Abstract
With advancing age, peripheral conduit and resistance arteries lose the ability to effectively dilate owing to endothelial dysfunction. This vascular senescence contributes to increased risk of cardiovascular disease (CVD) with aging. L-arginine plays a role in numerous physiological processes including nitrogen detoxification, immunocompetence, growth hormone (GH) secretion, and insulin secretion. Recently, a considerable amount of attention has been placed on the ability of this amino acid to affect vascular endothelial function. The purpose of this review will be to examine the use of L-arginine as a novel nutritional strategy to potentially stave progression of vascular dysfunction with aging and CVD. Emphasis will be placed on the ability of L-arginine to modulate the vascular inflammatory and systemic hormonal milieu, which in turn may have a positive effect on vascular endothelial function.
Keywords: vascular, endothelial, inflammation, growth hormone
Aging and Vascular Function
Cardiovascular disease (CVD) remains the most salient killer in the United States, and aging is a primary risk factor.1,2 Despite unprecedented improvements in detection and treatment of CVD, mortality from CVD is expected to worsen due to the immutable effect of aging. There are currently over 35 million Americans 65 years of age or older, and the majority of these individuals have some form of CVD.1,2 Even in the absence of other risk factors, aging per se is considered an independent proatherogenic stimulus, increasing morbidity and mortality from myocardial infarction and stroke.3
With advancing age, there is pervasive vascular dysfunction that manifests as diminished peripheral vasodilatory capacity stemming from endothelial dysfunction.4 The vascular endothelium is a single cell layer responsible for numerous autocrine, paracrine, and endocrine functions including regulation of vascular tone, vascular inflammation, cell growth, thrombosis, and platelet function.5 Nitric oxide (NO), a potent vasoactive hormone released by endothelial cells in response to shear stress, plays a key role in maintaining the vascular wall in a quiescent state via inhibition of inflammation, cellular proliferation, and thrombosis.6 Endothelial dysfunction with aging may result from reduced NO production/bioavailability.5 Loss of NO contributes to a shifting of the vascular wall from a quiescent phenotype toward one that involves an activated host defense response.6 Vascular expression of chemokines, cytokines, and adhesion molecules is increased, leading to leukocyte recruitment, platelet aggregation, and the initiation of the atherosclerotic process.6 Thus, endothelial dysfunction occurs early in the atherogenic process and is a prepotent factor in the development of any morphological atherosclerotic changes.6
Available data indicate that endothelial dysfunction is highly prevalent if not ubiquitous in elderly individuals7 and occurs with aging even in the absence of disease.8 As such, endothelial dysfunction may be regarded as primary phenotypic expression of normal human aging, and it has been suggested that this vascular senescence is the likely culprit underlying, in large part, the increased CVD risk associated with aging.9 Endothelial dysfunction has also been implicated in age-associated declines in cognitive function (ie, memory loss10), physical function (ie, reduced performance of activities of daily living),11 and in the pathogenesis of numerous diseases of aging such as hypertension, stroke, erectile dysfunction, and renal dysfunction.6,12 Endothelial dysfunction has been shown prognostic of future cardiovascular events,12–14 and persistent impairment of endothelial function despite optimized pharmacological therapy to reduce atherosclerotic risk factors has an adverse impact on outcome.15 Conversely, interventions that improve endothelial function improve clinical outcome.16,17 Thus, interventions that improve this surrogate end point with aging may prove invaluable.
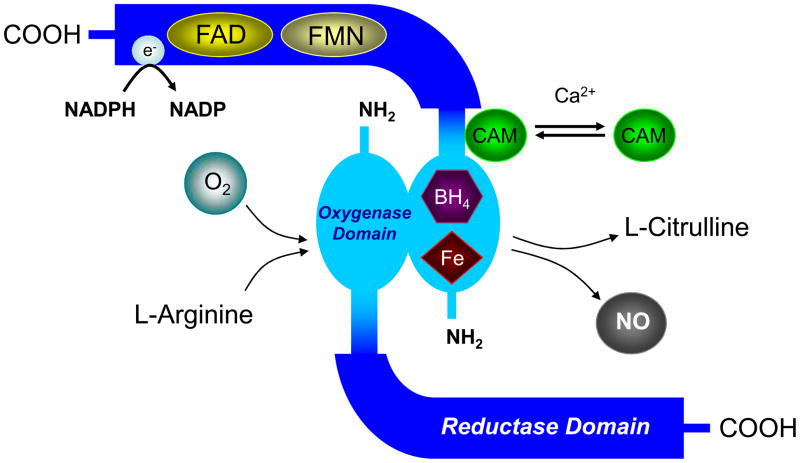
The synthesis of NO requires a precise admixture of substrate and cofactors. Shear stress, due to the viscous nature of blood flow dragging along the vascular wall, activates endothelial nitric oxide synthase (eNOS) via phosphorylation. L-arginine is hydroxylated to N-hydroxy-L-arginine and then further oxidized to NO and L-citrulline. Nitric oxide diffuses into vascular smooth muscle cells, activates guanylate cyclase, and induces cyclic guanosine 3′ ,5′-monophosphate (GMP)-mediated smooth muscle relaxation via the activation of cGMP-dependent protein kinase G (PKG) and subsequent protein phosphorylation of potassium channels, decreased cytosolic calcium levels, and myosin light chain dephosphorylation.18,19 Ultimately this regulates regional blood flow (ie, flow-mediated dilation). Other substrate and cofactors required for this reaction include oxygen, nicotinamide adenine dinucleotide phosphate (NADPH), flavin, heme, and tetrahydrobiopterin (BH4). Tetrahydrobiopterin binds to the heme group of the N-terminal oxidase domain of NOS, stabilizing the dimer molecule (Figure 1). Binding shifts NOS into a high spin state, increasing enzyme activity and increasing the affinity of NOS for arginine.20 Under normal conditions, eNOS, in the presence of sufficient BH4, accepts and stores electrons from NADPH to transform cosubstrates O2 and L-arginine into NO and L-citrulline21 (Figure 1). Tetrahydrobiopterin accepts electrons from a flavin in the C-terminus reductase domain of NOS during the synthesis of NO and L-citrulline, acting as an important redox agent. Dysfunction in any of these steps with aging may set the reaction awry, reduce NO bioavailability, attenuate vasodilatation, and alter regional circulation and tissue perfusion20 (Figure 2).
Figure 1.
Schematic representation of nitric oxide synthase (NOS) dimer formation. BH4 indicates tetrahydrobiopterin; CAM = calmodulin; FAD = flavin adenine dinucleotide; Fe = iron; FMN = flavin mononucleotide; NO = nitric oxide.
Stochiometry of the NOS reaction: L-Arginine + O2 + NADPH → N-OH-L-Arginine + NADP+ + H2O
N-OH-L-Arginine + O2 + 0.5 NADPH → L-Citrulline + NO• + 0.5 NADP+ + H2O
Electron transfer in NOS:NADPH → FAD → FMN → Heme (Fe)
Figure 2.
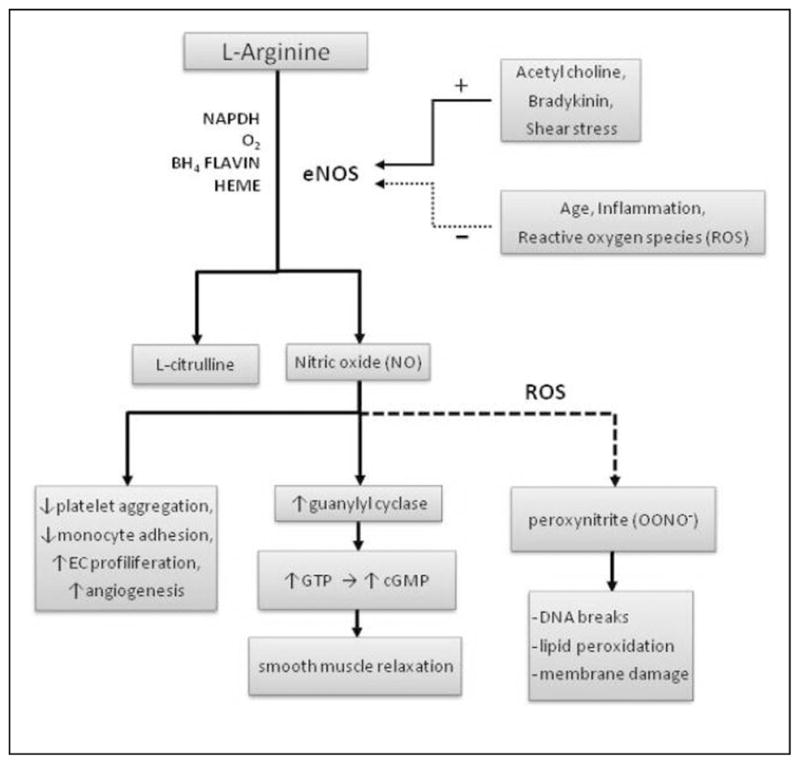
L-arginine and modulators of vascular function.
Reduced substrate may be rate limiting and reduce NO formation and subsequently endothelial-dependent vasodilation. L-arginine (2-amino-5-guanidinovaleric acid) is a nonessential amino acid. The typical Western diet will supply a person with ~3 to 6 g of L-arginine per day.22 Common dietary sources of L-arginine include soy proteins, meats, nuts, dairy, and seafood with a bioavailability of approximately 60%.22 Under catabolic conditions such as surgery or trauma in which growth and/or repair is accelerated, L-arginine may become conditionally essential.23 Aging is associated with deterioration of numerous organ systems, such as lean tissue loss (ie, sarcopenia). Moreover, aging and CVD has become synonymous with inflammation.3 Typically, inflammation leads to recovery and restoration of tissue integrity, but if repair is imperfectly matched to systemic demand, the inflammatory process could result in persistent tissue damage.24 As such, inflammation and oxidative stress have been identified as key factors in the pathogenesis of vascular damage with aging, even in the absence of risk factors or clinical disease.3 Therefore, aging per se may be considered a catabolic/inflammatory state. The use of L-arginine as a nutritional strategy to protect the vasculature from the ravages of aging and disease will now be explored.
L-Arginine and Vascular Function
L-arginine supplementation may have clinical utility with respect to improving symptomology associated with angina, atherosclerosis, coronary artery disease (CAD), erectile dysfunction, heart failure, and intermittent claudication/peripheral vascular disease.22,25,26 This may be related to its effects on vascular endothelial function.
Adams et al27 examined the effect of oral L-arginine on endothelium-dependent dilation in men with CAD. They found that a dose of 7 g taken 3 times per day (21 g/d) for 3 days increased plasma levels of L-arginine as well as improved endothelium-dependent dilation. A similar study done by Clarkson et al28 examined the effects of L-arginine using the same dose (three 7-gram doses per day) over a 4-week time period in hypercholesterolemic young adults. They found that plasma L-arginine levels doubled (115 ±103 to 231 ±125 μmol/L), while endothelial-dependent dilation increased nearly 3-and-a-half fold (1.7% ±1.3% to 5.6% ±3.0%).
In a prospective, double-blind, randomized crossover trial, the effect of L-arginine (16 g daily for 14 days) or placebo on vascular endothelial function was examined in 12 healthy old participants (age 73.8 ±2.7 years).29 L-Arginine significantly improved endothelial-dependent vasodilation (5.7% ±1.2%), whereas placebo had no effect. After L-arginine, plasma levels of L-arginine increased significantly (114.9 ±11.6 vs 57.4 ±5.0 μmol/L), but placebo had no effect. In older patients with stable CAD, L-arginine supplementation has also been shown to improve flow-mediated dilation.30–32
Older patients with CAD and concomitant renal dysfunction may not benefit from L-arginine supplementation.33 Similarly, Gates et al34 has shown that acute intravenous infusion of L-arginine has no effect on endothelial-dependent vasodilation in healthy older individuals. Blum et al35 examined the effects of L-arginine on endothelium-dependent vasodilation in healthy postmenopausal women. Participants took 9 g of L-arginine per day for 1 month. They found that plasma L-arginine increased but with no concomitant change in flow-mediated dilation. Chin-Dusting et al36 and Adams et al37 have shown that L-arginine has no effect on plasma levels of L-arginine, forearm blood flow, or endothelial-dependent vasodilation in healthy men.
At first glance, results from the literature appear to suggest equivocal findings with respect to the effects of L-arginine on vascular function. However, this is far from true. As recently concluded by a meta-analysis, the effects of L-arginine are dependent on initial endothelial health.38 L-arginine supplementation does not appear capable of impacting endothelial-dependent flow-mediated dilation in healthy individuals with more preserved vascular endothelial function. Participants with a high FMD may already have sufficient NO activity, which explains the failure of L-arginine supplementation to increase FMD in such participants. However, in persons with more pronounced overt endothelial dysfunction, L-arginine may be useful.
L-arginine may not be warranted for all aging populations. Although studies have shown that short-term L-arginine therapy improves endothelial function in older patients with peripheral artery disease,39 Wilson et al40 have demonstrated that long-term administration of L-arginine actually has a detrimental effect on endothelial function in this cohort. Six months of L-arginine therapy (3 g/d) reduced NO availability (assessed as flow-mediated vasodilation, plasma and urinary nitrogen oxides, and plasma citrulline formation). It has been suggested that additional L-arginine may lessen the sensitivity of smooth muscle cells to NO release due to shear stress (arginine tolerance).40–42 There may be a counterregulatory nitrate tolerance related to more prolonged exposure as is seen with sustained administration of exogenous NO donors such as nitrogly-cerin.40 As suggested by Wilson et al,40 a transient increase in NO levels could inhibit NO synthase activity by nitrosylation of NO synthase itself or the arginine transporter to counter any arginine-induced increase in NO production. However, this is not a universal finding. Lerman et al43 have shown that long-term L-arginine administration has a favorable effect on coronary endothelial function in patients with coronary endothelial dysfunction and nonobstructive CAD. L-arginine supplementation is also associated with higher postinfarction mortality.44 Therefore, much more research is needed to examine the safety and efficacy of long-term L-arginine administration before this nutritional intervention be recommended as a general panacea for the aging and diseased vasculature.
L-arginine has shown promise as a vascular prophylaxis against endothelial dysfunction induced by acute stressors. For example, acute cigarette smoking and consumption of a high-fat meal are known to significantly reduce endothelial function. Oral L-arginine taken prior to smoking or with a high-fat meal prevents the deleterious effect of these perturbations on endothelial function.45–48
Inflammation and Vascular Function With Aging: L-Arginine as an Anti-Inflammatory Agent
The beneficial effect of L-arginine on the vasculature may also be related to other ancillary properties independent of NO genesis (Figure 2). The mechanisms that govern age-associated deterioration of vascular function are multifaceted, and inflammation/oxidative stress has been identified as a key factor.3 In the absence of adequate L-arginine/BH4 and in the presence of reactive oxygen species (ROS) generated with aging, NOS becomes uncoupled from arginine oxidation, and superoxide is produced from the oxidase domain, leading to the formation of peroxynitrite instead of NO.21,49 Superoxide may further perpetuate BH4 oxidation and eNOS uncoupling.21 Numerous in vitro experiments have demonstrated a causative role of inflammation/oxidative stress in modulating vascular function. In vivo studies have followed en suite highlighting that (1) chronic inflammatory diseases are associated with vascular dysfunction (ie, reduced vasodilatory capacity); (2) acute inflammation/oxidative stress directly impairs vascular function50–53; (3) anti-inflammatory/antioxidant interventions restore and/or prevent inflammation-mediated vascular dysfunction.54 Interventions that reduce inflammation and oxidative stress may have significant clinical utility as a means of restoring/attenuating age-associated declines in endothelial function.
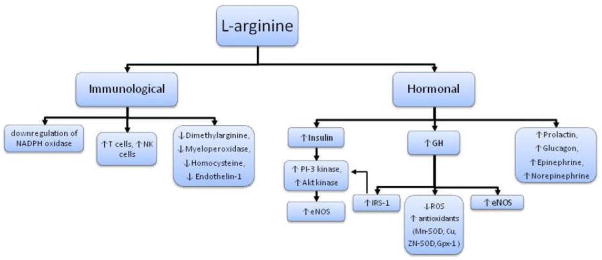
L-arginine has been shown to enhance immune function.55 With increasing plasma concentrations of L-arginine, the ability of lymphocytes to increase the activity of natural killer cells and lymphokine-activated killer cells increases in vivo.55 L-arginine has also been shown to enhance T-cell function and has an immunopreserving effect under conditions of protein malnutrition.56 The ability of L-arginine to affect inflammation and oxidative stress remains sparsely examined. Studies using animal models have demonstrated that L-arginine prevents sodium-induced upregulation of NADPH oxidase.57 Nicotinamide adenine dinucleotide phosphate oxidase is considered one of the principal generators of ROS within the aging vascula-ture.24 In patients with chronic kidney and CVD, L-arginine has been shown to reduce several important markers of inflammation and oxidative stress such as asymmetric dimethylarginine (the endogenous inhibitor of NO), myeloperoxidase (a heme enzyme present in inflammatory cells and catalytic sink for NO), and homocysteine (a stimulator of ROS that causes vascular damage and atherosclerosis).58,59 L-arginine also reduces endothelin-1, a potent vasoconstrictor43 and important modulator of endothelial dysfunction (possibly more important than NO) with advancing age. These preliminary findings in select patient populations would suggest that L-arginine may have anti-inflammatory and antioxidant properties. Whether this contributes in part to the favorable effect of L-arginine on vascular endothelial function requires further study.
Pleotropic Properties of L-Arginine: Effects on Vascular Function
L-arginine can stimulate the secretion of various hormones including growth hormone (GH), insulin, glucagon, epinephrine, norepinephrine, and prolactin.60 Many amino acids stimulate insulin secretion, but L-arginine has been shown to be the most potent.60,61 While insulin resistance is associated with endothelial dysfunction, insulin itself can improve endothelial-dependent vasodilation independent of glucose via a release of NO as a result of the activation of phosphatidylinositol 3 (PI-3) kinase and Akt kinase.62,63
With aging, there is a well-noted decline in GH secretion, and this is responsible for age-associated declines in muscle mass and bone mineral density as well as increases in adiposity. Diminution of GH has also been linked to deterioration of various cardiovascular and immunological functions.64 L-arginine is a potent stimulator of GH and has been used in a clinical setting to determine the responsiveness of the GH axis when a deficiency is suspected.65,66 Numerous studies have demonstrated that patients with GH deficiency have reduced endothelial dysfunction, and augmenting GH in these patients restores endothelial function.67–71 Acute infusion of GH increases endothelial-dependent vasodilation.72 Growth hormone contributes to the late phase of L-arginine-induced, NO-mediated vasodilation.73 Animal models have demonstrated that GH deficiency induces a pro-oxidative phenotype by reducing endothelial cell expression of antioxidant mechanisms, such as Mn-SOD, Cu, Zn-SOD, glutathione peroxidase 1 (GPx)-1, and eNOS.74 Endothelial cell production of ROS (O2− and H2O2) by mitochondria is also enhanced.74 Treatment with GH significantly reduces cellular O2− and H2O2 production and ROS generation by mitochondria and upregulates expression of Mn-SOD, Cu, Zn-SOD, GPx-1, and eNOS.74 Growth hormone can also signal directly through insulin receptor substrate (IRS)-1 to activate PI-3 kinase.75 Endothelial cells express abundant GH receptors, and thus GH may act directly through receptor-mediated eNOS phosphorylation, causing smooth muscle relaxation.75 Growth hormone increases the expression of eNOS mRNA, reduces ADMA, and increases endothelial progenitor cells.76 Thus, L-arginine-mediated increases in GH may improve endothelial function by (1) directly activating eNOS; (2) upregulating eNOS protein expression; and (3) preserving NO bioavail-ability via its antioxidant properties77 (Figure 3).
Figure 3.
Pleotropic properties of L–arginine.
Summary and Conclusions
In healthy young and older individuals with preserved vascular endothelial function as well as select patient populations (ie, peripheral artery disease and chronic kidney disease), L-argi-nine has no effect on endothelial-dependent vasodilation and may have detrimental effects on clinical outcome (ie, post-myocardial infarction [MI]). In select aging patient populations with overt endothelial dysfunction and reduced L-arginine stores, L-arginine may improve vascular function via its ability to augment NO. L-arginine may also improve endothelial function via its ancillary effects on systemic hormonal modulation (ie, GH augmentation) and anti-inflammatory/antioxidant phenotypic changes in endothelial cells. Long-term safety and efficacy of L-arginine therapy remains ill examined. Therefore, recommendation of L-arginine as a general vascular panacea for all aging persons is not warranted at this time, and the quest for the fountain of vascular youth should continue.
Acknowledgments
The authors received no financial support for the research and/or authorship of this article.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘‘set up’’ for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappa B. J Appl Physiol. 2008;105(4):1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffernan KS, Vieira VJ, Valentine RJ. Microvascular function and ageing L-arginine, tetrahydrobiopterin and the search for the fountain of vascular youth. J Physiol. 2008;586(8):2041–2042. doi: 10.1113/jphysiol.2008.151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuvin JT, Karas RH. Clinical utility of endothelial function testing: ready for prime time? Circulation. 2003;107(25):3243–3247. doi: 10.1161/01.CIR.0000075928.54461.33. [DOI] [PubMed] [Google Scholar]
- 6.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 7.Kuvin JT, Patel AR, Sliney KA, et al. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38(7):1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 8.Yavuz BB, Yavuz B, Sener DD, et al. Advanced age is associated with endothelial dysfunction in healthy elderly subjects. Gerontology. 2008;54(3):153–156. doi: 10.1159/000129064. [DOI] [PubMed] [Google Scholar]
- 9.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46(3):454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 10.Kearney-Schwartz A, Rossignol P, Bracard S, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40(4):1229–1236. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- 11.Welsch MA, Dobrosielski DA, Arce-Esquivel AA, et al. The association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc. 2008;40(7):1237–1243. doi: 10.1249/MSS.0b013e31816c5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(2):233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Reina G, Gripari P, Tumminello G, Vicenzi M, Arena R. Prognostic value of flow-mediated dilatation following myocardial infarction. Int J Cardiol. 2008;132(1):45–50. doi: 10.1016/j.ijcard.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53(4):323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 16.Suessenbacher A, Frick M, Alber HF, Barbieri V, Pachinger O, Weidinger F. Association of improvement of brachial artery flow-mediated vasodilation with cardiovascular events. Vasc Med. 2006;11(4):239–244. doi: 10.1177/1358863x06075006. [DOI] [PubMed] [Google Scholar]
- 17.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40(3):505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 18.Michael SK, Surks HK, Wang Y, et al. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A. 2008;105(8):6702–6707. doi: 10.1073/pnas.0802128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang KM, Wang GR, Lu P, et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. 2003;9(12):1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 20.Heffernan KS, Vieira VJ, Valentine RJ. Microvascular function and ageing L-arginine, tetrahydrobiopterin and the search for the fountain of vascular youth. J Physiol. 2008;586(8):2041–2042. doi: 10.1113/jphysiol.2008.151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586(4):1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu G, Meininger CJ. Arginine nutrition and cardiovascular function. J Nutr. 2000;130(11):2626–2629. doi: 10.1093/jn/130.11.2626. [DOI] [PubMed] [Google Scholar]
- 23.Di Pasquale M. The Anabolic Edge. New York: CRC Press; 1997. Amino Acids and Proteins for the Athlete. [Google Scholar]
- 24.Marchesi C, Paradis P, Schiffrin EL. Role of the renin–angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29(7):367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Gornik HL, Creager MA. Arginine and endothelial and vascular health. J Nutr. 2004;134(10 suppl):2880S–2887S. doi: 10.1093/jn/134.10.2880S. discussion 2895S. [DOI] [PubMed] [Google Scholar]
- 26.Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162(1):1–15. doi: 10.1016/s0021-9150(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 27.Adams MR, McCredie R, Jessup W, Robinson J, Sullivan D, Celermajer DS. Oral L-arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis. 1997;129(2):261–269. doi: 10.1016/s0021-9150(96)06044-3. [DOI] [PubMed] [Google Scholar]
- 28.Clarkson P, Adams MR, Powe AJ, et al. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97(8):1989–1994. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8(2):77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell AJ, Zapien MP, Pearce GL, MacCallum G, Stone PH. Randomized trial of a medical food for the dietary management of chronic, stable angina. J Am Coll Cardiol. 2002;39(1):37–45. doi: 10.1016/s0735-1097(01)01708-9. [DOI] [PubMed] [Google Scholar]
- 31.Yin WH, Chen JW, Tsai C, Chiang MC, Young MS, Lin SJ. L-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clin Nutr. 2005;24(6):988–997. doi: 10.1016/j.clnu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Sozykin AV, Noeva EA, Balakhonova TV, Pogorelova OA, Men’shikov M. Effect of L-arginine on platelet aggregation, endothelial function adn exercise tolerance in patients with stable angina pectoris [in Russian] Ter Arkh. 2000;72(8):24–27. [PubMed] [Google Scholar]
- 33.Jahangir E, Vita JA, Handy D, et al. The effect of l-arginine and creatine on vascular function and homocysteine metabolism. Vasc Med. 2009;14(3):239–248. doi: 10.1177/1358863X08100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102(1):63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 35.Blum A, Hathaway L, Mincemoyer R, et al. Effects of oral L-arginine on endothelium-dependent vasodilation and markers of inflammation in healthy postmenopausal women. J Am Coll Cardiol. 2000;35(2):271–276. doi: 10.1016/s0735-1097(99)00553-7. [DOI] [PubMed] [Google Scholar]
- 36.Chin-Dusting JP, Alexander CT, Arnold PJ, Hodgson WC, Lux AS, Jennings GL. Effects of in vivo and in vitro L-arginine supplementation on healthy human vessels. J Cardiovasc Pharmacol. 1996;28(1):158–166. doi: 10.1097/00005344-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Adams MR, Forsyth CJ, Jessup W, Robinson J, Celermajer DS. Oral L-arginine inhibits platelet aggregation but does not enhance endothelium-dependent dilation in healthy young men. J Am Coll Cardiol. 1995;26(4):1054–1061. doi: 10.1016/0735-1097(95)00257-9. [DOI] [PubMed] [Google Scholar]
- 38.Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral L-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009;89(1):77–84. doi: 10.3945/ajcn.2008.26544. [DOI] [PubMed] [Google Scholar]
- 39.Boger RH, Bode-Boger SM, Thiele W, Creutzig A, Alexander K, Frolich JC. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol. 1998;32(5):1336–1344. doi: 10.1016/s0735-1097(98)00375-1. [DOI] [PubMed] [Google Scholar]
- 40.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116(2):188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 41.Fahs CA, Heffernan KS, Fernhall B. Hemodynamic and vascular response to resistance exercise with L-arginine. Med Sci Sports Exerc. 2009;41(4):773–779. doi: 10.1249/MSS.0b013e3181909d9d. [DOI] [PubMed] [Google Scholar]
- 42.Mariotti F, Huneau JF, Szezepanski I, et al. Meal amino acids with varied levels of arginine do not affect postprandial vascular endothelial function in healthy young men. J Nutr. 2007;137(6):1383–1389. doi: 10.1093/jn/137.6.1383. [DOI] [PubMed] [Google Scholar]
- 43.Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97(21):2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 44.Schulman SP, Becker LC, Kass DA, et al. L-arginine therapy in acute myocardial infarction: The Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295(1):58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 45.Lin CC, Tsai WC, Chen JY, Li YH, Lin LJ, Chen JH. Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int J Cardiol. 2008;127(3):337–341. doi: 10.1016/j.ijcard.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Marchesi S, Lupattelli G, Siepi D, et al. Oral L-arginine administration attenuates postprandial endothelial dysfunction in young healthy males. J Clin Pharm Ther. 2001;26(5):343–349. doi: 10.1046/j.1365-2710.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- 47.Siasos G, Tousoulis D, Vlachopoulos C, et al. The impact of oral l-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am J Hypertens. 2009;22(6):586–592. doi: 10.1038/ajh.2009.57. [DOI] [PubMed] [Google Scholar]
- 48.Siasos G, Tousoulis D, Vlachopoulos C, et al. Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol. 2008;126(3):394–399. doi: 10.1016/j.ijcard.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 49.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clapp BR, Hirschfield GM, Storry C, et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111(12):1530–1536. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]
- 51.Kals J, Kampus P, Kals M, et al. Inflammation and oxidative stress are associated differently with endothelial function and arterial stiffness in healthy subjects and in patients with atherosclerosis. Scand J Clin Lab Invest. 2008:1–8. doi: 10.1080/00365510801930626. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Liuba P, Aburawi EH, Pesonen E, Andersson S, Yla-Herttuala S, Holmberg L. Residual adverse changes in arterial endothelial function and LDL oxidation after a mild systemic inflammation induced by influenza vaccination. Ann Med. 2007;39(5):392–399. doi: 10.1080/07853890701390111. [DOI] [PubMed] [Google Scholar]
- 53.Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92(4):441–444. doi: 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kharbanda RK, Walton B, Allen M, et al. Prevention of inflammation-induced endothelial dysfunction: a novel vasculo-protective action of aspirin. Circulation. 2002;105(22):2600–2604. doi: 10.1161/01.cir.0000017863.52347.6c. [DOI] [PubMed] [Google Scholar]
- 55.Park KG, Hayes PD, Garlick PJ, Sewell H, Eremin O. Stimulation of lymphocyte natural cytotoxicity by L-arginine. Lancet. 1991;337(8742):645–646. doi: 10.1016/0140-6736(91)92456-c. [DOI] [PubMed] [Google Scholar]
- 56.Daly JM, Reynolds J, Sigal RK, Shou J, Liberman MD. Effect of dietary protein and amino acids on immune function. Crit Care Med. 1990;18(2 suppl):S86–S93. [PubMed] [Google Scholar]
- 57.Fujii S, Zhang L, Igarashi J, Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension. 2003;42(5):1014–1020. doi: 10.1161/01.HYP.0000094557.36656.D0. [DOI] [PubMed] [Google Scholar]
- 58.El-Mesallamy HO, Abdel Hamid SG, Gad MZ. Oxidative stress and asymmetric dimethylarginine are associated with cardiovascular complications in hemodialysis patients: improvements by L-arginine intake. Kidney Blood Press Res. 2008;31(3):189–195. doi: 10.1159/000135655. [DOI] [PubMed] [Google Scholar]
- 59.West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135(2):212–217. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- 60.McConell GK. Effects of L-arginine supplementation on exercise metabolism. Curr Opin Clin Nutr Metab Care. 2007;10(1):46–51. doi: 10.1097/MCO.0b013e32801162fa. [DOI] [PubMed] [Google Scholar]
- 61.Borucki K, Aronica S, Starke I, Luley C, Westphal S. Addition of 2.5g l-arginine in a fatty meal prevents the lipemia-induced endothelial dysfunction in healthy volunteers. Atherosclerosis. 2009;205(1):251–254. doi: 10.1016/j.atherosclerosis.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Kuboki K, Jiang ZY, Takahara N, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101(6):676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 63.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98(4):894–898. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;541:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 65.Collier SR, Casey DP, Kanaley JA. Growth hormone responses to varying doses of oral arginine. Growth Horm IGF Res. 2005;15(2):136–139. doi: 10.1016/j.ghir.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Kanaley JA. Growth hormone, arginine and exercise. Curr Opin Clin Nutr Metab Care. 2008;11(1):50–54. doi: 10.1097/MCO.0b013e3282f2b0ad. [DOI] [PubMed] [Google Scholar]
- 67.Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton RN. Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab. 1999;84(2):453–457. doi: 10.1210/jcem.84.2.5456. [DOI] [PubMed] [Google Scholar]
- 68.Pfeifer M, Verhovec R, Zizek B. Growth hormone (GH) and atherosclerosis: changes in morphology and function of major arteries during GH treatment. Growth Horm IGF Res. 1999;9(suppl A):25–30. doi: 10.1016/s1096-6374(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 69.Evans LM, Davies JS, Goodfellow J, Rees JA, Scanlon MF. Endothelial dysfunction in hypopituitary adults with growth hormone deficiency. Clin Endocrinol (Oxf) 1999;50(4):457–464. doi: 10.1046/j.1365-2265.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 70.Smith JC, Evans LM, Wilkinson I, et al. Effects of GH replacement on endothelial function and large-artery stiffness in GH-deficient adults: a randomized, double-blind, placebo-controlled study. Clin Endocrinol (Oxf) 2002;56(4):493–501. doi: 10.1046/j.1365-2265.2002.01514.x. [DOI] [PubMed] [Google Scholar]
- 71.Evans LM, Davies JS, Anderson RA, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol. 2000;142(3):254–262. doi: 10.1530/eje.0.1420254. [DOI] [PubMed] [Google Scholar]
- 72.Napoli R, Guardasole V, Angelini V, et al. Acute effects of growth hormone on vascular function in human subjects. J Clin Endocrinol Metab. 2003;88(6):2817–2820. doi: 10.1210/jc.2003-030144. [DOI] [PubMed] [Google Scholar]
- 73.Bode-Boger SM, Boger RH, Loffler M, Tsikas D, Brabant G, Frolich JC. L-arginine stimulates NO-dependent vasodilation in healthy humans—effect of somatostatin pretreatment. J Investig Med. 1999;47(1):43–50. [PubMed] [Google Scholar]
- 74.Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295(5):H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li G, Del Rincon JP, Jahn LA, et al. Growth hormone exerts acute vascular effects independent of systemic or muscle insulin-like growth factor I. J Clin Endocrinol Metab. 2008;93(4):1379–1385. doi: 10.1210/jc.2007-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thum T, Fleissner F, Klink I, et al. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I. J Clin Endocrinol Metab. 2007;92(11):4172–4179. doi: 10.1210/jc.2007-0922. [DOI] [PubMed] [Google Scholar]
- 77.Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the anti-oxidative defense system. Mech Ageing Dev. 2003;124(10–12):1013–1024. doi: 10.1016/j.mad.2003.07.001. [DOI] [PubMed] [Google Scholar]