Abstract
We describe the pharmacological and pharmacokinetic profiles of SCH 486757, a nociceptin/orphanin FQ peptide (NOP) receptor agonist that has recently entered human clinical trials for cough. SCH 486757 selectively binds human NOP receptor (Ki = 4.6 ± 0.61 nM) over classical opioid receptors. In a guinea pig capsaicin cough model, SCH 486757 (0.01–1 mg/kg) suppressed cough at 2, 4, and 6 h post oral administration with a maximum efficacy occurring at 4 h equivalent to codeine, hydrocodone, dextromethorphan and baclofen. The antitussive effects of SCH 486757 (3.0 mg/kg, p.o.) was blocked by the NOP receptor antagonist J113397 (12 mg/kg, i.p.) but not by naltrexone (10 mg/kg, p.o.). SCH 486757 does not produce tolerance to its antitussive activity after a 5-day BID dosing regimen. After acute and chronic dosing paradigms, SCH 486757 (1 mg/kg) inhibited capsaicin-evoked coughing by 46 ±9% and 40 ± 11%, respectively. In a feline mechanically-evoked cough model, SCH 486757 produces a maximum inhibition of cough and expiratory abdominal electromyogram amplitude of 59 and 61%, respectively. SCH 486757 did not significantly affect inspiratory electromyogram amplitude. We examined the abuse potential of SCH 486757 (10 mg/kg, p.o.) in a rat conditioned place preference procedure which is sensitive to classical drugs of abuse, such as amphetamine and morphine. SCH 486757 was without effect in this model. Finally, SCH 486757 displays a good oral pharmacokinetic profile in the guinea pig, rat and dog. We conclude that SCH 486757 has a favorable antitussive profile in preclinical animal models.
Keywords: Cough, NOP receptor, Nociceptin/orphanin FQ, SCH 486757, Antitussive
1. Introduction
Cough is a serious and prominent pathophysiological feature of a diverse range of airway diseases including upper respiratory viral disease, bronchial carcinoma, pulmonary bacterial infections, asthma, chronic obstructive pulmonary disease, chronic bronchitis, and postnasal drip. Additionally, cough is frequently symptomatic of extrapulmonary conditions such as gastroesophageal reflux disease or a significant side effect associated with certain antihypertensive medications, such as angiotensin converting enzyme inhibitors (Lindgren and Andersson, 1989; Irwin and Richter 2000; Novitsky et al., 2002). As a result, cough continues to top the list as one of the most symptomatic complaint causing patients to seek medical attention from a health care provider (Cherry and Woodwell 2002; Morice 2002). Unfortunately, treatment options are inadequate consisting of over the counter products which make up a myriad of available “cough and cold” products or older prescription drugs that contain opioids. Many of the over the counter products have inconsequential or dubious efficacies and recently the Food and Drug Administration has recommended that children two years old and younger should not be given over the counter cough and cold products due to the possibility of serious life threatening adverse events, including convulsions, rapid heart rate and decreased levels of consciousness (Rimsza and Newberry 2008; Kuehn, 2008; Schaefer et al., 2008). Opioids, such as codeine, are effective antitussive drugs but their perceived standing as the “gold standard” is currently being debated in the research community (Bolser and Davenport, 2007). Moreover, opioids have significant liabilities (i.e. addiction potential, gastrointestinal constipation, respiratory depression, etc.) that may limit their use as a cough suppressant. Thus, new and innovative pharmacological approaches to cough suppression are needed to fill a significant unmet medical need.
We have previously proposed that selective non-peptide orally available nociceptin/orphanin FQ peptide (NOP) receptor agonists may be useful for the treatment of cough (McLeod et al., 2002, 2003, 2004, 2009). NOP is a G protein coupled receptor that has been found to share homology with classical opioid receptors, namely, μ (MOP), κ (KOP) and δ (DOP) receptors (Bunzow et al., 1994; Mollereau et al., 1994; Meunier et al., 1995; Costentin, 1995). However, it is clear from the scientific literature that a pharmacological distinction can be made between NOP and MOP receptors. Specifically, opioid peptides do not display significant affinity for NOP receptors and nociceptin/orphanin FQ (the endogenous ligand for NOP) does not simulate classical opioid receptors. Like MOP receptors, NOP receptors are widely distributed within and outside of the central nervous system. Indeed, activation of central NOP receptors has been shown to produce several behavioral effects such as pain modulation, stimulation of food intake, sedation and modulation of anxiety states (Calo et al., 2000). In the periphery, the pharmacology of NOP receptors may also have diverse pharmacology including effects on cardiovascular, genitourinary and gastrointestinal systems (Giuliani et al., 2000; Kapusta, 2000).
In the respiratory system vagal airway C-fibers, which play a role in the initiation of cough, are endowed with nociceptin/orphanin FQ receptors. Activation of these receptors triggers activation of inward-rectifying K+ channels resulting in cell membrane hyperpolarization of these sensory nerves and attenuation of tussigenic nerve activity (Fisher et al., 1998; Jia et al., 2002; Canning, 2007). The most noteworthy support for the progression and development of a selective NOP receptor agonist for cough is based on results from experimental animal models. McLeod et al. (2001) demonstrated that nociceptin/orphanin FQ given centrally (into the cerebroventricular system) or peripherally by the intravenous route suppresses capsaicin-induced cough in guinea pigs. Similar results in the guinea pig were reported with the nociceptin/orphanin FQ peptide against cough evoked by acid inhalation (Lee et al., 2006). Bolser et al. (2001) demonstrated that nociceptin/orphanin FQ attenuates cough frequency and cough intensity due to mechanical stimulation of the airways in the cat. Using a non-peptide selective nociceptin/orphanin FQ standard, Ro-64-6198 ((1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one) given by the intra-peritoneal route, we confirmed that capsaicin-evoked cough in the guinea pig could be suppressed by NOP receptor activation (McLeod et al., 2004). However, Ro-64-6198 is not likely a suitable compound to advance into a clinical proof of concept cough study as the drug is not readily orally absorbed. We recently reported on the preclinical antitussive profile of the selective NOP receptor agonist, SCH 225288 (McLeod et al., 2009). This compound is orally absorbed from the gastrointestinal tract, demonstrated antitussive properties in the guinea pig (irritant induced), and cat (mechanically-evoked) and showed a strong tendency to inhibit canine infectious tracheobronchitis cough (McLeod et al., 2009). Nonetheless, the pharmacokinetics of SCH 225288 have not been optimized to advance to clinical testing.
Presently, we describe the pharmacological and pharmacokinetic profiles of SCH 486757 (8-[Bis(2-Chlorophenyl)Methyl]-3-(2-Pyrimidinyl)-8-Azabicyclo-[3.2.1.]Octan-3-Ol) whose structure is displayed in Fig. 1. SCH 486757 is a non-peptide, orally active NOP receptor agonist with excellent selectivity over classical opioid receptors. The drug reduces cough in experimental cough models through activation of NOP receptors. In contrast to opioids, SCH 486757 does not display potential for abuse/dependency as per results from a rat conditioned place preference model. Finally, SCH 486757 displays a good oral pharmacokinetic profile in several animal species. Consequently, SCH 486757 has been advanced to evaluation in human clinical proof of concept cough trials.
Fig. 1.
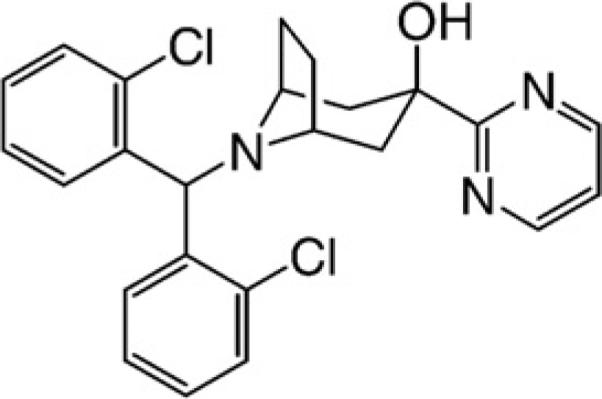
Chemical structure of SCH 487675 ((8-[Bis(2-Chlorophenyl)Methyl]-3-(2-Pyrimidinyl)-8-Azabicyclo-[3.2.1.]Octan-3-Ol)).
2. Materials and methods
2.1. Binding assay
NOP and opioid receptor binding assays were performed as described by Fawzi et al. (1997) and Corboz et al. (2000).Briefly, Chinese hamster ovary cell membranes expressing the human NOP receptors were incubated with [125I][Tyr14]nociceptin/orphanin FQ and increasing concentrations of compounds in binding assay buffer containing 50 mM 4-(2hydroxyethyl)-1-piperazine-ethane-sulfonic-acid (HEPES) pH 7.4, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM NaCl, 0.025% bacitracin, and 0.1% bovine serum albumin. Assays were performed at room temperature for 1 h and were terminated by rapid filtration over glass fiber filter (grade GF/B) membranes. Radioactivity retained on filters was determined in a Packard Top-Count microplate scintillation counter. Ki values were determined using curve fitting and data analysis by the program GraphPad Prism (GraphPad Software, San Diego, Ca, USA). All assays were performed in duplicates. Total and non-specific binding was determined in quadruplicates.
Opioid receptor binding assays were performed on Chinese hamster ovary cell membranes expressing the human opioid receptors (Receptor Biology, Beltsville, MD, USA) as described by Corboz et al. (2000).Briefly, Chinese hamster ovary cell membranes were incubated with [3H] diprenorphine and increasing concentrations of compounds in binding assay buffer for 1 h at room temperature. Assays were terminated by rapid filtration over glass fiber filter (grade GF/B) membranes and radioactivity retained on filters was counted in a Packard Top-Count. Ki values were determined using curve fitting and analysis of data by the program GraphPad Prism as described above.
2.2. Additional receptor Counterscreen binding characterization
SCH 486757 was run against a panel of receptor binding assays (MDS Pharma Services, Taipei, Taiwan). Methods used in all receptor assays were adapted from the scientific literature and are available upon request from MDS Pharma Services. SCH 486757 was tested in duplicated at 10 μM. If SCH 486757 was active (i.e. greater that 50% inhibition) then semi-quantitative analysis and determination of Ki values were performed.
2.3. Functional GTPγS binding assay
Guanosine 5′-g thiotriphosphate (GTPγS) binding assays were performed as described by Fawzi et al. (1997). Chinese hamster ovary cell membranes expressing human NOP and MOP receptors were incubated for 30 min with compounds and [35S]GTPγS in buffer containing 50 mM HEPES (pH 7.4), 120 mM NaCl, 3 mM MgCl2, 0.2 mM EGTA, 1 μM guanosine 5′-diphosphate, and 1 mg/mL bovine serum albumin. Reactions were terminated by filtration as described above.
2.4. Cough studies
2.4.1. Animal care and use
These studies were performed in accordance to the National Institute of Heath Guide to the Care and Use of Laboratory Animals and the Animal Welfare Act in an AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care)-accredited program.
2.4.2. Capsaicin-evoked cough in guinea pigs
The antitussive profile of oral SCH 486757 was evaluated in conscious guinea pigs against capsaicin-induced cough using established methods (McLeod et al., 1998). Briefly, overnight fasted guinea pigs were placed in a transparent 14 in. × 4 in. Plexiglas cylinder chamber and exposed to aerosolized capsaicin (300 μM, for 4 min) produced by an Ultra-NeB 99 Devilbiss nebulizer (Somerset, PA) to elicit cough. Each animal was exposed only once to capsaicin. The number of coughs was detected by a microphone placed in the chamber and verified by a trained observer. The signal from the microphone was relayed to a polygraph that provided a record of the number of coughs. The antitussive dose response characteristics of SCH oral (p.o.) 486757 (0.01–1.0) were studied at 2, 4, 6 and 24-hour post treatment. As comparators, the antitussive activities of hydrocodone (1.0–30 mg/kg, p.o.), codeine (1–60 mg/kg, p.o.), dextromethorphan (3.0–100 mg/kg, p.o.) and baclofen (0.03–3.0 mg/kg, p.o.) were also examined. In two separate studies, the nociceptin/orphanin FQ receptor antagonist, ((1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one)) (J113397; Bigoni et al., 2000) and the classical opioid receptor antagonist naltrexone were used to determine the receptor subtype involved in the antitussive effects of SCH 486757 (3.0 mg/kg, p.o.). Doses of J113397 and naltrexone used in the current study were selected based on previous experience with these drugs in the guinea pig (McLeod et al., 2001, 2009). Intra-peritoneally (i.p.) administration of J113397 (12 mg/kg) was given in two equal divided doses (i.e. 6.0 mg/kg, i.p.), 15 min before and 30 min after SCH 486757. Naltrexone (10 mg/kg, p.o.) was given 15 min before SCH 486757. In these experiments, cough responses to capsaicin were examined 2 h after SCH 486757 treatment. In each series of experiments, treatment responses were compared to vehicle control animals exposed only to capsaicin.
In a separate set of guinea pig studies, the potential for SCH 486757 to produce tolerance to its antitussive effect after a 5-day twice a day (BID) dosing regimen was studied. Guinea pigs were administered repeated doses of SCH 486757 (1 mg/kg, p.o.). These animals received SCH 486757 (1 mg/kg, p.o., BID) for 4 consecutive days (subacute regimen). On the fifth day, they were given only the morning dose of SCH 486757 and cough responses were evaluated 2 h later. A second group of guinea pigs were administered methylcellulose (0.4%) for 4 days and on the fifth day given a single dose of SCH 486757 (1 mg/kg, p.o.; acute regimen). Subsequent cough activity was evaluated in these animals at 2 h post treatment.
2.4.3. Feline mechanically-induced cough studies
Feline mechanically-induced cough studies were conducted using methods previously described (Bolser et al., 1993). Cats were anesthetized with pentobarbital sodium (35 mg/kg, i.p.). Supplemental anesthetic was administered intravenously as required (5.0 mg/kg). Intravenous atropine sulfate (0.3 mg/kg) was administered to block reflex airway secretions. The trachea, femoral artery, and femoral vein were cannulated in all animals. In some animals, the left vertebral artery was cannulated. Electromyograms from the parasternal (inspiratory) and rectus abdominis (expiratory) muscles were recorded with bipolar tungsten wire electrodes. The electromyograms were amplified, filtered (0.5–10 kHz), and integrated with a resistance–capacitance circuit (100 ms time constant). The integrated electromyograms were displayed on a chart recorder. Cough was defined as a large burst of electromyogram activity in the diaphragm immediately followed by a burst of electromyogram activity in the rectus abdominis muscle. Coughing was produced by mechanical stimulation of the intrathoracic trachea with a thin flexible polyethylene cannula for 10 s per stimulus trial.
The antitussive activity of SCH 486757 (1–300 μg/kg) was evaluated from cumulative dose responses obtained after intravertebral artery administration. Control values were obtained by averaging the number of coughs during five consecutive mechanical stimulus trials after vehicle administration. One minute elapsed between stimulus trials. Stimulus trials were applied at 1-minute intervals after each dose of compound for a total of five stimulus trials between doses. Approximately 5 min elapsed between each dose of compound. Cough measurements included cough number (i.e. the number of coughs per stimulus trial), abdominal expiratory muscle electromyogram amplitude, and inspiratory muscle electromyogram amplitude. The cough response after vehicle or each dose of compound was assessed by averaging cough number, abdominal muscle electromyogram amplitude, and inspiratory muscle electromyogram amplitude during the five stimulus trials.
2.5. Rat conditioned place preference
The conditioned place preference apparatus consisted of Plexiglas boxes (86 × 32 × 31 cm; L × W × H) divided into two equally sized visually distinctive compartments at both ends and one smaller central compartment. Each rat was pre-exposed to the entire apparatus for 15 min with free access to the two end compartments. Then the compartment doors were shut. Rats were randomly and equally assigned to receive test compound in one of the two sides of the apparatus. Each rat received an oral 0.4% methylcellulose vehicle first and was then placed into the non-drug paired side for 30 min. One hour later, each rat received SCH 486757 at a dose of 10 mg/kg, and then 2 h later the rats were placed into the drug paired side for another 30 min. This conditioning procedure was repeated 3 more times for a total of 4 pairings over 4 consecutive days. Twenty-four hours after the final pairing, rats were placed into the conditioned place preference apparatus with the compartment doors opened and each drug-free rat was placed into the smaller middle compartment and allowed free access to the chambers for 15 min. The time spent exploring both the drug paired side and non-drug paired side was recorded and analyzed for treatment effects. Rats exhibiting any preference for a drug should spend significantly more time exploring the DPS.
To validate the conditioned place preference apparatus and procedure, studies using two known drugs of abuse, amphetamine and morphine, were conducted in the conditioned place preference procedure, prior to the study with SCH 486757. Both drugs have historically been shown to produce a robust conditioned place preference (Spyraki et al., 1982; Bardo et al., 1995). In both cases, the experimental procedure was identical to that used to test SCH 486757, except there was no pretreatment time.
2.6. Pharmacokinetic studies
2.6.1. Pharmacokinetic profile in the guinea pig
A pharmacokinetic study was conducted in the guinea pig with SCH 486757 to determine systemic exposure and the pharmacokinetic profile after oral dosing in this species. SCH 486757 was dosed orally to guinea pigs at 0.03 mg/kg (n = 4) and 0.30 mg/kg (n = 5) in a 0.4% (w/v) methylcellulose suspension. Blood samples were collected at 1, 2, 3, 4, 6 and 24 h post dose. Plasma was isolated from the samples and the plasma was stored in tubes and frozen at −20 °C until assayed via a high-performance liquid chromatography-atmospheric pressure ionization tandem mass spectrometry (HPLC-API-MS/MS) procedure.
2.6.2. Pharmacokinetic profile in the rat
A pharmacokinetic study was conducted in the rat with SCH 486757 to determine systemic exposure and the pharmacokinetic profile after oral dosing. SCH 486757 was dosed to male Sprague–Dawley rats at 10 mg/kg (n = 3) in a 0.4% (w/v) methylcellulose suspension. Blood samples were collected at 0.25, 0.5 1, 2, 4, 6, 8 and 24 h post dose. Plasma was isolated from the samples and the plasma was stored in tubes and frozen at −20 °C until assayed via a HPLC-API-MS/MS procedure.
2.6.3. Pharmacokinetic profile in the dog
A pharmacokinetic study was conducted in the dog with SCH 486757 to determine systemic exposure and the pharmacokinetic profile after oral dosing. SCH 486757 was dosed orally to male Beagle dogs at 3 mg/kg (n = 3) in a 0.4% (w/v) methylcellulose suspension. Blood samples were collected at 0.25, 0.5 1, 2, 4, 6, 8, 24, 48 and 72 h post dose. Plasma was isolated from the samples and the plasma was stored in tubes and frozen at −20 °C until assayed via a HPLC-API-MS/MS procedure. In addition, a pharmacokinetic study was conducted in the dog with SCH 486757 to determine systemic exposure and the pharmacokinetic profile after intravenous dosing. In these studies SCH 486757 was given to male Beagle dogs at 3 mg/kg (n = 3) in a 20% (w/v) hydroxypropyl-β-cyclodextrin solution. Blood samples were collected at 0.125, 0.25, 0.5 1, 2, 4, 6, 8, 24, 48 and 72 h post dose. Plasma was isolated from the samples and the plasma was stored in tubes and frozen at −20 °C until assayed via a HPLC-API-MS/MS procedure.
2.6.4. HPLC-API/MS/MS assay of plasma samples
Plasma samples were assayed for SCH 486757 using HPLC-API-MS/MS methodology as has been described previously (Korfmacher et al., 1997; Korfmacher, 2005; Ackermann, 2008; Hsieh, 2008). Typically, a 50-μL plasma sample was placed in a 1-mL 96-well plate cell and was then subjected to protein precipitation by adding 150 μL of acetonitrile which contained the internal standard. After vortexing for about 1 min, the samples were centrifuged at 12,000 g for 10 min and then 100 μL of the supernatant was transferred to a clean 350-μL 96-well plate. The samples and standards were then injected onto the HPLC-API-MS/MS system (typical injection volumes were 10–25 μL). The HPLC system consisted of a Shimadzu LC-10AD pump and a LEAP CTC HTS PAL autosampler. Chromatographic separation of SCH 486757 and the internal standard was obtained on a Phenomenex MAXRP (4 μ particle size) 30×2 mm (diameter) HPLC column using a gradient mobile phase system. The mobile phase consisted of two solvents, A and B. Solvent A was 20:80:methanol:(water+10 mM ammonium acetate); solvent B was methanol+10 mM ammonium acetate+0.6 mL of 10% acetic acid/L. The gradient was 99% A at the start, then ramped to 95% A at 0.1 min, then ramped to 5% A at 1.2 min and held until 2.1 min and then ramped back to 99% A at 2.3 min and held for 0.2 min. The mobile phase flow rate was held at 1.0 mL/min throughout the assay. The HPLC effluent was sent directly to the API-MS/MS source. The API-MS/MS system was a Thermo-Finnigan TSQ 7000 system operated in the electrospray (ESI) mode. The selected reaction monitoring (SRM) transition for SCH 486757 was m/z 440 to m/z 235 with a collision energy setting of 26 eV. Calibration samples were prepared to match the expected levels of the samples (typically 0.5–500 ng/mL) by spiking known concentrations of SCH 486757 into drug-free rat, dog or guinea pig plasma (as needed, depending on the sample set). The calibration curve was processed and assayed along with the samples and the resulting standard curve was obtained after using least-squares linear regression with suitable weighting (typically 1/x2) and used peak area ratios of the analyte and internal standard vs the nominal concentrations.
2.7. Drugs
[125I][Tyr14]nociceptin/orphanin FQ (2200 Ci/mmol) was obtained from Amersham-Pharmacia Biotech (Cardiff, UK). [3H]Diprenorphine (58 Ci/mmol) was purchased from New England Nuclear (Boston, MA, USA). Capsaicin was purchased from Sigma Chemical Co. (St. Louis MO, USA) and J113397 ((1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one)) and SCH 486757 ((8-[Bis(2-Chlorophenyl)Methyl]-3-(2-Pyrimidinyl)-8-Azabicyclo-[3.2.1.]Octan-3-Ol)) were synthesized at Schering Plough Research Institute. Drug doses refer to their respective free bases. Capsaicin was dissolved in 10% ethanol and 90% physiological saline (0.9%). For feline studies, SCH 486757 was dissolved in 2-hydroxypropyl-β-cyclodextrine. All other drugs were dissolved in physiological saline.
2.8. Statistics
Data from the cough studies are expressed as % inhibition of capsaicin-induced cough. Values displayed in the figures represent the Mean±S.E.M of 6–12 animals per group. Cough data were evaluated using a non-parametric Kruskal Wallis in conjunction with a Mann Whitney U. Statistical significance was set at P<0.05. Comparison for conditioned place preference studies was made using a paired t-test (significance level set at P<0.05).
3. Results
3.1. Binding studies
3.1.1. Receptor binding profile
SCH 486757 inhibits [125I] nociceptin/orphanin FQ binding to the human NOP receptor in a concentration-dependent manner. In competition binding assays to the human NOP receptor expressed in Chinese hamster ovary cells, SCH 486757 displaces [125I] nociceptin/orphanin FQ binding with a Ki of 5 nM (Table 1). In similar assays using cloned human MOP, KOP, and DOP opioid receptors, SCH 486757 displays a 211-, 128-, and 3206-fold selectivity respectively over human NOP receptor binding (Table 1). SCH 486757 inhibits [125I] nociceptin/orphanin FQ binding to the NOP receptor from several different species with Ki values similar to that of the human receptor (Table 2). SCH 486757 did not show significant affinity for variety receptors and ion channels in MDS experiments (Table 3). These studies indicate that SCH 486757 is highly selective for the NOP receptor. In a functional cell based assay, SCH 486757 causes full activation of the human NOP receptor leading to an increase in [35S] guanosine 5′-g thiotriphosphate binding with an EC50 of 79 ± 12 nM (n = 12). In similar functional assays in a cell line expressing the human MOP opioid receptor, SCH 486757 acts as a weak partial agonist (i.e. 10 μM SCH 486757 induces <50% of maximal agonist-induced response; n = 4). These results indicate that SCH 486757 is a selective, full agonist at the NOP receptor.
Table 1.
Selectivity of SCH 486757 against opioid receptors.
| Receptor | Ki (nM) | Fold selectivity |
|---|---|---|
| NOP | 4.6 ±0.61 (16) | 1 |
| MOP | 972 ±40 (4) | 211 |
| KOP | 590 ±40 (4) | 128 |
| DOP | 14747 ±2558 (4) | 3206 |
Receptor binding (Ki) values of SCH 486757 for human, NOP, MOP, DOP and KOP receptors. Values represent the Mean±S.E.M. The number of experiments is shown in parentheses.
Table 2.
Species selectivity of SCH 486757.
| NOP receptor | Source | Ki (nM) |
|---|---|---|
| Human | Clone (CHO cells) | 4.6±0.61 (16) |
| Guinea pig | Brain (cortex) | 5.4±0.32 (3) |
| Cat | Brain | 6.7±0.67 (3) |
| Dog | Brain | 5.3±0.71 (3) |
| Rat | Clone (CHO cells) | 6.6±0.15 (3) |
Comparative receptor binding (Ki) values of SCH 486757 for human, guinea pig, cat, dog mouse and rat NOP receptors. Values represent the Mean±S.E.M. The number of experiments is shown in parentheses.
Table 3.
MDS Pharma Services Counterscreen Profile.
| Receptor | Ki (μM) |
|---|---|
| Serotonin 5-HT6 (human) | 2.35 |
| Dopamine D3 (human) | 3.66 |
| Sodium channel, site 2(rat) | 5.58 |
| Sigma,(non-selective-guinea pig) | 7.12 |
| Dopamine transporter(human) | 8.43 |
Receptor binding characterization of SCH 486757. Please note that SCH 486757 did not display significant activity at the following MDS Pharma enzyme and receptor binding assays: benzodiazepine, bradykinin (B1 and B2) dopamine (D1, D2, and D4), GABA transporter, GABAA (agonist site, benzodiazepine site, and chloride channel), GABAB, glutamate (non-selective, AMPA, kainite, NMDA agonist, NMDA glycine, NMDA phencyclidine, and NMDA polyamine), glycine (strychnine-sensitive), serotonin (5-HT1 non-selective, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2, 5-HT2A, 5-HT3, 5-HT4, 5-HT5A, 5-HT7, and 5-HT transporter), tachykinin (NK1, NK2, and NK3).
3.2. Guinea pig cough studies
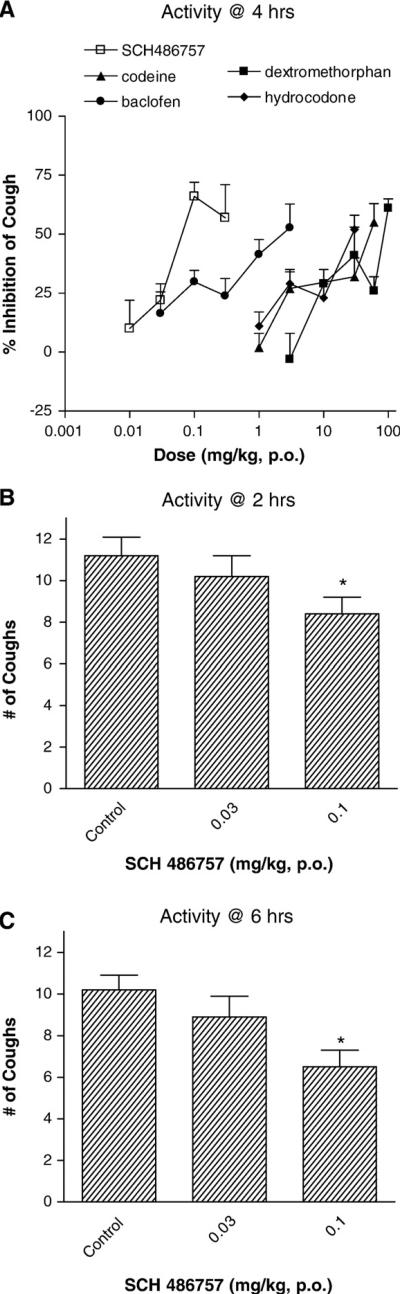
The antitussive duration of SCH 486757 was evaluated in the guinea pig. At 2, 4, 6 and 24 h after oral administration, SCH 486757 produced a dose-dependent attenuation of cough (Fig. 2). The maximum potency (ED50 = 0.04 mg/kg, p.o.) and efficacy (66 ± 6% cough suppression) of SCH 486757 against capsaicin-induced cough were observed at 4 h (Fig. 2A). Significant antitussive activity was observed at 2 and 6 h at a dose of 0.1 mg/kg (Fig. 2C). At 24 h SCH 486757 (1 mg/kg, p.o.) did not inhibited cough (data not shown). Fig. 2A illustrates the comparative antitussive activities of codeine and SCH 486757 at 4 h. Both SCH 486757 and codeine produced comparable antitussive efficacies (Fig. 2A). It must be pointed out that doses of codeine and dextromethorphan higher than 60 mg/kg and 100 mg, respectively, produced significant CNS effects (i.e. lethargy, ataxia). When compared to other standard antitussive drugs such as dextromethorphan, hydrocodone and baclofen, SCH 486757 was more potent.
Fig. 2.
Antitussive effect of orally administered SCH 486757 and various antitussive standards in the guinea pig. Panel A illustrates the dose-dependent antitussive effect of SCH 486757 (0.01–0.3 mg/kg, p.o.) against capsaicin-evoked cough 4 h after administration. Codeine, hydrocodone, dextromethorphan and baclofen were studied as antitussive comparators. Panels B and C display the cough suppressant actions of at SCH 486757 at 2 and 6 h post oral administration. Each bar represents the Mean ± S.E.M. (*P<0.05 compared to control animals; n = 7–15 animals per treatment group).
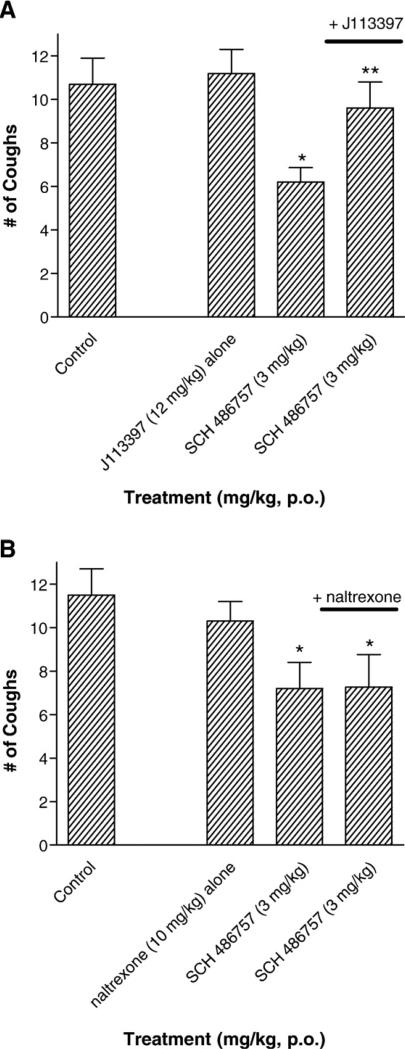
The contribution of NOP and MOP opioid receptors to the antitussive effect of SCH 486757 was also studied. Guinea pigs were pretreated with either J113397 (NOP receptor antagonist) or naltrexone (MOP opioid receptor antagonist). Fig. 3 shows that the antitussive effect of SCH 486757 (3 mg/kg, p.o.) was blocked by J113397 (12 mg/kg, i.p.) but not by naltrexone (10 mg/kg, p.o.). Neither naltrexone nor J113397 given alone had any actions on capsaicin-induced cough responses (Fig. 3).
Fig. 3.
Effect of NOP and MOP receptor blockade on the antitussive effect of orally administered SCH 486757. Panel A illustrates the antitussive effect of SCH 486757 (3 mg/kg, p.o.) administered alone and in guinea pigs treated with J113397 (12 mg/kg, i.p.). In separate studies the antitussive effect of SCH 486757 in the absence or presence of naltrexone (10 mg/kg, p.o.) is shown in Panel B. Each bar represents the Mean±S.E.M. (*P<0.05 compared to control animals; **P<0.05 compared to SCH 476757 treatment alone; n = 6–11 animals per treatment group).
SCH 486757 does not produce tolerance to its antitussive activity after a 5-day twice a day dosing regimen. After acute and chronic dosing paradigms, SCH 486757 (1 mg/kg) inhibited capsaicin-evoked coughing by 46 ± 9% and 40 ± 11%, respectively. There was no difference between these values.
3.3. Antitussive activity in the cat
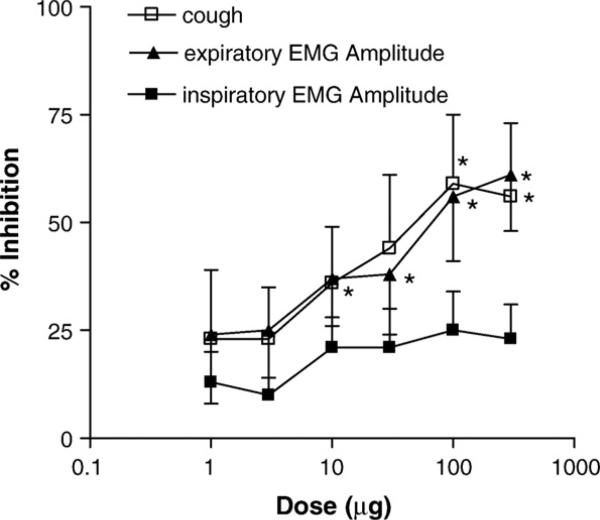
The antitussive activity of SCH 486757 was evaluated against mechanically-induced cough in the cat. Fig. 4 shows the antitussive effect of intra-arterial SCH 486757 on cough number, abdominal electromyogram amplitude, and inspiratory muscle electromyogram amplitude after intra-vertebral artery (0.001–0.3 mg/kg; 45% hydroxypropyl-β-cyclodextrin vehicle) administration. SCH 486757 inhibited both cough number and abdominal electromyogram amplitude in a dose-dependent manner with a maximum efficacy of approximately 60%. At the doses tested SCH 486757 had no significant effect on inspiratory electromyogram amplitude.
Fig. 4.
Influence of intra-arterial SCH 486757 on cough frequency in the cat. Figure shows that SCH 486757 (1–300 μg) produces a dose-related antitussive effect when administered intra-arterial routes. Values on the graph represent the Mean ± S.E.M. (*P<0.05 relative to vehicle control responses).
3.4. Abuse liability potential
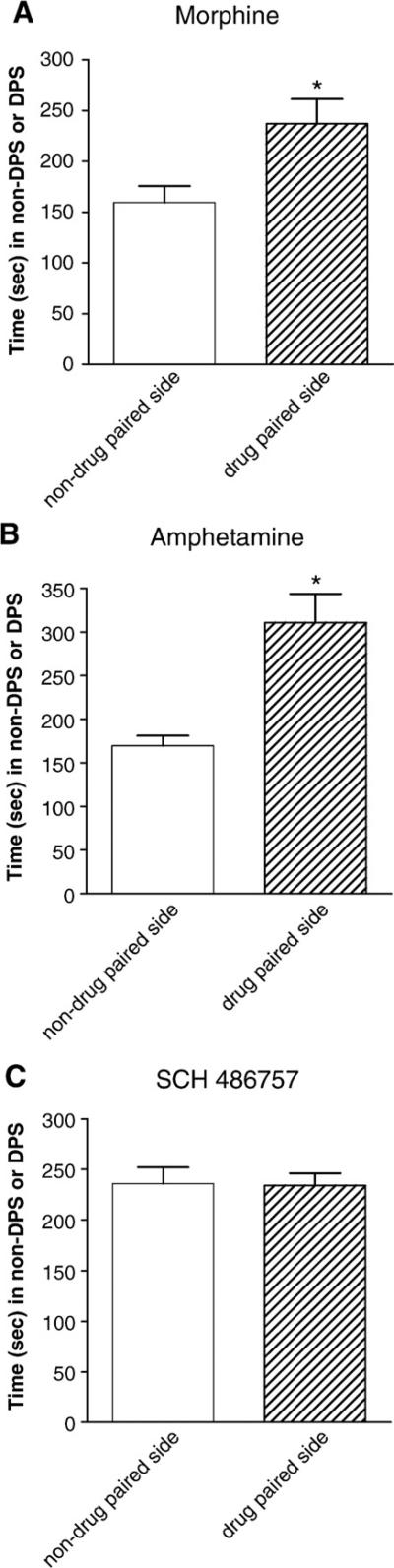
Pre-exposure of rats to conditioned place preference apparatus prior to all three drug studies revealed that the apparatus was unbiased since the drug-free animals spent equal time in both sides. SCH 486757, at a dose of 10 mg/kg, did not produce any conditioned place preference as measured by time spent in the drug paired side and additionally, SCH 486757 did not alter the number of entries into the drug paired side (Fig. 5). In contrast, both amphetamine (3 mg/kg) and morphine (10 mg/kg) induced a marked conditioned place preference. Pairings with both amphetamine and morphine produced significant increases in the time spend in the drug paired side.
Fig. 5.
Effect of SCH 486757 on side preference in the conditioned placed test. Data represent time (Mean +/− S.E.M.; n = 8–16) spent on each side on the conditioned placed apparatus. *P<0.05 compared to non-drug paired side. The figure shows that morphine (Panel A) and amphetamine (Panel B) increased the time spent on the drug paired side. In contrast, SCH 486757 had no effect on conditioned place preference.
3.5. Pharmacokinetic profile in the guinea pig, rat and dog
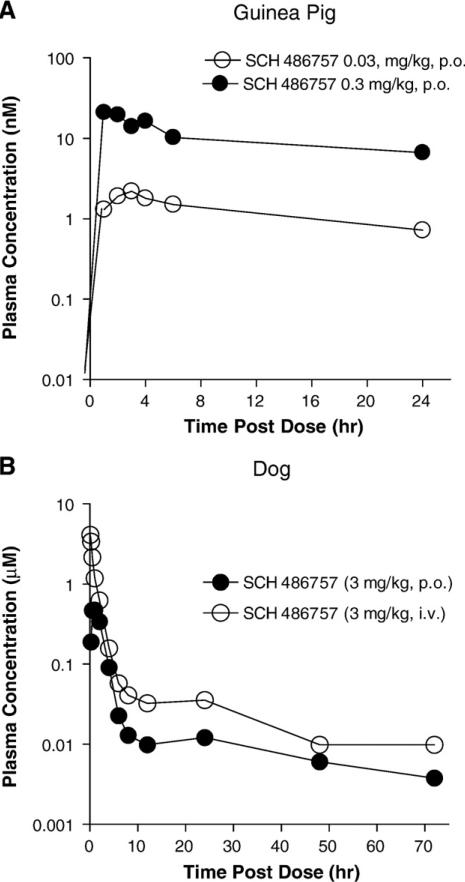
The pharmacokinetic profile in the guinea pig showed a dose-related increase for the two doses tested. For 0.03 mg/kg p.o., the Cmax, Tmax and AUC values were 3.1 nM, 3 h and 27 nM × hour, respectively. For 0.30 mg/kg dose level the Cmax, Tmax and AUC values were 31 nM, 3 h and 242 nM × hour, respectively. The mean pharmacokinetic profiles for the two doses are shown in Fig. 6. Our rat pharmacokinetic profiling indicated that there was plasma exposure of SCH 486757 after a 10 mg/kg dose. The Cmax, Tmax and AUC values were 127 nM, 3.3 h and 1290 nM × hour, respectively. The SCH 486757 (3 mg/kg, p.o.) dog pharmacokinetic profile is shown in Fig. 6.The Cmax, Tmax and AUC values were 540 nM, 0.8 h and 1840 nM × hour, respectively. The dogs that were dosed intravenously provided a measure for the half-life (t1/2) of SCH 486757 in this species. The mean AUC, percent bioavailability (F%) and t1/2 values for SCH 486757 were 5680 nM × hour, 32% and 7.9 h, respectively.
Fig. 6.
Pharmacokinetic profile in the guinea pig and dog. Panel A displays the mean pharmacokinetic profile of SCH 486757 (0.03 mg/kg and 0.3 mg/kg, p.o.; n = 4–5) in guinea pigs. Panel B illustrates the plasma exposure characteristics of SCH 486757 (3 mg/kg) after oral (n = 3) and intravenous (n = 3) dosing in the dog.
4. Discussion
Cough suppressants are among the most widely used drugs in the world (Choudry and Fuller, 1992). However, the marginal efficacy of over the counter products and the significant side effect profile (e.g. sedation, gastrointestinal constipation, respiratory depression, and addiction liability) of the opioid prescription drugs strongly support an urgent need for the development of new, effective cough suppressant therapies. Currently, we describe the pharmacological profile of SCH 486757, a drug recently entered into human clinical proof of concept studies (P03069 and P04887) as a novel agent for the treatment of cough (chemical structure shown in Fig. 1). The present preclinical characterization of SCH 486757 demonstrates that SCH 486757 displays high affinity binding for human, guinea pig, rat, dog and cat NOP receptors. Additionally, SCH 486757 has 211-, 178- and 3206-fold selectivity for NOP over MOP, KOP and DOP receptors and displays insignificant binding in a variety of additional receptor and ion channel assays. Finally, in vitro SCH 486757 is a full NOP agonist but only a weak partial MOP agonist in functional human guanosine 5′-g thiotriphosphate membrane binding studies.
In experimental cough models, SCH 486757 is a potent and efficacious antitussive agent. Against capsaicin-evoked cough in conscious guinea pigs, SCH 486757 produced a level of antitussive efficacy equivalent to codeine, hydrocodone, dextromethorphan and baclofen. However, SCH 486757 was more potent than these agents. The cough suppressing activity of SCH 486757 is mediated by activation of NOP receptors as we show that the antitussive effect of the drug was blocked by a NOP receptor antagonist (Ozaki et al., 2000). In contrast, the classical opioid receptor antagonist naltrexone had no effect on the antitussive activity of SCH 486757. In the cat, SCH 486757 produces a maximum inhibition of mechanically induced cough and expiratory abdominal electromyogram amplitude of 59 and 61%, respectively. SCH 486757 did not significantly affect inspiratory electromyogram amplitude suggesting that the drug does not behave as a respiratory depressant. Indeed, in safety pharmacology rat studies SCH 486757 did not affect blood gases or respiratory function parameters at oral doses up to 30 mg/kg (data not shown). Taken together, our studies are consistent with previous experiments indicating that NOP receptor agonists, the endogenous peptide nociceptin/orphanin FQ and small molecules, from varying chemical structures, are effective in inhibiting cough in experimental models (McLeod et al., 2001, 2004, 2009; Bolser et al., 2001; Lee et al., 2006; Ho et al., 2009; Yang et al., 2009).
Opioid antitussive agents often produce mechanism based drug dependency. Presently, we examined the abuse potential of SCH 486757 in a rat conditioned place preference procedure. This assay is sensitive to classical drugs of abuse, such as amphetamine and morphine. SCH 486757 administration did not affect the proportion of time rats spent in the non-drug paired side and drug paired side. In contrast, both amphetamine and morphine induced significant preference for the drug paired side when tested in the same conditioned place preference procedure. In summary, these studies show that SCH 486757 does not show potential to induce abuse liabilities. Furthermore, there are no scientific reports to our knowledge indicating that NOP receptor agonists have abuse liability potential. On the contrary, recent studies suggest that NOP receptor agonists may have anti-abuse potential, based on the findings with nociceptin/orphanin FQ (Ciccocioppo et al., 2003, 2004; Reinscheid et al., 1996; Zhao et al., 2003).
SCH 486757 was well tolerated in the guinea pig, rat, cat and dogs with no overt behavioral effects. Plasma concentrations of SCH 486757 were determined in guinea pig plasma following an oral dose level of 0.03 mg/kg. Concentrations were also measured following a higher, 0.3-mg/kg dose. The plasma concentrations during the 2- to 4-hour time period, during which cough is suppressed averaged about 2 nM with an average Cmax of 3 nM. Interesting this Cmax concentration is below the NOP binding receptor affinity for SCH 486757. This may suggest that SCH 486757 may metabolize to an active metabolite or that the drug may have central effects. The rat and dog are species often used in the toxicological assessment of drugs advancing through a development pipeline. Thus, it is important to evaluate the pharmacokinetic profile of the advancing agent in these animals. We show that the pharmacokinetic profile of SCH 486757 was acceptable in both the rat and dog.
The ideal antitussive should possess efficacy equivalent to codeine, absence of gastrointestinal side effects, oral activity, a pharmacokinetic profile that supports once a day or twice a day dosing, absence of CNS or respiratory depression liability, and no tolerance or abuse liability. Given the importance of cough as a respiratory defensive mechanism, the drug should not merely ablate cough responses. The ideal antitussive should shift cough responses to a less sensitive trigger while maintaining cough mechanisms intact. From a preclinical perspective SCH 486757 appears to fulfill several of these characteristics. In summary, we show SCH 486757 to be a selective orally active NOP receptor agonist. SCH 486757 does not destroy the integrity of cough reflexes but only attenuates irritant- and mechanically-mediated cough in the guinea pig and cat, respectively. The drug is well tolerated, has a respectable pharmacokinetic profile and does not appear to elicit MOP receptor related side effects, such as addiction liability. Nonetheless, the utility of NOP receptor agonists as antitussive agents has to be placed in the context of efficacy and safety results generated from human clinical trials.
References
- Ackermann B. Current applications of liquid chromatography/mass spectrometry in pharmaceutical discovery after a decade of innovation. Annu. Rev. Anal. Chem. 2008;1:357–396. doi: 10.1146/annurev.anchem.1.031207.112855. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci. Biobehav. Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bigoni R, Calo G, Rizzi A, Guerrini R, DeRisi C, Hashimoto Y, Hashiba E, Lambert DG, Regoli D. In vitro characterization of J113397, a non-peptide nociceptin/orphanin FQ receptor antagonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;361:565–568. doi: 10.1007/s002100000220. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Curr. Opin. Allergy Clin. Immunol. 2007;7:32–36. doi: 10.1097/ACI.0b013e3280115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Sultan AM, DeGennaro FC, Kreutner K, Egan RW, Siegel MI, Chapman RW. Antitussive effect of GABAB agonist in the cat and guinea pig. Br. J. Pharmacol. 1993;110:491–495. doi: 10.1111/j.1476-5381.1993.tb13837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, McLeod RL, Tulshian DB, Hey JA. Antitussive action of nociceptin in the cat. Eur. J. Pharmacol. 2001;430:107–111. doi: 10.1016/s0014-2999(01)01244-4. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Calo G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ. Encoding of the cough reflex. Pulm. Pharmacol. Ther. 2007;20:396–401. doi: 10.1016/j.pupt.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey 2000 Summary. Advance data from vital and health statistics. 2002;328:1–32. [PubMed] [Google Scholar]
- Choudry NB, Fuller RW. Sensitivity of the cough reflex in patients with chronic cough. Eur. Respir. J. 1992;5:296–300. [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Massi M. The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: a review of recent work in alcohol-preferring rats. Physiol. Behav. 2003;79:121–128. doi: 10.1016/s0031-9384(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Helig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behavior by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology. 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corboz MR, Rivelli MA, Egan RW, Tulshian D, Matasi J, Fawzi AB, Benbow L, Smith-Torhan A, Zhang H, Hey JA. Nociceptin inhibits capsaicin-induced bronchoconstriction in isolated guinea pig lung. Eur. J. Pharmacol. 2000;18:171–179. doi: 10.1016/s0014-2999(00)00505-7. [DOI] [PubMed] [Google Scholar]
- Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Fawzi AB, Zhang H, Weig B, Hawes B, Graziano M. N/OFQ activation of the human ORL1 receptor expresses in Chinese hamster ovary cells: functional homology with opioid receptors. Eur. J. Pharmacol. 1997;336:233–242. doi: 10.1016/s0014-2999(97)01227-2. [DOI] [PubMed] [Google Scholar]
- Fisher A, Forssman WG, Undem BJ. N/OFQ-induced inhibition of tachykinergic neurotransmission in guinea pig bronchus. J. Pharmacol. Exp. 1998;268:85–89. [PubMed] [Google Scholar]
- Giuliani S, Lecci A, Maggi CA. N/OFQ and neurotransmitter release in the periphery. Peptides. 2000;21:977–984. doi: 10.1016/s0196-9781(00)00237-0. [DOI] [PubMed] [Google Scholar]
- Ho GD, Anthes J, Bercovici A, Caldwell JP, Cheng KC, Cui X, Fawzi A, Fernandez X, Greenlee WJ, Hey JA, Korfmacher W, Lu SX, McLeod RL, Ng F, Smith-Torhan A, Tan Z, Tulshian D, Varty GB, Xu L, Zhang H. The discovery of tropane derivatives as nociceptin receptor ligands for the management of cough and anxiety. Bioorg Med Chem Lett. 2009;19:2519–2523. doi: 10.1016/j.bmcl.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Hsieh Y. HPLC-MS/MS in drug metabolism and pharmacokinetic screening. Expert Opin. Drug. Metab. Toxicol. 2008;4:93–101. doi: 10.1517/17425255.4.1.93. [DOI] [PubMed] [Google Scholar]
- Irwin RS, Richter JE. Gastroesophageal reflux and cough. Am. J. Gastroenterol. 2000;95:S9–S14. doi: 10.1016/s0002-9270(00)01073-x. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wang X, Aponte SI, Rivelli MA, Yang R, Rizzo C, Corboz MR, Priestley T, Hey JA. Nociceptin inhibits capsaicin-induced guinea pig airway contraction through an inward-rectifier potassium channel. Br. J. Pharmacol. 2002;135:764–770. doi: 10.1038/sj.bjp.0704515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta DR. Neurohumoral effects of orphanin FQ/N/OFQ: relevance to cardiovascular and renal function. Peptides. 2000;21:1081–1099. doi: 10.1016/s0196-9781(00)00246-1. [DOI] [PubMed] [Google Scholar]
- Korfmacher WA. Principles and applications of LC-MS in new drug discovery. Drug Discov. Today. 2005;10:1357–1367. doi: 10.1016/S1359-6446(05)03620-2. [DOI] [PubMed] [Google Scholar]
- Korfmacher WA, Cox KA, Bryant MS, Veals J, Ng K, Watkins R, Lin C-C. HPLC-API/MS/MS: a powerful tool for integrating drug metabolism into the drug discovery process. Drug. Discov. Today. 1997;2:532–537. [Google Scholar]
- Kuehn BM. Citing serious risks, FDA recommends no cold and cough medicines for infants. JAMA. 2008;299:887–888. doi: 10.1001/jama.299.8.887. [DOI] [PubMed] [Google Scholar]
- Lee MG, Undem BJ, Brown C, Carr MJ. Effect of nociceptin in acid-evoked cough and airway sensory nerve activation in guinea pigs. Am. J. Respir. Crit. Care Med. 2006;173:271–275. doi: 10.1164/rccm.200507-1043OC. [DOI] [PubMed] [Google Scholar]
- Lindgren BR, Andersson RG. Angiotensin-converting enzyme inhibitors and their influence on inflammation, bronchial reactivity and cough. A research review. Med. Toxicol. Adverse Drug Exp. 1989;4:369–380. doi: 10.1007/BF03259918. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Mingo G, O'Reilly S, Ruck LA, Bolser DC, Hey JA. Antitussive action of antihistamines is independent of sedative and ventilation activity in the guinea pig. Pharmacology. 1998;57:57–64. doi: 10.1159/000028226. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Parra LE, Mutter JC, Erickson CH, Carey GJ, Tulshian DB, Fawzi AB, Smith-Torhan A, Egan RW, Cuss FM, Hey JA. Nociceptin inhibits cough in the guinea-pig by activation of ORL(1) receptors. Br. J. Pharmacol. 2001;132:1175–1178. doi: 10.1038/sj.bjp.0703954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod RL, Bolser DC, Jia Y, Parra LE, Mutter JC, Tulshian DB, Egan RW, Hey JA. Antitussive effect of nociceptin/orphanin FQ in experimental cough models. Pulm. Pharmacol. Ther. 2002;15:213–216. doi: 10.1006/pupt.2002.0357. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Tulshian DB, Hey JA. Novel pharmacological targets and potential progression of new antitussive drugs. Expert Opin Ther Patents. 2003;13:1501–1512. [Google Scholar]
- McLeod RL, Jia Y, Fernandez X, Parra LE, Wang X, Tulshian DB, Fawzi AB, Smith-Torhan A, Hey JA. Antitussive profile of the NOP agonist Ro64-6198 in the guinea pig. Pharmacology. 2004;71:143–149. doi: 10.1159/000077448. [DOI] [PubMed] [Google Scholar]
- McLeod RL, Tulshian DB, Ho GD, Fernandez X, Bolser DC, Parra LE, Zimmer JC, Erickson CH, Fawzi AB, Jyappa H, Lehr C, Erskine J, Smith-Torhan A, Zhang H, Hey JA. A novel NOP receptor agonist (SCH 225288) attenuates guinea pig irritant-evoked, feline mechanically-induced and canine infectious tracheobronchitis cough. Pharmacology. 2009;84:153–161. doi: 10.1159/000235601. [DOI] [PubMed] [Google Scholar]
- Meunier J-C, Mollereau C, Toll C, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, Guillemot J-C, Ferrara P, Monserrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family-cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Morice AH. Epidemiology of chronic cough. Eur. Respir. Rev. 2002;85:222–225. [Google Scholar]
- Novitsky YW, Zawachi JK, Irwin RS, French CT, Hussey VM, Callery MP. Chronic cough due to gastroesophageal reflux disease: efficacy of antireflux surgery. Surg. Endoscop. 2002;16:567–571. doi: 10.1007/s00464-001-8328-y. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H. In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur. J. Pharmacol. 2000;402:45–53. doi: 10.1016/s0014-2999(00)00520-3. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Ardati A, Monsma FJ, Jr., Civelli O. Structure–activity relationship studies on the novel neuropeptide orphanin FQ. J. Biol. Chem. 1996;271:14163–14168. doi: 10.1074/jbc.271.24.14163. [DOI] [PubMed] [Google Scholar]
- Rimsza ME, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics. 2008;122:e318–e322. doi: 10.1542/peds.2007-3813. [DOI] [PubMed] [Google Scholar]
- Schaefer MK, Shehab N, Cohen AL, Budnitz DS. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783–787. doi: 10.1542/peds.2007-3638. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res. 1982;253:195–203. doi: 10.1016/0006-8993(82)90686-2. [DOI] [PubMed] [Google Scholar]
- Yang S-W, Ho G, Tulshian DB, Greenlee WJ, Tan Z, Zhang H, Fawzi A, Anthes J, Varty G, Fernandez X, McLeod RL, Hey JA. Identification of 3-substituted N-benzhydrl-nortropane analogs as nociceptin receptor ligands for the management of cough and anxiety. Bioorg Med Chem Lett. 2009;19:2482–2486. doi: 10.1016/j.bmcl.2009.03.057. [DOI] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–2385. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]