Abstract
Two luminescent terbium(III) complexes have been prepared from chiral ligands containing 2-hydroxyisophthalamide (IAM) antenna chromophores and their non-polarized and circularly-polarized luminescence properties have been studied. These tetradentate ligands, which form 2:1 ligand/TbIII complexes, utilize diaminocyclohexane (cyLI) and diphenylethylenediamine (dpenLI) backbones, which we reasoned would impart conformational rigidity and result in TbIII complexes that display both large luminescence quantum yield (Φ) values and strong circularly polarized luminescence (CPL) activities. Both TbIII complexes are highly emissive, with Φ values of 0.32 (dpenLI-Tb) and 0.60 (cyLI-Tb). Luminescence lifetime measurements in H2O and D2O indicate that while cyLI-Tb exists as a single species in solution, dpenLI-Tb exists as two species: a monohydrate complex with one H2O molecule directly bound to the TbIII ion and a complex with no water molecules in the inner coordination sphere. Both cyLI-Tb and dpenLI-Tb display increased CPL activity compared to previously reported TbIII complexes made with chiral IAM ligands. The CPL measurements also provide additional confirmation of the presence of a single emissive species in solution in the case of cyLI-Tb, and multiple emissive species in the case of dpenLI-Tb.
Keywords: Lanthanides, Terbium, Luminescence, Circularly polarized luminescence (CPL), Chirality
Introduction
Circularly polarized luminescence (CPL), the emission analog of circular dichroism (CD), combines the sensitivity of luminescence techniques with the specificity of chiroptical spectroscopy.[1–7] The luminescence of lanthanide complexes is especially sensitive to changes in coordination geometry and molecular environment, and as such, CPL-active lanthanide complexes are excellent candidates for luminescent probes that can report on their chiral surroundings.[8] To maximize the sensitivity of a luminescent lanthanide-based chiroptical probe the complex should possess both a large luminescence quantum yield and strong CPL activity. Additionally, solubility and stability of the LnIII complex in aqueous solution is very important for practical applications. Such systems, however, remain elusive; often complexes are optimized in respect to either emission intensity or CPL activity, though not both (see below). A system that combines a large luminescence quantum yield with strong CPL activity in aqueous solution would represent a significant advance in the field of LnIII-based CPL.
We have previously shown that TbIII complexes of 2-hydroxyisophthalamide (IAM)-based ligands are exceptionally bright, displaying some of the highest luminescence quantum yield (Φ) values of LnIII complexes in aqueous solution reported to date (Φ ≈ 0.60).[9] While chiral IAM ligands afford CPL-active TbIII complexes that retain the brightness of the parent achiral forms, their CPL activity is relatively modest. CPL activity is commonly reported as the luminescence dissymmetry factor, glum, defined as glum = 2(IL − IR)/(IL + IR), where IL and IR are the intensities of left- and right-polarized emission respectively. Among chiral TbIII-IAM complexes with large Φ values, |glum| values ≤ 0.078 are observed.[10,11] As a strategy to increase CPL activity we designed chiral IAM ligands that are more rigid than the previously reported tetrapodal octadenate ligands since CPL activity had been shown to increase with the conformational rigidity of the complex.[12,13] These ligands, cyLI-IAM and dpenLI-IAM (Figure 1), utilize diaminocyclohexane and diphenylethylenediamine backbones respectively. Tetradentate IAM ligands of this type form 2:1 ligand/ LnIII (ML2) complexes,[14] analogous to the previously reported octadentate ligands that form 1:1 ligand/LnIII complexes. Tetradendate ligands offer the advantage of being more readily synthesized than higher denticity ligands and consequently by utilizing tetradentate ligands we are able to easily investigate how small structural changes in the ligand scaffolds influence the photophysical behavior of the resulting TbIII complexes. Herein we report the syntheses of cyLI- and dpenLI-IAM and their TbIII complexes along with their absorption and luminescence properties in the presence of both circularly-polarized and non-polarized light.
Figure 1.
Chemical structures of cyLI- and dpenLI-IAM ligands.
Results and Discussion
CyLI- and dpenLI-IAM were synthesized following the procedure shown in Scheme 1. By starting with the dithiazolide precursor 1[15] one is able to substitute both ends of the molecule with two distinct amines. Dilute solutions of (1R,2R)-(+)-1,2-diphenylethylenediamine and (1R,2R)-(+)-1,2-diaminocyclohexane were each added to 1 over 36 h to produce the mono-substituted species, 2a and 2b, respectively. These two species were each then combined with methylamine to yield 3a and 3b. The methyl-protected ligands were then both deprotected using BBr3 to yield the final ligands. The TbIII ML2 complexes (here referred to as cyLI-Tb and dpenLI-Tb) used for the spectroscopic measurements were prepared in situ by combining 1 equiv. of TbCl3 (in 1 m HCl) with 2 equiv. of ligand (in a basic aqueous solution) in 0.1 m Tris buffered H2O (pH = 7.4). The complexes were characterized using mass spectrometry (ES−) in addition to the optical techniques that are described in the upcoming sections. The ability to rely on these optical techniques is especially valuable in the study of TbIII-IAM complexes since 1H NMR spectroscopy is problematic because of signal broadening caused by the paramagnetic TbIII center.[16]
Scheme 1.
Syntheses of cyLI- and dpenLI-IAM ligands.
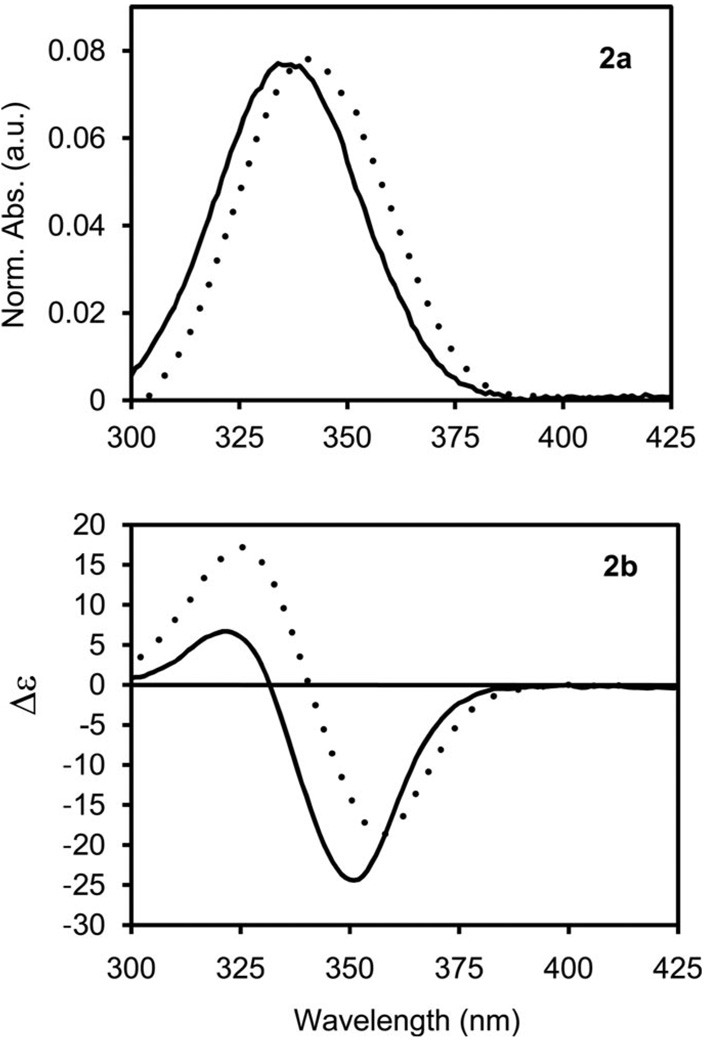
The absorption spectra of cyLI-Tb and dpenLI-Tb display broad transitions similar to those previously observed for Tb-IAM complexes in aqueous solution, which are attributed to π-π* transitions in the IAM chromophore (Figure 2, a).[9,17] As with other Tb-IAM complexes, the broad absorption bands of cyLI-Tb and dpenLI-Tb allow for excitation with wavelengths up to ca. 390 nm, which is more favorable for practical biological applications compared to many other high quantum yield TbIII complexes that require more damaging higher energy excitation wave-lengths.[18,19] Additionally, both cyLI-Tb and dpenLI-Tb show CD activity as expected (Figure 2, b). The CD spectra show strong Cotton effects[20] for both complexes between 300–380 nm.
Figure 2.
Absorption spectra (2a) and CD spectra (2b) of cyLI-Tb (solid) and dpenLI-Tb (dashed) in aqueous solution [C = 10−5 m, 0.1 m Tris (pH = 7.4)] at 298 K.
To determine the efficiency of TbIII sensitization, the emission behavior of cyLI-Tb and dpenLI-Tb was investigated. A summary of the luminescence quantum yield and lifetime values is given in Table 1. The luminescence spectra display the characteristic bands corresponding to transitions from the 5D4 electronic level to the 7FJ (J = 0–6) manifold of TbIII (Figure 3). The two complexes show slight differences in the relative intensities and fine structures of the peaks, which points to variation in the coordination environments experienced by the TbIII ions in each complex.[21] This difference in coordination environment is supported by the luminescence quantum yield (Φ) values for the two complexes: cyLI-Tb has a quantum yield of 0.60, while dpenLI-Tb has a quantum yield of 0.32. The cyLI-Tb value is consistent with the high quantum yield values observed for TbIII complexes with octadentate IAM ligands.[9,11,17]
Table 1.
Photophysical properties of the TbIII complexes [10−5 m (Tris buffer, pH 7.4, λex = 340 nm)].
| Complex | QY (Φ) | τ (r.t.), ms | q[a] | τ (77 K), ms[b] | ||
|---|---|---|---|---|---|---|
| H2O | D2O | H2O | D2O | |||
| cyLI-Tb | 0.60 | 1.66 | 1.84 | 0 | 1.77 | 2.05 |
| dpenLI-Tb | 0.32 | 1.51 | 1.68 | 0 | 1.89 | 2.21 |
| (84%) | (88%) | (37%) | (37%) | |||
| 0.550 | 1.68 | 1 | 1.89 | 2.21 | ||
| (16%) | (12%) | (63%) | (63%) | |||
Calculated from r.t. values using q = 5×(1/τH2O − 1/τD2O − 0.06).[21]
Contained 10% (v/v) glycerol.
Figure 3.
Luminescence spectra of cyLI-Tb (solid) and dpenLI-Tb (dashed) in aqueous solution (0.1 m Tris, pH = 7.4) at 298 K normalized to the intensity of the J = 5 peak.
To further investigate this difference in emission intensity the luminescence lifetimes were measured both in H2O and D2O at room temperature (r.t.) and at 77 K (Table 1). Mono-exponential lifetime decays are exhibited by cyLI-Tb, while dpenLI-Tb exhibits bi-exponential decays, indicating that for the former, only one luminescent species is present in solution, while for the latter, two luminescent species are present. The number of bound water molecules (q) was estimated for each of the complexes based on the lifetimes measured at room temperature using the formula developed by Beeby et al.,[22] and q values of zero were obtained for cyLI-Tb and for one of the two dpenLI-Tb species. The other dpenLI-Tb species, however, has approximately one water molecule in the inner coordination sphere, which quenches TbIII emission and contributes to the low luminescence quantum yield observed for dpenLI-Tb. The overall quantum yield for dpenLI-Tb is the weighted sum of the quantum yields of the q = 0 and q = 1 species. Since the latter experiences quenching due to bound water, it has a lower quantum yield than a q = 0 species.
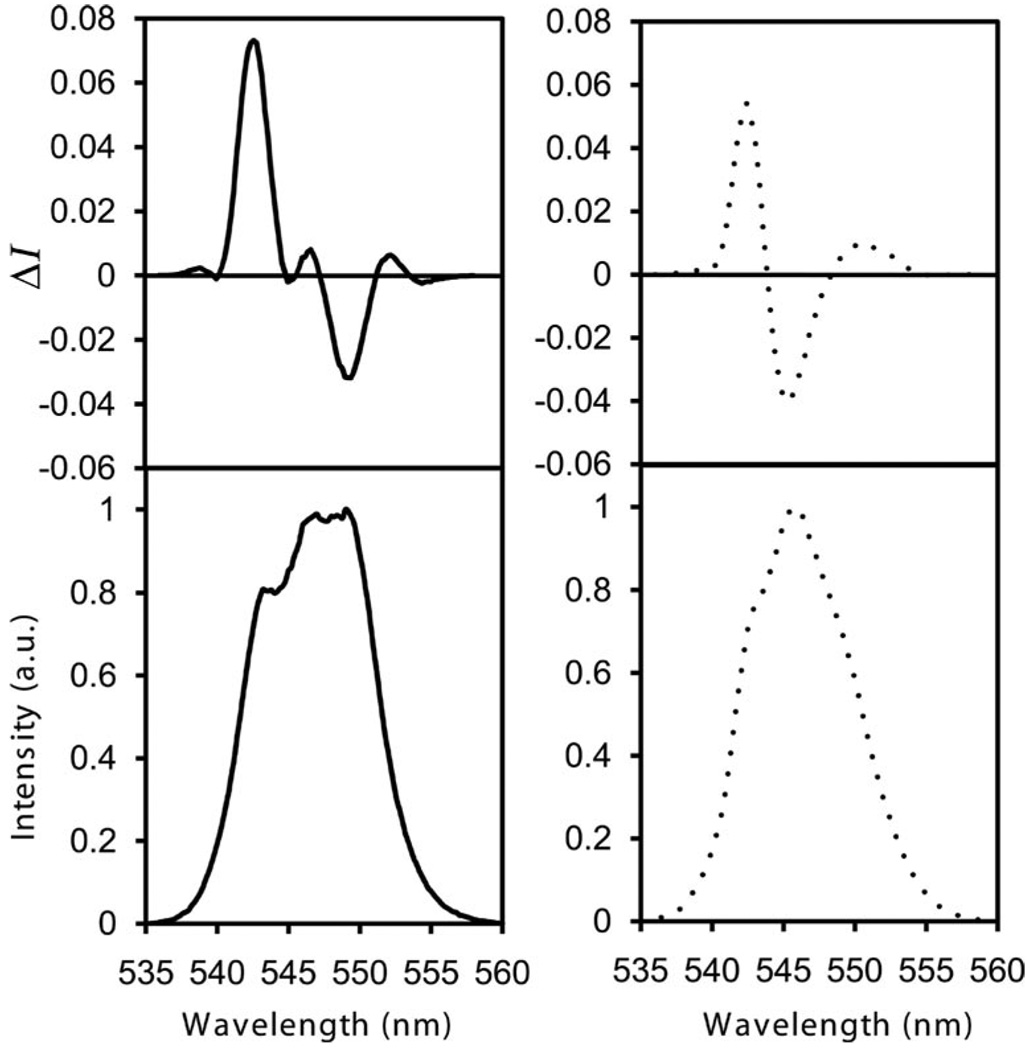
The CPL spectra of cyLI-Tb and dpenLI-Tb are plotted in Figure 4 for the magnetic dipole allowed 5D4→7F5 transition. The glum values are summarized in Table 2. The CPL signals provide additional confirmation that stable chiral species are present in solution. Both spectra display several peaks corresponding to crystal-field splitting; the difference in sign, shape and magnitude of the CPL signal are consistent with the fact that the two systems do not exhibit the same crystal field structure in solution. The CPL signal exhibited by cyLI-Tb is similar whether via direct excitation of the TbIII ion (λex = 488 nm) or indirect excitation via the ligand absorption bands (λex = 341 nm), which indicates that the same species in solution is responsible for the CPL activity detected. Additionally, the glum values obtained for this complex are the same upon excitation with right-, left-, and plane-polarized light, which is consistent with the presence of only one species in solution;[23] had the solution contained a mixture of diastereomers, the CPL signal would have been dependent on the polarization of the excitation beam.[24] In contrast, the glum values obtained for dpenLI-Tb are dependent on the polarization of the excitation beam and on whether direct or indirect excitation is used, which indicates that more than one species in solution is responsible for the CPL signal detected. These results support the lifetime data, which indicate the presence of two emitting species in solution that differ in hydration number and therefore also coordination environment.
Figure 4.
CPL (top) and total luminescence spectra (bottom) for the 5D4→7F5 transition for cyLI-Tb (solid) and dpenLI-Tb (dashed) in aqueous solution.
Table 2.
Summary of CPL data for cyLI-Tb and dpenLI-Tb.
| Complex | λex | Transition | λ [nm] | glum |
|---|---|---|---|---|
| cyLI-Tb | 341 nm | 5D4→7F5 | 542.0 | +0.20 |
| 548.6 | −0.063 | |||
| dpenLI-Tb | 343 nm | 5D4→7F5 | 542.4 | +0.16 |
| 545.4 | −0.082 | |||
| 551.0 | +0.043 |
Conclusions
Tetradentate IAM ligands yield highly luminescent TbIII complexes with large glum values in aqueous solution at physiologically relevant pH, which may be attributed to the increased rigidity of the ligand frameworks. Varying the chiral amine that serves as the ligand backbone was found to impact solvent access to the TbIII center, which was determined through luminescence lifetime, luminescence quantum yield and CPL measurements. The CyLI-Tb complex is particularly interesting, since it has an extremely high quantum yield, a glum value over twofold larger than the largest observed for a comparably emissive Tb-IAM complex, and consists of a single emitting species in solution.
Experimental Section
General Methods
All chemicals were obtained from commercial suppliers and used without further purification. 1H and 13C NMR spectra, elemental analyses, and mass spectra were obtained at the corresponding analytical facility in the College of Chemistry, University of California, Berkeley.
2a
(2-Methoxy-1,3-phenylene)bis[(2-thioxothiazolidin-3-yl)methanone][15] (1) (5.0 g, 12.5 mmol) was dissolved in 10 mL of CH2Cl2. A 250 mL solution of (1R,2R)-(+)-1,2-diaminocyclohexane (0.128 g, 1.1 mmol) in a 95:5 CH2Cl2/MeOH solution was cannulated into the solution of 1 over 36 h. The solvents were then removed under vacuum and the reaction mixture was dissolved in CH2Cl2 and washed with 1 m NaOH. The product was purified by silica gel chromatography (2% MeOH/98% CH2Cl2); yield 0.620 g (84 %). 1H NMR (400 MHz, CDCl3): δ = 1.35 (m, 4 H, CH2), 1.74 (m, 2 H, CHH), 2.15 (m, 2 H, CHH), 3.35 (m, 4 H, NCH2CH2S), 3.76 (s, 6 H, OCH3), 3.98 (m, 2 H, CH2CHN), 4.55 (m, 4 H, NCH2CH2S), 7.07 (t, J = 8 Hz, 2 H, ArH), 7.30 (dd, J = 5, 2 Hz, 2 H, ArH), 7.60 (d, J = 8 Hz, 2 H, NH), 7.89 (dd, J = 8, 2 Hz, 2 H, ArH) ppm. 13C NMR (100 MHz, CDCl3): δ = 24.8, 29.2, 32.6, 53.4, 55.7, 63.1, 124.1, 127.1, 129.1, 132.1, 134.0, 155.7, 165.0, 167.4, 201.3 ppm. MS (FAB+): m/z = 673 [MH+].
2b
Compound 1[15] (7.96 g, 20.0 mmol) was dissolved in 10 mL of CH2Cl2. A 250 mL solution of (1R,2R)-(+)-1,2-diphenylethylenediamine (0.425 g, 2.0 mmol) in a 95:5 CH2Cl2/MeOH solution was cannulated into the solution of 1 over 36 h. The solvents were then removed under vacuum and the reaction mixture was dissolved in CH2Cl2 and washed with 1 m NaOH. The product was purified by silica gel chromatography (15% EtOAc/85% CH2Cl2); yield 0.470 g (30 %). 1H NMR (400 MHz, CDCl3): δ = 3.30 (m, 4 H, NCH2CH2S), 3.86 (s, 6 H, OCH3), 4.52 (m, 4 H, NCH2CH2S) 5.50 (m, 2 H, CHNH), 6.95 (t, J = 8 Hz, 2 H, ArH), 7.16 (m, 10 H, ArH), 7.85 (dd, J = 8, 2 Hz, 2 H, ArH), 7.89 (dd, J = 8, 2 Hz, 2 H, ArH), 8.80 (q, J = 5 Hz, 2 H, NHCH3), 9.21 (m, 2 H, NH) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 29.7, 53.6, 57.5, 64.2, 118.7, 120.2, 126.9, 128.3, 131.5, 136.6, 142.4, 158.6, 167.2, 175.6, 201.8 ppm. MS (FAB+): m/z = 771 [MH+].
3a (Representative Procedure)
To a solution of 2a (0.60 g, 0.89 mmol) in 15 mL of CH2Cl2 was added 1.0 mL of methylamine (40% aq. soln.). The reaction mixture was stirred at room temperature for 6 h and was then washed with 1 m NaOH. The resulting off-white residue was applied to a silica column and the product was eluted with 4% MeOH/96% CH2Cl2; yield 0.402 g (91 %). 1H NMR (400 MHz, [D6]DMSO): δ = 1.30 (m, 2 H, CHH), 1.44 (m, 2 H, CHH), 1.71 (m, 2 H, CHH), 1.94 (m, 2 H, CHH), 2.76 (d, J = 5 Hz, 6 H, NHCH3), 3.70 (s, 6 H, OCH3), 3.89 (m, 2 H, CH2CHNH), 7.17 (t, J = 8 Hz, 2 H, ArH), 7.53 (m, 4 H, ArH), 8.21 (m, 4 H, NH) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 24.4, 26.2, 31.7, 52.3, 62.4, 123.4, 130.2, 130.4, 131.0, 131.1, 154.8, 165.4, 166.1 ppm. MS (FAB+): m/z = 497 [MH+].
3b
1H NMR (400 MHz, [D6]DMSO): δ = 2.81 (d, J = 4 Hz, 6 H, NHCH3), 3.80 (s, 6 H, OCH3), 5.51 (m, 2 H, CHNH), 6.95 (t, J = 8 Hz, 2 H, ArH), 7.16 (m, 10 H, ArH), 7.85 (dd, J = 8, 2 Hz, 2 H, ArH), 7.89 (dd, J = 8, 2 Hz, 2 H, ArH), 8.80 (q, J = 5 Hz, 2 H, NHCH3), 9.21 (m, 2 H, NH) ppm. 13C NMR (100 MHz, [D6]-DMSO): δ = 26.0, 57.5, 64.2, 116.7, 118.1, 120.4, 126.8, 127.3, 127.5, 130.5, 132.4, 138.6, 159.2, 165.6, 168.3 ppm. MS (FAB+): m/z = 595 [MH+].
cyLI-IAM (Representative Procedure)
A suspension of 3a (0.325 g, 0.66 mmol) in 30 mL of CH2Cl2 was cooled to −78 °C and 1.0 mL (10.6 mmol) of BBr3 was added. The reaction mixture was warmed to room temperature and stirred for 48 h. The volatiles were removed under vacuum and a few milliliters of 1 m HCl were added, causing the product to precipitate out of solution. The product was recrystallized from hot H2O; yield 0.229 g (74 %). 1H NMR (400 MHz, [D6]DMSO): δ = 1.31 (m, 2 H, CHH), 1.48 (m, 2 H, CHH), 1.73 (m, 2 H, CHH), 1.98 (m, 2 H, CHH), 2.81 (d, J = 4 Hz, 6 H, NHCH3), 3.94 (m, 2 H, CH2CHNH), 6.93 (t, J = 8 Hz, 2 H, ArH), 7.91 (t, J = 9 Hz, 4 H, ArH), 8.61 (m, 2 H, CH2CHNH), 8.69 (m, 2 H, NHCH3), 14.71 (s, 2 H, OH) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 24.4, 26.1, 31.5, 52.4, 117.9, 118.0, 118.7, 132.2, 132.7, 159.3, 166.6, 167.7 ppm. MS (FAB+): m/z = 469 [MH+]. Anal. calcd. (found) for C24H28N4O6·0.5H2O: C, 60.36 (60.15); H, 6.12 (6.06); N, 11.73 (11.42).
cyLI-Tb
[C48H52N8O12Tb]−, MS (ES−): m/z = 1091.2 [M−].
dpenLI-IAM
1H NMR (400 MHz, [D6]DMSO): δ = 2.83 (d, J = 4 Hz, 6 H, NHCH3), 5.60 (m, 2 H, CHNH), 6.97 (t, J = 8 Hz, 2 H, ArH), 7.20 (m, 10 H, ArH), 7.88 (dd, J = 8, 2 Hz, 2 H, ArH), 7.96 (dd, J = 8, 2 Hz, 2 H, ArH), 8.86 (q, J = 5 Hz, 2 H,NHCH3), 9.25 (m, 2 H, NH), 14.67 (s, 2 H, OH) ppm. 13C NMR (100 MHz, [D6]DMSO): δ = 26.1, 57.5, 116.9, 118.2, 119.9, 127.2, 127.6, 128.0, 131.5, 133.4, 139.6, 159.3, 165.8, 168.6 ppm. MS (FAB+): m/z = 567 [MH+]. Anal. calcd. (found) for C32H30N4O6·0.5H2O·MeOH: C, 65.66 (65.46); H, 5.18 (5.35); N, 9.28 (9.48).
dpenLI-Tb
[C64H56N8O12Tb]−, MS (ES−): m/z = 1287.3 [M−].
UV/Vis Absorption, Circular Dichroism, Emission, and Circularly Polarized Luminescence Spectra. General Methods
Absorption spectra were recorded on a Cary 300 UV/Vis spectrophotometer using a 1-cm quartz cell. Emission spectra were recorded on a FluoroLog-3 (JobinYvon) fluorimeter using a 1-cm Supracil quartz luminescence cell (room-temperature measurements). The TbIII complexes (10 mm) were prepared in situ in 0.1 m Tris-buffered H2O (pH 7.4). The complexes were characterized using mass spectrometry (ES−). Quantum yields were determined by the optically dilute method[25] using the following equation:
where A is the absorbance at the excitation wavelength (λ), I is the intensity of the excitation light at the same wavelength, n is the refractive index and D is the integrated intensity. Quinine sulfate in 1.0 n sulfuric acid was used as the reference (Qr = 0.546).[26] Circularly polarized luminescence and total luminescence spectra were recorded on an instrument according to literature procedures,[23,24,27] operating in a differential photon-counting mode. CPL measurements were performed at 295 K in H2O (0.1 m Tris, pH = 7.4) with analyte concentrations of 10−4 to 10−5 m.
Acknowledgments
This research is supported by the Director of the U. S. Department of Energy at LBNL, Office of Science, Office of Basic Energy Sciences, and the Division of Chemical Sciences, Geosciences, and Biosciences (Contract No. DE-AC02-05CH11231. G. M. thanks the National Institute of Health, Minority Biomedical Research Support (1 SC3 GM089589-01) and the Henry Dreyfus Teacher-Scholar Award for financial support.
References
- 1.Brittain HG. In: Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice. 3rd ed. Bünzli J-CG, Choppin GR, editors. Amsterdam: Elsevier; 1989. [Google Scholar]
- 2.Richardson FS, Riehl JP. Chem. Rev. 1977;77:772–792. [Google Scholar]
- 3.Riehl JP, Richardson FS. Chem. Rev. 1986;86:1–16. [Google Scholar]
- 4.Riehl JP, Richardson FS. Methods Enzymol. 1993;226:539–553. doi: 10.1016/0076-6879(93)26024-4. [DOI] [PubMed] [Google Scholar]
- 5.Brittain HG. Appl. Spectrosc. Rev. 2007;35:175–201. [Google Scholar]
- 6.Dekkers HPJM. In: Circular Dichroism. Berova N, Nakanishi K, Woody RW, editors. Weinheim: Wiley-VCH; 2000. [Google Scholar]
- 7.Riehl JP, Muller G. In: Handbook on the Physics and Chemistry of Rare Earths. Gschneider KA, Bünzli J-CG, Pecharsky VK, editors. vol. 43. Amsterdam: North-Holland Publishing Company; 2005. p. 289. [Google Scholar]
- 8.Muller G. Dalton Trans. 2009:9692–9707. doi: 10.1039/b909430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petoud S, Cohen SM, Bünzli J-C, Raymond K. J. Am. Chem. Soc. 2003;125:13324–13325. doi: 10.1021/ja0379363. [DOI] [PubMed] [Google Scholar]
- 10.Petoud S, Muller G, Moore EG, Xu J, Sokolnicki J, Riehl JP, Le UN, Cohen SM, Raymond KN. J. Am. Chem. Soc. 2007;129:77–83. doi: 10.1021/ja064902x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seitz M, Moore EG, Ingram AJ, Muller G, Raymond KN. J. Am. Chem. Soc. 2007;129:15468–15470. doi: 10.1021/ja076005e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickins RS, Howard JAK, Lehmann CW, Moloney JM, Parker D, Peacock RD. Angew. Chem. Int. Ed. Engl. 1997;36:521–523. [Google Scholar]
- 13.Lunkley JL, Shirotani D, Yamanari K, Kaizaki S, Muller G. J. Am. Chem. Soc. 2008;130:13814–13815. doi: 10.1021/ja805681w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel APS, Xu J, Raymond KN. Inorg. Chem. 2009;48:687–698. doi: 10.1021/ic801904s. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SM, O’Sullivan B, Raymond KN. Inorg. Chem. 2000;39:4339–4346. doi: 10.1021/ic000239g. [DOI] [PubMed] [Google Scholar]
- 16.Attempts to characterize IAM-TbIII complexes using 1H NMR spectroscopy yielded spectra with no distinct resonances that could be assigned unambiguously to IAM protons and thus these results were uninformative.
- 17.Samuel APS, Moore EG, Melchior M, Xu J, Raymond KN. Inorg. Chem. 2007;46:7535–7544. doi: 10.1021/ic800328g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takalo H, Mukkala V-M, Meriö L, Rodríguez-Ubis J-C, Seadno R, Juanes O, Brunet E. Helv. Chim. Acta. 1997;80:372–387. [Google Scholar]
- 19.Xiao M, Selvin PR. J. Am. Chem. Soc. 2001;123:7067–7073. doi: 10.1021/ja0031669. [DOI] [PubMed] [Google Scholar]
- 20.Snatzke G. In: Circular Dichroism. 2nd ed. Berova N, Nakanishi K, Woody RW, editors. New York: Wiley-VCH; 2000. [Google Scholar]
- 21.Bünzli J-CG. In: Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice. Bünzli J-CG, Choppin GR, editors. Amsterdam: Elsevier; 1989. [Google Scholar]
- 22.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, Sousa ASd, Williams JAG, Woods M. J. Chem. Soc. Perkin Trans. 1999;2:493–503. [Google Scholar]
- 23.Do K, Muller FC, Muller G. J. Phys. Chem. A. 2008;112:6789–6793. doi: 10.1021/jp804463e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seitz M, King D, Ingram AJ, Moore EG, Muller G, Raymond KN. Inorg. Chem. 2009;48:8469–8479. doi: 10.1021/ic901079s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crosby GA, Demas JN. J. Phys. Chem. 1971;75:991–1024. [Google Scholar]
- 26.Meech SR, Philips D. J. Photochem. 1983;23:193–217. [Google Scholar]
- 27.Bonsall SD, Houcheime M, Straus DA, Muller G. Chem. Commun. 2007;35:3673–6378. doi: 10.1039/b704346e. [DOI] [PMC free article] [PubMed] [Google Scholar]