were likely to have less than 20 remaining teeth after treatment of tooth decay, abscesses, or other non-periodontal pathoses. Serum samples were obtained from women at baseline prior to dental care (13 weeks to 16 weeks 6 days gestation) and following study dental care at 29–32 weeks. Samples were stored at −80°C in aliquots and shipped in batches on dry ice via overnight courier to a central laboratory. None of the study women who were lost to follow-up (N=7), who withdrew consent (N=2), or who had an elective abortion (N=2) provided a 29–32 week serum sample.
Calibrated and blinded examiners (9 across four clinical sites) measured probing depth (PD), the distance from the gingival margin to the cementoenamel junction (GM-CEJ), and bleeding on probing (BOP) at 6 sites on all teeth excluding third molars. Clinical attachment loss (CAL) was calculated from the PD and GM-CEJ measures. BOP was scored as present or absent.
The clinical results of the trial have been reported elsewhere.14 In brief, control and treatment groups did not differ significantly (P > 0.1) at baseline in terms of their mean number of natural teeth (26.8 versus 26.7) or percentage of tooth sites with PD >= 4mm (24.8% versus 26.5%), CAL >=2mm (41.2% versus 43.6%), or bleeding on probing (69.0% versus 69.6%). When compared to controls, treatment group participants had significantly (P < 0.001) greater reductions in the percentage of sites with PD >= 4mm (11.5% versus 0.5%), CAL >= 2mm (9.7% versus 0.8%) and BOP (22.7% versus 2.1%).
Laboratory Methods
Serum samples were assayed for C-reactive protein (CRP), prostaglandin E2 (PGE2), matrix metalloproteinase-9 (gelatinase B, MMP-9), fibrinogen, endotoxin, interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α. All analyses were conducted at a central laboratory at the University of Kentucky. CRP was analyzed using a capture ELISA as described elsewhere.15, 16 Mouse monoclonal antibodies to IL-1β, IL-6, IL-8, PGE2‡‡ and TNF-᧧ were used for ELISA. MMP-9 was assayed using a commercial ELISA kit‡‡ according to the manufacturer’s instructions. Endotoxin activity was assessed using a commercial kit|| || as an indicator of local and systemic challenge with this inflammatory stimulant, and as a correlate with increases in gram-negative bacteria in the host. The assay was semi-quantitative with a range of endotoxin units (EtxU) in each category as follows: Category 1 = less than 0.0125 EtxU; Category 2 = 0.0125 to 1 EtxU; Category 3 = 1 to 5 EtxU; Category 4 = 5 to 10 EtxU; and Category 5 = greater than 10 EtxU.
Statistical Analyses
For CRP, PGE2, MMP-9, and fibrinogen, log (base 10) of the measurements were analyzed. Endotoxin, which was measured using a semi-quantitative assay, was analyzed as both a categorical and continuous measure. Here we present only results from the latter analyses, because they would have higher power to find associations with other measures. Cox regressions with likelihood ratio tests were used to study the association between gestational age at delivery and each biomarker (at baseline, at 29–32 weeks, and its change from baseline to 29–32 weeks). Full-term pregnancies were censored at 37 weeks gestation. Adjusted analyses of baseline serum data included clinical site, race/ethnicity, age, and body mass index (BMI, kilograms/meter2). Adjusted analyses of the follow-up serum data (29–32 weeks and change from baseline) also included periodontal treatment group assignment. Associations between the biomarkers and birthweight were examined using simple linear regression in unadjusted analyses and multiple linear regression in adjusted analyses.
Associations between changes in the periodontal measures and these biomarkers were tested using linear regression. Change in periodontal status was summarized as the change from baseline in the percent of sites with PD ≥ 4 mm and the percent with BOP. To calculate change from baseline in the clinical measures, we used data from the 29–32 week examination when it was available; data from the 21–24 week examination was substituted when 29–32 week data was not available. Only 5 of the 620 women with both baseline and 29–32 week serum data, however, did not have 29–32 week clinical periodontal data.
We also tested for association between change in periodontal measures and change in the serum biomarkers by comparing women in the upper and lower quartiles of change in the percent of tooth sites with PD >=4mm, where the quartiles were determined separately for the treatment and control groups. In a two-way ANOVA, the outcome was change in the serum biomarker and the independent variables were group (treatment or control), quartile of change in % PD >= 4mm, and the interaction. For these latter analyses, the 25th and 75th percentiles were −19.64 and −3.04 percentage points, respectively, for the treatment group and −5.95 and 5.55 percentage points for the control group. The median change was −10.60 for the treatment group and −1.19 for the control group.
Few women had detectable levels of IL-1β, IL-6, IL-8, and TNF-α at either baseline or 29–32 weeks of gestation. For these biomarkers, we compared treatment groups and explored associations with preterm birth using chi-square and Fisher’s exact tests as detailed below. In all cases, reported P-values are not adjusted for multiple comparisons. Eighteen women randomized to the periodontal treatment group did not receive their assigned treatment, but none of these women provided a 29–32 week serum sample. Thus, intent-to-treat and per-protocol analyses of the available data are identical. Analyses of the 29–32 week samples also excluded 19 women (14 control, 5 treatment) whose pregnancies ended in a spontaneous abortion or stillbirth; only 4 of these women were still pregnant and provided a serum sample at 29–32 weeks of gestation.
RESULTS
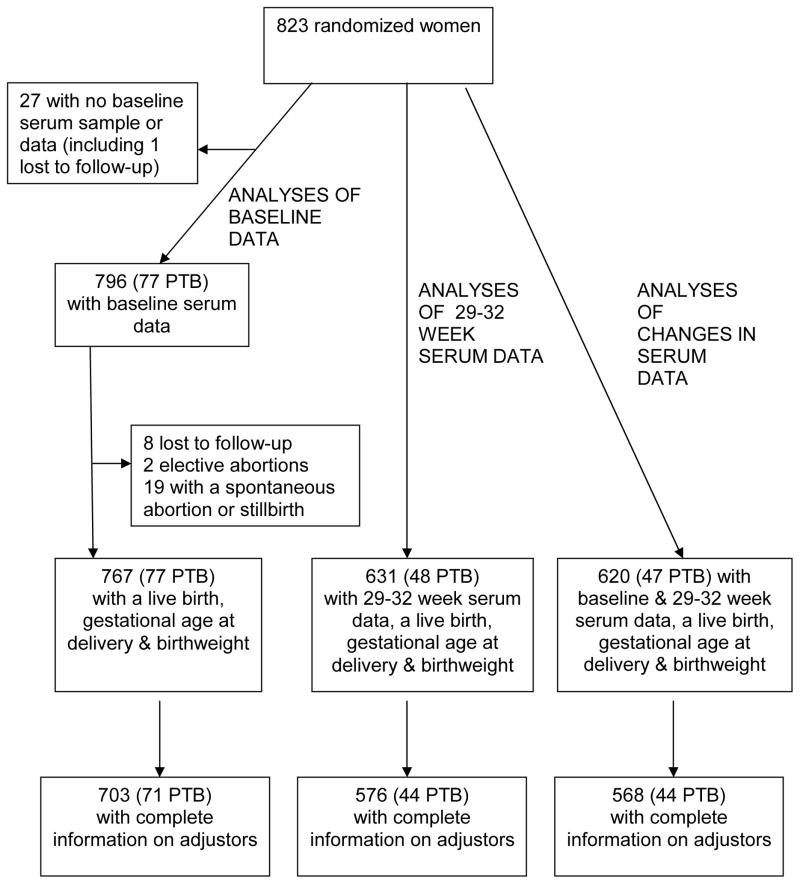
Figure 1 describes sample sizes for the analyses. Of the 823 randomized women, 82 delivered a live preterm infant, 19 experienced a spontaneous abortion (loss before 20 weeks’ gestation) or stillbirth (loss between 20 weeks and 36 weeks 6 days), 2 electively ended their pregnancy, and 9 were lost to follow-up or withdrew consent. Baseline serum data were available for 796 of the 823 randomized women; 27 lacked a baseline serum sample or data. Fewer women had follow-up serum data and complete information on the covariates used in the adjusted analyses. The smallest sample for any analysis reported here (N = 568) was for women who delivered a live infant, had complete birth data, baseline and follow-up serum data, and complete information on adjusters.
Figure 1.
Samples sizes for analyses of baseline serum data, 29–32 week serum data and change from baseline. PTB = Live preterm birth.
Relationship between serum markers and periodontal treatment
Levels of the serum markers did not differ significantly between treatment and control groups at baseline (Table 1). Only 2.0 to 8.1% of women had detectable levels of IL-1β, IL-6, IL-8, and TNF-α at baseline. For these markers, we compared groups in terms of biomarker presence or absence (Table 1b) and found no significant differences. Changes in biomarker levels from baseline also did not differ significantly (P > 0.05) between treatment and control groups (Table 2). Average CRP level decreased and average PGE2 and fibrinogen levels increased significantly in both treatment and control groups. The average changes, however, were small and did not differ between treatment groups (Table 2a). For IL-1β, IL-6, IL-8, and TNF-α, we compared groups in terms of the proportion of participants for whom the biomarker was detectable at baseline but not at 29–32 weeks. The treatment groups did not differ in terms of changes in these biomarkers (P > 0.05, Table 2b).
Table 1.
| Table 1a. Baseline Levels of Serum Biomarkers, by Treatment Group, for Markers with High Frequency of Detection. | |||
|---|---|---|---|
| Treatment Group (N = 402) | Control Group (N = 394) | P value | |
| CRP, pg/ml | 0.76 (0.46)* | 0.75 (0.46) | 0.81† |
| PGE2, pg/ml | 2.27 (0.51) | 2.28 (0.49) | 0.80 |
| MMP-9, μg/ml | −0.24 (0.29) | −0.23 (0.28) | 0.62 |
| Fibrinogen, pg/ml | 1.23 (0.24) | 1.25 (0.25) | 0.35 |
| Endotoxin, ETX units | 1.79 (1.09) | 1.77 (1.11) | 0.77 |
| Table 1b. Presence of Serum Biomarkers at Baseline, by Treatment Group, for Markers with Low Frequency of Detection. | |||
|---|---|---|---|
| Treatment Group (N = 402) | Control Group (N = 394) | P value | |
| IL-1β | 23 (5.7%)§ | 29 (7.4) | 0.39** |
| IL-6 | 27 (6.7) | 28 (7.1) | 0.89 |
| IL-8 | 16 (4.0) | 8 (2.0) | 0.15 |
| TNF-α | 28 (7.0) | 32 (8.1) | 0.59 |
Mean (standard deviation) of common log (base 10) for CRP, PGE2, MMP9, and Fibrinogen
From two-sample t-test
Number (percent) of samples above the lower limit of detection
From Fisher’s Exact Test
Table 2.
| Table 2a. Change from Baseline in Serum Biomarkers, by Treatment Group, for Markers with High Frequency of Detection | ||||
|---|---|---|---|---|
| Treatment Group (N = 302) | Control Group (N = 318) | P value, unadjusted analysis | P value, adjusted analysis | |
| CRP | −0.06 (0.39)*† | −0.08 (0.36)† | 0.60 | 0.65§ |
| PGE2 | 0.08 (0.56)† | 0.06 (0.54)† | 0.71 | 0.34 |
| MMP-9 | −0.02 (0.26) | −0.02 (0.31) | 0.97 | 0.93 |
| Fibrinogen | 0.30 (0.29)† | 0.27 (0.32)† | 0.35 | 0.35 |
| Endotoxin | 0.05 (1.54) | 0.02 (1.54) | 0.76 | 0.67 |
| Table 2b. Reduction of Detectable Serum Biomarkers, by Treatment Group, for Markers with Low Frequency of Detection | |||
|---|---|---|---|
| Treatment Group (N = 302) | Control Group(N = 318) | P value | |
| IL-1β | 10/16 (62.5%)¶ | 13/23 (56.5) | 0.75** |
| IL-6 | 12/20 (60.0) | 16/23 (69.6) | 0.54 |
| IL-8 | 8/9 (88.9) | 4/5 (80.0) | 1.00 |
| TNF-α | 15/23 (65.2) | 14/25 (56.0) | 0.57 |
Mean change (standard deviation), in the same units as Table 1; Positive value indicates an increase from baseline, negative values a decrease.
Significantly different from baseline, P < 0.05
Comparing groups; from adjusted analysis including clinical site (MN, KY, MS, NY), clinic by group interaction, age, BMI and race/ethnicity (Total N = 586, see Fig. 1).
Proportion (%) of women with baseline and follow-up biomarker data who had readings above the lower limit of detection at baseline and below the lower limit at 29–32 weeks.
Comparing Groups; unadjusted comparison using Fisher’s exact test
Relationships between serum biomarkers and pregnancy outcomes
The fraction of live preterm births did not differ significantly between women with undetectable or detectable levels of IL-1β, IL-6, IL-8, or TNF-α at baseline (Table 3). The number of women with positive samples, however, was small and the results should be viewed with appropriate caution. The number of women with detectable levels of these cytokines in 29–32 weeks samples was even smaller (11 for IL-8, to 33 for IL-1β) and these biomarkers were not analyzed further. None of the other biomarkers (at baseline, at 29–32 weeks, or change from baseline) was significantly associated with gestational age at delivery in unadjusted (data not shown) or adjusted analyses (Table 4; for all hazard ratios, the 95% confidence interval includes 1.0). For example, a 10-fold increase in baseline CRP was associated with a non-significant 30% decrease in the hazard of delivery, which loosely speaking is a relative risk of 0.70 for preterm delivery (Table 4, upper left data cell). For fibrinogen, the adjusted hazard ratio was 0.72 at baseline and 1.68 at 29–32 weeks. Thus, while higher fibrinogen levels at baseline were associated with a lowered risk for preterm delivery, at 29–32 weeks higher fibrinogen was associated with an elevated risk. None of these hazard ratios, however, differed significantly from 1.0 (P > 0.05).
Table 3.
Number (%) of women with a live preterm birth, by serum biomarker detection at baseline (13 – 17 weeks gestation).
| Biomarker Detectable: Preterm Delivery/Total (%) | Biomarker Undetectable: Preterm Delivery/Total (%) | P value | |
|---|---|---|---|
| IL-1β | 7/49 (14.3) | 70/718 (9.8) | 0.32† |
| IL-6 | 3/52 (5.8) | 74/715 (10.4) | 0.47 |
| IL-8 | 3/23 (13.0) | 74/744 (10.0) | 0.50 |
| TNF-α | 5/57 (8.8) | 72/710 (10.1) | 1.00 |
From Fisher’s exact test
Table 4.
Change in Hazard of Pre-term Delivery Associated with Change in the Serum Biomarkers
| Adjusted Hazard Ratio for Preterm Delivery (95% Confidence Interval)* | |||
|---|---|---|---|
| Baseline | 29–32 weeks | Change from Baseline | |
| CRP | 0.70 (0.40, 1.27)† | 1.09 (0.52, 2.36) | 1.36 (0.60, 3.06) |
| PGE2 | 1.08 (0.66, 1.86) | 0.78 (0.43, 1.52) | 0.64 (0.37, 1.13) |
| MMP-9 | 0.59 (0.24, 1.45) | 0.58 (0.24, 1.43) | 1.05 (0.35, 3.16) |
| Fibrinogen | 0.72 (0.25, 2.03) | 1.68 (0.51, 5.53) | 1.28 (0.48, 3.53) |
| Endotoxin | 0.90 (0.70, 1.14) | 0.86 (0.64, 1.12) | 0.95 (0.79, 1.15) |
Adjusted for clinic, race/ethnicity, age and BMI. Hazards for 29–32 week data and change from baseline also adjusted for periodontal treatment group.
Hazard ratios and confidence limits refer to a 10-fold (one log base 10 unit) increase in the level of the biomarker, except for endotoxin, where they refer to a one-step change in the endotoxin scale.
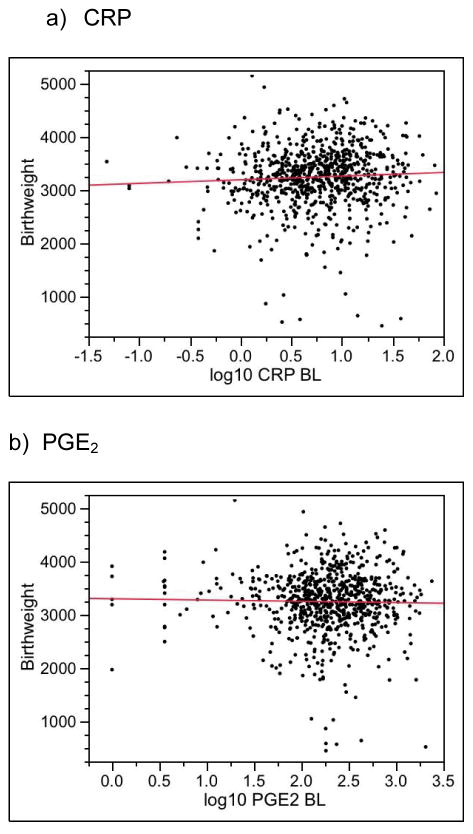
Figure 2 plots birthweight as a function of the biomarkers at baseline. No marker was significantly associated with birthweight (Table 5, column 1 gives P values). Similarly, birthweight was not significantly associated with any biomarker at 29–32 weeks or with any change from baseline (Table 5, columns 2 and 3).
Figure 2.
Birthweight versus Baseline Serum Biomarkers. None of the adjusted slopes (adjusted for clinic, race/ethnicity, age and body-mass index) differed significantly from zero (P > 0.05, see Table 5, column 1 for P values).
Table 5.
P values for Association between Serum Markers and Birthweight
| Baseline | 29–32 weeks | Change from Baseline | |
|---|---|---|---|
| CRP | 0.76* | 0.19 | 0.14 |
| PGE2 | 0.62 | 0.44 | 0.91 |
| MMP-9 | 0.72 | 0.68 | 0.46 |
| Fibrinogen | 0.30 | 0.08 | 0.22 |
| Endotoxin | 0.87 | 0.17 | 0.45 |
P value from multiple linear regression with birthweight as the dependent variable and the serum marker (log10), age, sex and BMI as independent variables. Treatment group also included as an independent variable in the analysis of the 29–32 week data (columns 2 and 3). P values in column 1 correspond to data shown in Figure 2.
Relationship between change in periodontal status and serum biomarkers
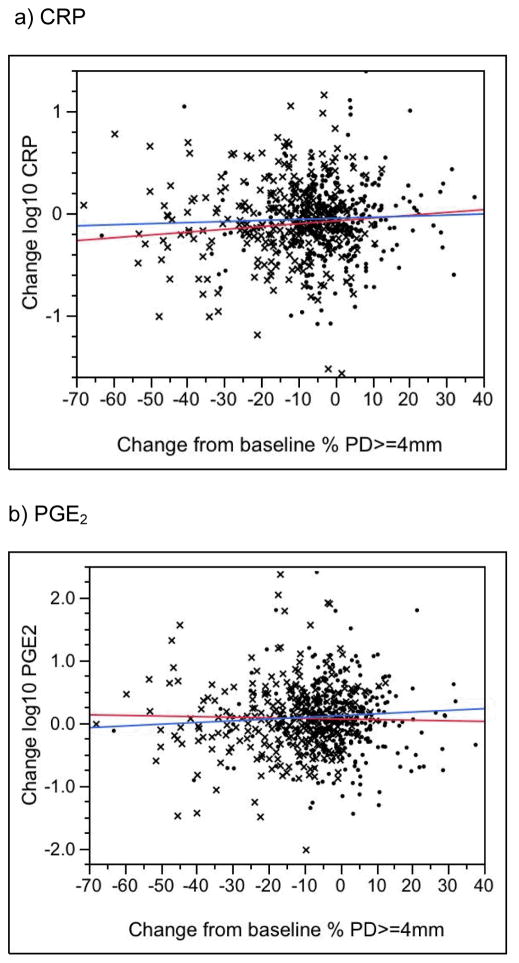
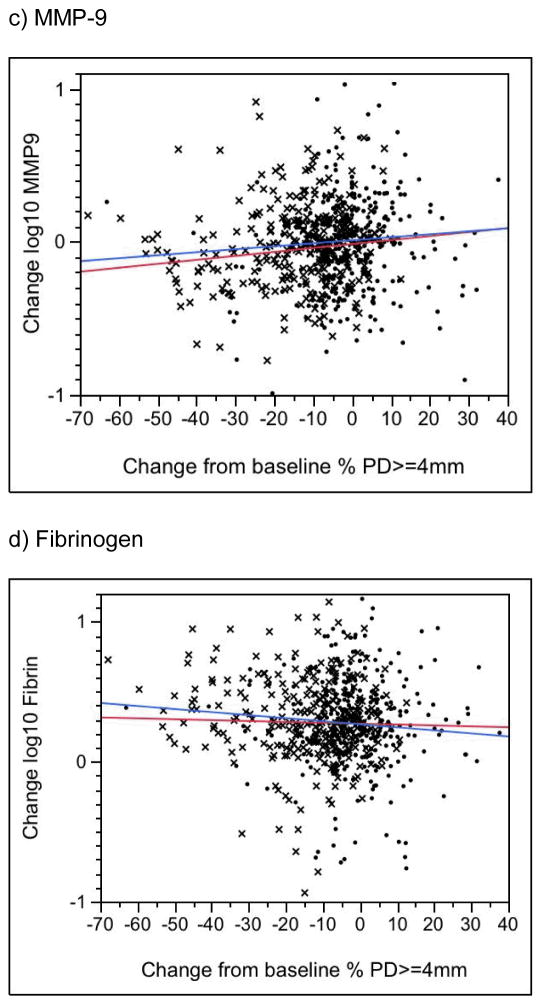
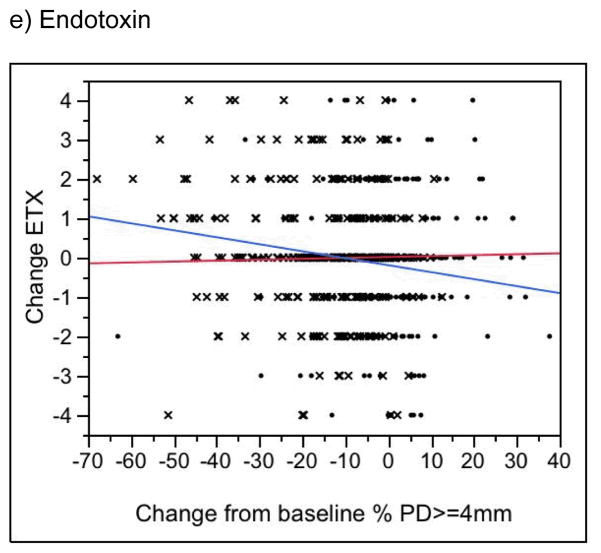
Changes in CRP, PGE2, MMP-9 and fibrinogen levels were not associated with changes in periodontal status in either the treatment or control group (Figure 3a–d, P > 0.05; results for BOP not shown). In the treatment group only, change in periodontal status was significantly associated with change in endotoxin levels (P = 0.005 for change in percent of sites with PD >= 4mm [Fig. 3e]; P = 0.03 for change in percent of sites with BOP [data not shown]). Surprisingly, endotoxin levels tended to increase over time in treatment group women who experienced the greatest improvements in the clinical periodontal measures (see, e.g., the blue line in Figure 2e). No similar association was seen in the controls (P > 0.1). Finally, for both treatment and control groups, for changes in all biomarkers, we found no significance difference between those in the lowest (best) and highest (worst) quartiles in terms of change from baseline in periodontal condition.
Figure 3.
Change in the Percent of Tooth Sites with a Probing Depth >= 4mm versus Change in the Serum Biomarker. A negative value on the horizontal axis indicates a reduction, or improvement, in the percentage of tooth sites with probing depths >= 4mm. A negative value on the vertical axis indicates a reduction in the biomarker level from baseline to 29–32 weeks. X’s denote treatment group participants, dots denote controls. The blue line is the regression slope for treatment group women; the red line is for controls. See text for P values.
DISCUSSION
We analyzed serum samples from pregnant women who received non-surgical treatment for periodontitis either before 21 weeks of gestation (treatment group) or after delivery (control group). None of the biomarkers differed significantly between groups at baseline. In both groups, CRP decreased and PGE2 and fibrinogen levels increased slightly but significantly between baseline and follow-up. MMP-9 and endotoxin levels did not change significantly over time in either group.
We also explored associations between the biomarkers and subsequent preterm delivery and birthweight (Tables 3–5). None of the biomarkers at baseline was significantly associated with either outcome. Hazard ratios for preterm delivery were computed for a 10-fold increase in CRP, PGE2, MMP-9 or fibrinogen level at either baseline or follow-up, or in their changes over time (Table 4). While none of the hazard ratios was significant, some may seem substantially different from 1. However, of these 4 biomarkers, the standard deviations of their levels and their changes from baseline ranged from 0.24 to 0.56 logs (Tables 1a and 2a). Thus, the computed hazard ratios correspond to differences that are approximately 2 to 4 times the relevant standard deviation. For example, a 10-fold difference in MMP-9 at baseline was associated with a non-significant 41% increase in risk for preterm delivery (Table 4, first data column). The standard deviation of baseline common log MMP-9 was 0.29 in the treatment group (a 100.29-fold difference) and 0.28 in controls (Table 1a). Thus, this non-significant increase in risk is the difference between two women at the extreme tails of the distribution of change in fibrinogen, separated by over 3 standard deviations.
Finally, associations between changes in the biomarkers and periodontal measures were tested separately in treatment and control subjects. With the exception of endotoxin in the treatment group, none of the associations was statistically significant. Paradoxically, treatment group participants with the largest reductions in pocketing and bleeding on probing also tended to have the largest increases in serum endotoxin activities over time. Previous studies suggest that periodontitis is associated with endotoxemia, measured directly or indirectly by elevated concentrations of lipopolysaccharide binding proteins, soluble CD14 and antibodies to LPS from periodontal pathogens.17 While it is possible that women with the greatest response to treatment experienced the largest and most prolonged treatment-induced bacteremia, follow-up serum samples were obtained at least 8 weeks after treatment. While short-term spikes in serum inflammatory markers (e.g., CRP, Il-6) have been reported following scaling and root planing, levels appear to subside rapidly, on average within a week following treatment. 18
Endotoxin was measured using a semi-quantitative assay stratified into 5 category levels. We analyzed endotoxin as a categorical measure and found no significant associations with periodontal treatment, birth outcomes or changes in clinical periodontal measures (results not shown). We have presented results from analyzing the semi-quantitative measure of endotoxin because this gives greater statistical power to find associations. Despite this unconventional but statistically more powerful approach, we found no association between endotoxin and any birth outcome and only a counter-intuitive association with change in clinical periodontal status in the treatment group. The effect of periodontal treatment specifically on serum endotoxin has not been previously reported.
In the current study, periodontal treatment was not associated with change in any serum inflammatory marker. These findings are consistent with several studies of non-pregnant populations in the literature, where the effect of treatment on systemic markers of inflammation continues to be debated. For example, Ide et al19 found that treatment did not significantly reduce serum/plasma levels of fibrinogen, CRP, TNF-α, IL-6 or IL-1β. Similarly, Tonetti et al 18 and D’Auito et al 20 showed that scaling and root planing alone did not significantly reduce serum levels of IL-1β, IL-6, or TNF-α one to six months after treatment. In contrast, Elter et al 21 demonstrated significant reductions in CRP and IL-6 one month following treatment, which included scaling and root planing and surgery. Paraskevas and colleagues22 concluded from their meta-analysis there was only modest evidence overall that mechanical periodontal therapy reduces serum CRP.
The current study’s failure to find a treatment effect on serum markers may be attributed to the type of treatment provided, which consisted of scaling and root planing followed by monthly tooth polishings. Topical or systemic antimicrobial agents were not used. In contrast, reductions in select serum markers have been observed with more extensive treatment protocols involving surgery or the widespread use of locally-delivered antimicrobial agents. 21, 23 For example, D’Aiuto et al23 found that levels of IL-6 decreased significantly from baseline in patients treated with scaling and root planing and an average of 80 applications of a locally-delivered minocycline product, but not in patients treated with scaling and root planing alone. Also, as we reported earlier 14 women in our treatment group had relatively extensive BOP following treatment. (Mean BOP was reduced in this group to 47.1% from 69.8%.) It is possible that we did not achieve some threshold in BOP improvements, above which these serum biomarkers could have been affected.
There also is some evidence that serum inflammatory biomarkers, including CRP and granulocyte-macrophage colony stimulating factor, increase during normal pregnancy and exceed levels found in non-pregnant women.24, 25 According to Belo et al,24 “raised CRP levels [in pregnancy] may result from different stimuli occurring at different phases of pregnancy; the implantation and monocyte/macrophage production of IL-6, the necrotic process associated with placenta ageing and the progressive increments in the levels of estrogens during gestation…” Thus, the lack of a significant periodontal treatment effect on these selected biomarkers in our study may have been masked by increases associated with pregnancy. We were unable to test this hypothesis because the study did not have non-pregnant controls; all study women were both pregnant and had periodontitis.
None of the serum biomarkers was significantly associated with preterm birth or birthweight. These findings are consistent with several obstetrics studies that failed to demonstrate the clinical utility of some of the same serum biomarkers to predict preterm delivery in asymptomatic women.26, 27 For example, the Preterm Prediction Study28 examined a variety of markers in serum obtained at 24 or 28 weeks of gestation. Alkaline phosphatase, alpha-fetoprotein and corticotrophin-releasing hormone were associated with subsequent delivery at less than 35 weeks of gestation, but CRP and IL-6 were not. Vogel and colleagues 12 reviewed the utility of biomarkers in serum, amniotic fluid and vaginal swab samples to predict preterm delivery in asymptomatic women. Although a combination of markers including cervical fetal fibronectin and serum alpha-fetoprotein were found to predict delivery before 37 weeks, many other serum markers, including CRP, had little clinical utility to predict preterm birth.
Many of the reported associations between inflammatory markers and preterm delivery have been based on analyses of amniotic fluid or vaginal or cervical secretions.29–31 Similarly, associations between serum markers and preterm delivery are frequently detected only in select groups, including those with previous preterm deliveries32 or who are symptomatic for preterm delivery at the time of sampling.33, 34 In the current study, we sampled all women, very few of whom would have been symptomatic at the time of sampling. Finally, while some investigators have reported correlations between specific biomarker levels in serum and vaginal fluids (cervical or cervicovaginal), others have not.13 For example, while Vogel et al13 found that IL-1β and GM-CSF levels in serum were positively correlated with one another, IL-1β levels in serum and vaginal fluid were not.
Previously we showed that scaling and root planing plus monthly tooth polishing in pregnant women with periodontitis significantly improved clinical and microbial measures of the disease.14, 35 Despite this, treatment was not associated with reduced rates of preterm birth or low birthweight.14 In the current study, we showed this same treatment did not alter systemic markers of inflammation, which themselves were not associated with preterm delivery or birthweight. While it is possible that more aggressive periodontal treatment, including the use of locally-delivered antimicrobial agents with or without surgery, might have reduced systemic markers of inflammation, we found no evidence that the magnitude of the periodontal treatment response was associated with changes in these biomarkers or, previously, 14 with preterm birth risk. In conclusion, non-surgical periodontal treatment in pregnant women delivered before 21 weeks of gestation does not reduce systemic (serum) markers of inflammation. In pregnant women with periodontitis, levels of these markers at 13–17 weeks and 29–32 weeks of gestation are not associated with risk for preterm birth or with infant birthweight.
Acknowledgments
Supported by NIH/NIDCR grant DE014338. We thank the trial participants and the entire OPT Study team, including: University of Minnesota, Minneapolis -P. Tschida, H. Voelker, I. Olson, J. Osborn, L. Wolff, E. Delmore, and S. Wewerka; Hennepin County Medical Center, Minneapolis - L. Long-Simpson, J. Anderson, K. Meyer, J. Danielson, T. Thompson; University of Kentucky, Lexington - D. Dawson, A. Buell, D. Mischel, P. Stein, L. Cunningham, D. Dawson; University of Mississippi, Jackson - S. Vance, G. Young, A. Garner, N. Wood, K. Holmes, R. Johnson; Harlem Hospital/Columbia University, New York - S. Lassiter, J. Mays, J. Jackson, E. Rijo, M. Bolden, C. Spicer.
This study was supported by grant UO1 DE014338 from the National Institute for Dental and Craniofacial Research of the National Institutes for Health.
Footnotes
Biosource™, Invitrogen Corporation, Carlsbad, CA
Quantikine ELISA Kit, R&D Systems, Inc., Minneapolis, MN
Endosafe®, Charles River Laboratories International, Inc., Wilmington, MA
The authors report no conflict of interest.
References
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2005. Natl Vital Stat Rep. 2006;55(11):1–18. [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS Institute of Medicine. Preterm birth: causes, consequences, and prevention. Washington, D.C: National Academies Press; 2007. Committee on Understanding Premature Birth and Assuring Healthy Outcomes; p. 772. [PubMed] [Google Scholar]
- 3.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15 (Suppl 2):41–56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 4.Collins JG, Smith MA, Arnold RR, Offenbacher S. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect Immun. 1994;62(10):4652–4655. doi: 10.1128/iai.62.10.4652-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins JG, Windley HW, 3rd, Arnold RR, Offenbacher S. Effects of a Porphyromonas gingivalis infection on inflammatory mediator response and pregnancy outcome in hamsters. Infect Immun. 1994;62(10):4356–4361. doi: 10.1128/iai.62.10.4356-4361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offenbacher S, Boggess KA, Murtha AP, et al. Progressive Periodontal Disease and Risk of Very Preterm Delivery. Obstet Gynecol. 2006;107(1):29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 7.Xiong X, Buekens P, Fraser W, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113(2):135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 8.Wimmer G, Pihlstrom BL. A critical assessment of adverse pregnancy outcome and periodontal disease. J Clin Periodontol. 2008;35(8 Suppl):380–397. doi: 10.1111/j.1600-051X.2008.01284.x. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff M, Searle K. The role of inflammation in preterm birth--focus on periodontitis. BJOG. 2006;113 (Suppl 3):43–45. doi: 10.1111/j.1471-0528.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- 10.Koj A. Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317(2):84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 11.Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36(4):611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- 12.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 13.Vogel I, Goepfert AR, Thorsen P, et al. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 2007;75(2):133–140. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355(18):1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 15.Ebersole JL, Cappelli D, Mott G, Kesavalu L, Holt SC, Singer RE. Systemic manifestations of periodontitis in the non-human primate. J Periodontal Res. 1999;34(7):358–362. doi: 10.1111/j.1600-0765.1999.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 16.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107(2):347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pussinen PJ, Paju S, Mantyla P, Sorsa T. Serum microbial- and host-derived markers of periodontal diseases: a review. Curr Med Chem. 2007;14(22):2402–2412. doi: 10.2174/092986707781745604. [DOI] [PubMed] [Google Scholar]
- 18.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 19.Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30(4):334–340. doi: 10.1034/j.1600-051x.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 20.D’Aiuto F, Parkar M, Tonetti MS. Acute effects of periodontal therapy on bio-markers of vascular health. J Clin Periodontol. 2007;34(2):124–129. doi: 10.1111/j.1600-051X.2006.01037.x. [DOI] [PubMed] [Google Scholar]
- 21.Elter JR, Hinderliter AL, Offenbacher S, et al. The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J. 2006;151(1):47. doi: 10.1016/j.ahj.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35(4):277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 23.D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84(3):269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 24.Belo L, Santos-Silva A, Rocha S, et al. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005;123(1):46–51. doi: 10.1016/j.ejogrb.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Larsson A, Palm M, Hansson LO, Basu S, Axelsson O. Reference values for alpha1-acid glycoprotein, alpha1-antitrypsin, albumin, haptoglobin, C-reactive protein, IgA, IgG and IgM during pregnancy. Acta Obstet Gynecol Scand. 2008;87(10):1084–1088. doi: 10.1080/00016340802428146. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. Am J Obstet Gynecol. 2005;192(5 Suppl):S36–46. doi: 10.1016/j.ajog.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Tu FF, Goldenberg RL, Tamura T, Drews M, Zucker SJ, Voss HF. Prenatal plasma matrix metalloproteinase-9 levels to predict spontaneous preterm birth. Obstet Gynecol. 1998;92(3):446–449. doi: 10.1016/s0029-7844(98)00222-1. [DOI] [PubMed] [Google Scholar]
- 28.Moawad AH, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: the value of serum alkaline phosphatase, alpha-fetoprotein, plasma corticotropin-releasing hormone, and other serum markers for the prediction of spontaneous preterm birth. Am J Obstet Gynecol. 2002;186(5):990–996. doi: 10.1067/mob.2002.121727. [DOI] [PubMed] [Google Scholar]
- 29.Lange M, Chen FK, Wessel J, Buscher U, Dudenhausen JW. Elevation of interleukin-6 levels in cervical secretions as a predictor of preterm delivery. Acta Obstet Gynecol Scand. 2003;82(4):326–329. doi: 10.1034/j.1600-0412.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 30.Foulon W, Van Liedekerke D, Demanet C, Decatte L, Dewaele M, Naessens A. Markers of infection and their relationship to preterm delivery. Am J Perinatol. 1995;12(3):208–211. doi: 10.1055/s-2007-994454. [DOI] [PubMed] [Google Scholar]
- 31.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178(3):546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 32.Vogel I, Goepfert AR, Thorsen P, et al. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 2007;75(2):133–140. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Skrablin S, Lovric H, Banovic V, Kralik S, Dijakovic A, Kalafatic D. Maternal plasma interleukin-6, interleukin-1beta and C-reactive protein as indicators of tocolysis failure and neonatal outcome after preterm delivery. J Matern Fetal Neonatal Med. 2007;20(4):335–341. doi: 10.1080/14767050701227877. [DOI] [PubMed] [Google Scholar]
- 34.Kurkinen-Raty M, Ruokonen A, Vuopala S, et al. Combination of cervical interleukin-6 and -8, phosphorylated insulin-like growth factor-binding protein-1 and transvaginal cervical ultrasonography in assessment of the risk of preterm birth. BJOG. 2001;108(8):875–881. doi: 10.1111/j.1471-0528.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- 35.Novak MJ, Novak KF, Hodges JS, et al. Periodontal bacterial profiles in pregnant women: response to treatment and associations with birth outcomes in the obstetrics and periodontal therapy (OPT) study. J Periodontol. 2008;79(10):1870–1879. doi: 10.1902/jop.2008.070554. [DOI] [PubMed] [Google Scholar]