Abstract
A new type of Cu2+ fluorescent sensor, coucage, has been prepared with a photosensitive nitrophenyl group incorporated into the backbone of a coumarin-tagged tetradentate ligand. Coucage provides a selective fluorescence response for Cu2+ over other biologically relevant metal ions. Coordination of Cu2+ dims the fluorescence output until irradiation with UV light cleaves the ligand backbone, which relieves the copper-induced quenching to provide a turn-on response. Experiments in live MCF-7 cells show that coucage can be used for detecting changes in intracellular Cu2+ upon the addition of excess exogenous copper. If improvements can be made to increase its affinity for copper, this new type of turn-on sensor could be used as a tool for visualizing the cellular distribution of labile copper to gain insight into the mechanisms of copper trafficking.
Copper, the third most abundant transition metal in the human body, plays a critical role in many fundamental physiological processes; however, it also catalyzes the production of highly reactive oxygen species that damage biomolecules.1 Due to copper’s dual nature, cells have developed strict regulatory processes to control its cellular distribution.1 Alterations in copper homeostasis are linked to neurodegenerative diseases such as Menkes and Wilson diseases, Alzheimer’s, familial amyotrophic lateral sclerosis, and prion diseases.2 Being able to visualize the cellular distribution of copper in both its physiological oxidation states, Cu+ and Cu2+, would offer insight into how cells acquire, maintain, and utilize copper while suppressing its toxicity. Whereas reliable fluorescence sensors exist for Cu+, there are fewer options for detecting Cu2+ in living cells.3
A common strategy in designing fluorescent probes for metal ions is to link a ligand to a fluorophore such that metal binding causes an increase in fluorescence only in response to the target ion. Cell permeable fluorescent sensors have proven useful for investigating intracellular metal ion distribution, particularly for Ca2+,4 Zn2+,5 and Cu+.6 The development of this type of “turn-on” sensor for Cu2+, however, is hampered by the fluorescence quenching effect of this paramagnetic metal ion. As a consequence, many Cu2+ sensors have a “turn-off” mechanism,7 which is generally less sensitive, gives false-positive results, and offers limited spatial resolution. Several examples of turn-on sensors have appeared recently,3,8 but limitations include sensing mechanisms that operate only in organic solvent or at non-physiological pH,8a-d low quantum yields in aqueous solution,8e or potential off-target responses.8f-i Therefore, there is a need to develop new strategies that provide a fluorescent turn-on response in order to investigate intracellular Cu2+. We present here coucage, a new type of fluorescent sensor that uses UV light to uncage a Cu2+-dependent fluorescence response.
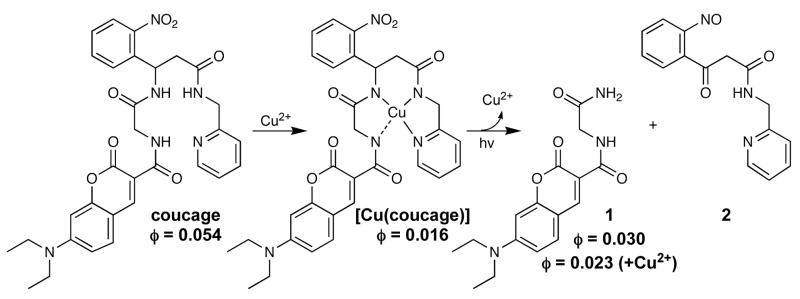
Coucage is based on our previously reported copper caging ligand H2cage,9 but adapted with coumarin as a fluorescence reporter that is quenched upon Cu2+ coordination. The nitrophenyl group incorporated into the backbone of the fluorescent tetradentate chelator is the caging element that blocks activity until activated with light.10 Exposure to UV light induces bond cleavage, as shown in Scheme 1, which triggers two-fold activity: release of copper by decreasing ligand denticity, and restoration of fluorescence by disengaging the copper-induced quenching.
Scheme 1.
Synthesis and Photolysis of [Cu(coucage)]
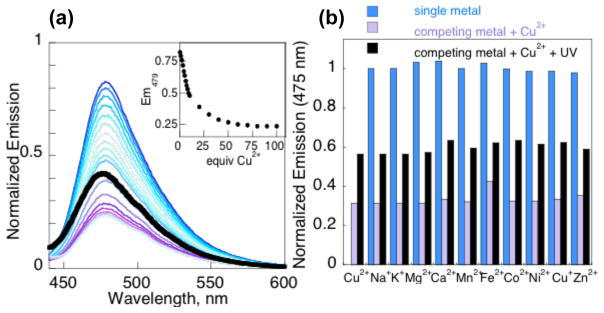
Coucage displays an absorbance band at 432 nm that gives a corresponding fluorescence emission maximum at 479 nm with a quantum yield of 0.054. Fig. 1a shows that its fluorescence at pH 7.4 is quenched by 75% when saturated with Cu2+, giving a quantum yield of 0.016 and a conditional dissociation constant, Kd, of 7.3 ± 0.9 μM. The 1:1 coucage:Cu2+ ratio for complex formation was confirmed by the method of continuous variation (Supp. Info.).
Figure 1.
a) Fluorescence decrease of 1 μM coucage with 0–100 equiv. Cu2+, along with the subsequent increase following UV exposure (thick black trace). Inset: Emission at 479 nm vs. added Cu2+. b) Blue bars: the unchanged fluorescence of 1 μM coucage in the presence of 1 mM Na+, K+, Mg2+, Ca2+ and Zn2+ or 50 μM for others; Purple bars: quenched emission upon addition of 50 μM Cu2+; black bars: restored fluorescence after 4 min of UV exposure. All samples prepared in 10 mM Hepes buffer at pH 7.4 with 10% DMSO and excited at 430 nm.
The depressed fluorescence of solutions containing coucage and Cu2+ can be restored to nearly half the original intensity by irradiation at 350 nm, as shown by the thick spectral trace in Fig. 1a. The emission maximum of photolyzed samples shifts slightly to 475 nm, with a quantum yield of 0.023. The fluorescence of the photolyzed products does not return to initial levels for at least two reasons, the first being that the quantum yield of independently synthesized photoproduct 1 (0.030) is inherently lower than coucage. The second is that Cu2+ retains some quenching effect on the photoproducts, although to a much lesser extent than on intact coucage (see Fig S5).
Unlike the response observed with Cu2+, no significant fluorescence changes are observed for coucage in the presence of other metal cations, as shown in Fig. 1b for Na+, K+, Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu+, and Zn2+. When Cu2+is added back to these solutions, the fluorescence decreases by 70% (Fig. 1b, purple bars), confirming coucage’s high selectivity for Cu2+ over other biologically important metal ions. The fluorescence can again be partially restored upon irradiation, as shown by the black bars in Fig. 1b.
The increase in fluorescence upon irradiation of [Cu(coucage)] is apparent immediately, and cleavage of the ligand backbone is complete in approximately 3 min. The quantum yield of photolysis for coucage and coucage in the presence of Cu2+ is 0.51 and 0.68 respectively, indicating that coordination by Cu2+ does not decrease photolysis efficiency, as previously observed for [Cu(cage)].9 Analysis of the reaction mixture by liquid chromatography mass spectrometry (LC-MS) revealed 1 and 2 as photoproducts (Fig. S1).
In order for coucage to bind tightly to Cu2+, all three amide protons must be deprotonated. The fact that only 75% fluorescence quenching is achieved at pH 7.4 suggests that the amide proton closest to the coumarin is not fully deprotonated at this pH, setting up a H+/Cu2+ competition that precludes maximum fluorescence quenching. Indeed, increasing the pH of coucage/Cu2+ solutions above 8 dramatically decreases fluorescence, leaving only a residual 10% signal by pH 9 (Fig. S4). Although the greatest fluorescence quenching is observed at high pH, coucage remains biologically applicable since a Cu2+ turn-off response is observed at pH 7.4.
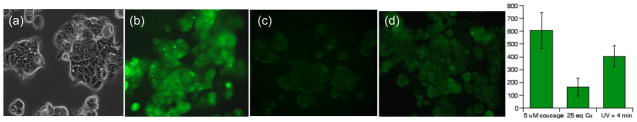
To test coucage in living cells, we treated human breast carcinoma MCF-7 cells with coucage and Cu2+ and observed the intracellular fluorescence of irradiated vs. non-irradiated cells using scanning confocal microscopy. MCF-7 cells incubated with coucage alone initially show a high fluorescence response, as shown in Fig. 2b (see also Supp Info). After addition of excess Cu2+ to the cell culture medium and incubation for 20 min, the intracellular fluorescence signal decreases by 70%, indicating that Cu2+ has coordinated to coucage inside the cells (Fig. 2c). Cu2+-treated cells exposed to UV light from a Rayonet photoreactor for 4 min exhibit bulk fluorescence restoration up to 67% of the original intensity, as seen in Fig. 2d. Control experiments in the absence of fluorophore show no background fluorescence, and photobleaching of coucage results in less than 2% intensity loss during the 3 s excitation times used to collect images. (Supp Info). Brightfield images after coucage, Cu2+, and UV exposure show that cells remain viable throughout the imaging experiment. In these experiments, cells receive only 0.28 kJ/m2 of UVA irradiation, which is significantly lower than the 50–300 kJ/m2 doses known to induce DNA damage and cell death.11 Cells were also irradiated directly on the microscope (See Fig. S14). Although this method provides a less distinct fluorescence increase, it demonstrates the possibility of observing the same cells before and after photolysis.
Figure 2.
Confocal fluorescence images of coucage and Cu2+ in MCF-7 cells; each panel shows an independent view from the same well. a) Bright-field transmission image. b) Cells incubated with 5 μM coucage for 20 min. c) Image taken 20 min after addition of 25 equiv of Cu2+ to coumarin-incubated sample. d) Image taken after 4 min of UV light exposure to coumarin/Cu2+-treated sample. Bar graph represents the average, background-corrected intensity from 10 randomly selected fields of view collected for each condition.
In conclusion, we have presented a new strategy for achieving a fluorescence turn-on response to detect Cu2+ in living cells. The sensor relies on a coumarin-tagged ligand that selectively binds Cu2+ over other biometals to induce fluorescence quenching, which is subsequently relieved upon UV irradiation to provide the turn-on response. In essence, the strategy reports on the memory of where Cu2+ had been available for chelation by the 7 μM binder. Because the probe is destroyed during the readout, this strategy inherently cannot provide real-time monitoring of cellular Cu2+ fluctuations. Experiments in live MCF-7 cells demonstrate that coucage is cell permeable and can detect an increase of intracellular Cu2+ under conditions of excess (between 25 and 125 μM) exogenous copper. Copper is imported in its reduced Cu+ oxidation state, and intracellularly is believed mostly to remain in its reduced form. However, subcellular microenvironments may support Cu2+, and the coucage strategy introduced here might find utility in providing snapshots of such Cu2+, provided that improvements can be made to the ligand to make it more sensitive. Future investigations are therefore aimed at improving the quenching efficiency of the copper complex at physiological pH and increasing the binding affinity in order to create a more sensitive probe, as well as applying photoactive fluorescent ligands to other biologically interesting metal ions.
Supplementary Material
Acknowledgments
We thank Dr. Sam Johnson and the Light Microscopy Core Facility at Duke for technical assistance. We thank the US National Science Foundation (CAREER 0449699), the Sloan Foundation, the Camille and Henry Dreyfus Foundation, and the US National Institutes of Health (GM084176) for funding various aspects of this work.
Footnotes
Supporting Information Available: Full experimental details, including synthesis of coucage and additional fluorescence and microscopy data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Balamurugan K, Schaffner W. Biochim Biophys Acta-Mol Cell Res. 2006;1763:737–746. doi: 10.1016/j.bbamcr.2006.05.001. [DOI] [PubMed] [Google Scholar]; b) Kim BE, Nevitt T, Thiele DJ. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 2.a) Barnham KJ, Masters CL, Bush AI. Nat Rev Drug Disc. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]; b) Gaggelli E, Kozlowski H, Valensin D, Valensin G. Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]; c) Madsen E, Gitlin JD. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 3.Que EL, Domaille DW, Chang CJ. Chem Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 4.Tsien RW, Tsien RY. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 5.a) Priya C, Sivaramapanicker S, Ayyappanpillai A.Chem Asian J 20072338–348.17441169 [Google Scholar]; b) Kikuchi K, Komatsu K, Nagano T. Curr Opin Chem Biol. 2004;8:182–191. doi: 10.1016/j.cbpa.2004.02.007. [DOI] [PubMed] [Google Scholar]; c) Nolan EM, Lippard SJ. Acc Chem Res. 2009;42:193–203. doi: 10.1021/ar8001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Proc Natl Acad Sci USA. 2005;102:11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Miller EW, Zeng L, Domaille DW, Chang CJ. Nat Protocols. 2006;1:824–827. doi: 10.1038/nprot.2006.140. [DOI] [PubMed] [Google Scholar]; c) Domaille DW, Zeng L, Chang CJ. J Am Chem Soc. doi: 10.1021/ja907778b. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Fabbrizzi L, Licchelli M, Pallavicini P, Perotti A, Taglietti A, Sacchi D. Chem Eur J. 1996;2:75–82. [Google Scholar]; b) Jung HS, Kwon PS, Lee JW, Kim JI, Hong CS, Kim JW, Yan SH, Lee JY, Lee JH, Joo T, Kim JS. J Am Chem Soc. 2009;131:2008–2012. doi: 10.1021/ja808611d. [DOI] [PubMed] [Google Scholar]; c) Torrado A, Walkup GK, Imperiali B. J Am Chem Soc. 1998;120:609–610. [Google Scholar]; d) Xie J, Menand M, Maisonneuve S, Metivier R. J Org Chem. 2007;72:5980–5985. doi: 10.1021/jo070315y. [DOI] [PubMed] [Google Scholar]; e) Khatua S, Choi SH, Lee J, Huh JO, Do Y, Churchill DG. Inorg Chem. 2009;48:1799–1801. doi: 10.1021/ic802314u. [DOI] [PubMed] [Google Scholar]
- 8.a) Li GK, Xu ZX, Chen CF, Huang ZT. Chem Commun. 2008:1774–1776. doi: 10.1039/b800258d. [DOI] [PubMed] [Google Scholar]; b) Lin W, Yuan L, Tan W, Feng J, Long L. Chem Eur J. 2009;15:1030–1035. doi: 10.1002/chem.200801501. [DOI] [PubMed] [Google Scholar]; c) Shao N, Jin JY, Wang H, Zhang Y, Yang RH, Chan WH. Anal Chem. 2008;80:3466–3475. doi: 10.1021/ac800072y. [DOI] [PubMed] [Google Scholar]; d) Kim MH, Jang HH, Yi S, Chang SK, Han MS. Chem Commun. 2009:4838–4840. doi: 10.1039/b908638b. [DOI] [PubMed] [Google Scholar]; e) Wang MX, Huang SH, Meng XM, Zhu MZ, Guo QX. Chem Lett. 2008;37:462–463. [Google Scholar]; f) Hyman LM, Stephenson CJ, Dickens MG, Shimizu KD, Franz KJ. Dalton Trans. 2010;39:568–576. doi: 10.1039/b914568k. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Yu M, Shi M, Chen Z, Li F, Li X, Gao Y, Xu J, Yang H, Zhou Z, Yi T, Huang C. Chem Eur J. 2008;14:6892–6900. doi: 10.1002/chem.200800005. [DOI] [PubMed] [Google Scholar]; h) Zhao Y, Zhang XB, Han ZX, Qiao L, Li CY, Jian LX, Shen GL, Yu RQ. Anal Chem. 2009;81:7022–7030. doi: 10.1021/ac901127n. [DOI] [PubMed] [Google Scholar]; i) Swamy KMK, Ko S, Kwon SK, Lee HN, Mao C, Kim J, Lee K, Kim J, Shin I, Yoon J. Chem Commun. 2008;45:5915–5917. doi: 10.1039/b814167c. [DOI] [PubMed] [Google Scholar]; j) Liu J, Lu Y. J Am Chem Soc. 2007;129:9838–9839. doi: 10.1021/ja0717358. [DOI] [PubMed] [Google Scholar]
- 9.Ciesienski KL, Haas KL, Dickens MG, Tesema YT, Franz KJ. J Am Chem Soc. 2008;130:12246–12247. doi: 10.1021/ja8047442. [DOI] [PubMed] [Google Scholar]
- 10.Lee HM, Larson DR, Lawrence DS. ACS Chem Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Kozmin S, Slezak G, Reynaud-Angelin A, Elie C, de Rycke Y, Boiteux S, Sage E. Proc Natl Acad Sci USA. 2005;102:13538–13543. doi: 10.1073/pnas.0504497102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Koch-Paiz CA, Amundson SA, Bittner ML, Meltzer PS, Fornace AJ. Mutation Res. 2004;549:65–78. doi: 10.1016/j.mrfmmm.2004.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.