Abstract
Background. New-generation drug-eluting stents (DES) may solve several problems encountered with first-generation DES, but there is a lack of prospective head-to-head comparisons between new-generation DES. In addition, the outcome of regulatory trials may not perfectly reflect the outcome in ‘real world’ patients.
Objectives. To compare the efficacy and safety of two new-generation DES in a ‘real world’ patient population.
Methods. A prospective, randomised, single-blinded clinical trial to evaluate clinical outcome after Endeavor Resolute vs. Xience V stent implantation. The primary endpoint is target vessel failure at one-year follow-up. In addition, the study comprises a two-year and an open-label five-year follow-up. (Neth Heart J 2010;18:360–4.)
Keywords: Drug-Eluting Stents; Coronary Artery Diseases; Angioplasty, Transliminal, Percutaneous Coronary; Thrombosis; Randomized Controlled Trial
The positive results of early drug-eluting stent (DES) trials led to widespread DES use.1-3 However, meta-analyses4,5 and long-term follow-up data6,7 demonstrated that DES improved morbidity but did not reduce mortality (compared with bare metal stents; BMS). Newer generation DES may solve the problems encountered with first-generation DES. In the present article, we describe the design of the TWENTE study (The real-World Endeavor Resolute versus Xience V drug-eluting steNt study in TwentE) that compares two newer generation DES, we comment on the rationale of the study, and briefly discuss several key issues of this trial.
Rationale of the study
Endeavor Resolute and Xience V are two new-generation DES. Pivotal clinical trials have demonstrated the safety and efficacy of both stents.8-12 The positive clinical results may reflect superior drug characteristics, polymer morphology,13,14 and biocompatibility,15-17 which may account for a better endothelialisation compared with older-generation DES.18 When we started the present study no data from prospective randomised head-to-head comparisons between these two DES in a real-world scenario were available.
Accordingly, the TWENTE study was designed to evaluate the clinical outcome of randomised use of Endeavor Resolute versus Xience V stents in a non-selected patient population.
Study design
General design
TWENTE is an ongoing, physician-initiated clinical trial. This study has a prospective, two arm, randomised, single-blinded design. The aim of the TWENTE study is to compare efficacy and safety of Endeavor Resolute versus Xience V DES in a real-world patient population with:
Single or multiple lesions to be treated in any stage of coronary artery disease, ranging from single vessel to complex three-vessel disease;
De-novo coronary lesions, restenoses following previous PCI, and/or lesions in venous or arterial coronary artery bypass grafts (CABG);
Various clinical syndromes, including stable angina pectoris, unstable angina pectoris with or without cardiac marker rise, non-ST-elevation myocardial infarction (non-STEMI), and status following recent STEMI (except during the initial 48 hours).
Patients will be monitored throughout the two-year study period for the occurrence of death, myocardial infarction (MI), re-intervention (re-PCI or CABG), stent thrombosis, and new-onset angina pectoris or worsening of symptoms. Then, an additional open-label follow-up (duration of three years) will be performed to evaluate the efficacy and safety of the study devices until five-year follow-up. Between 18 June 2008 and 18 May 2010, a total of 1196 patients were included in the TWENTE trial, which corresponds with an inclusion rate of 624 patients per year.
Study hypothesis
The hypothesis to be tested in this trial is that the zotarolimus-eluting stent (Endeavor-Resolute; Medtronic Vascular, Santa Rosa, CA, USA) is non-inferior to the everolimus-eluting stent (Xience V; Abbott Vascular, Santa Clara, CA, USA) as assessed by the primary endpoint target vessel failure (TVF) after one year (non-inferiority hypothesis), as outlined below.
Study population
Enrolment is planned in 1380 patients with symptomatic coronary artery disease and coronary (or graft) lesions >50%, in whom PCI with DES implantation is indicated. Patients are enrolled at the Thoraxcentrum Twente in Enschede, the Netherlands. Details of the inclusion and exclusion criteria are presented in table 1.
Table 1.
Inclusion and exclusion criteria.
Inclusion criteria
|
Exclusion criteria
|
NVVC=Netherlands Society of Cardiology, ESC=European Society of Cardiology, DES=drug-eluting stents.
Study devices
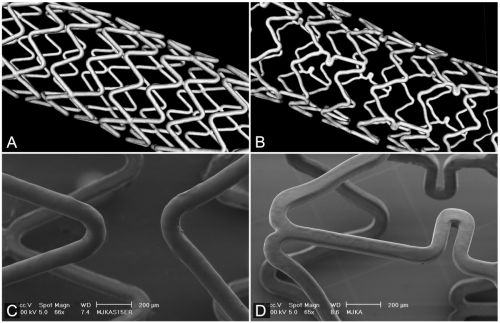
Endeavor Resolute (figures 1A and C) is based on the Driver cobalt chromium platform with a strut thickness of 91 μm, coated with a mixture of zotarolimus as the antiproliferative drug plus Biolinx polymer;12 the coating thickness is 5.6 μm. Xience V stents (figures 1B and D) consist of the Vision multi-link cobalt-chromium platform with a strut thickness of 81 μm, covered by a 7.8 μm thick layer of a mixture of fluoropolymer and everolimus as the antiproliferative drug.19
Figure 1.
Geometry and surface morphology of Endeavor Resolute and Xience V. Micro-computed tomography images of Endeavor Resolute (A) and Xience V (B). Scanning electron microscopic images of Endeavor Resolute (C) and Xience V (D) (images from ongoing bench side studies in DES, performed by C. von Birgelen and co-workers, University of Twente, Enschede, the Netherlands.
Ethics, informed consent, and randomisation
The study is conducted according to the principles of the Declaration of Helsinki (1964) and in accordance with the Medical Research Involving Human Subjects Act. The local medical ethics committee has approved the study protocol. Before participating, patients are informed about the purpose, and possible risks/benefits of the study. Written informed consent is obtained in all patients. Patients who meet the inclusion criteria and give informed consent are randomised between implantation of Endeavor Resolute vs. Xience V stents in a proportion of 1:1. Allocation to treatment is stratified by gender and performed by means of sealed envelopes, containing a computer-generated sequence that was produced with random block size. The two treatment groups are studied concurrently.
Treatment of patients
Patients who are not on oral aspirin therapy receive a loading dose of at least 300 mg prior to PCI. In elective PCI patients, clopidogrel therapy of 75 mg daily is started one week before the PCI. In urgent PCI, a loading dose of 600 mg clopidogrel is given as soon as possible, either before PCI or (at least) directly after the PCI is performed. The PCI procedure is performed according to routine clinical standards via the femoral or radial route, using 6 French guiding catheters. Prior to PCI, unfractionated heparin is administered intravenously, and an intracoronary bolus of nitroglycerin is given and repeated if necessary. Glycoprotein IIb/IIIa inhibitor use is left to the operator’s discretion. Following the index PCI procedure, patients are generally maintained on aspirin ≥80 mg daily during the entire trial (and preferably lifelong). If patients require oral anticoagulation therapy (e.g., for atrial fibrillation), aspirin ≥80 mg daily is prescribed for at least one to three months after PCI. Clopidogrel 75 mg daily is recommended and prescribed for a period of 12 months. Further medical treatment is performed according to current medical guidelines, clinical standards, and the judgment of the referring physicians.
Follow-up
Following the index PCI procedure, patients are contacted by telephone or seen in the outpatient clinic after 30 days and after 3, 12, and 24 months. In addition, there will be a five-year open-label follow-up. Data are collected on clinical endpoints (see below) and on (dis)continuation of the dual antiplatelet therapy.
Primary study endpoints
The primary endpoint of this study is defined as the composite (TVF) after one-year follow-up. Target vessel failure is defined as (in hierarchical order): target vessel related death, myocardial infarction, or clinically driven target vessel revascularisation by means of re-PCI or CABG. All clinical endpoints are defined according to the Academic Research Consortium (ARC) definitions and addendum.20,21
Secondary study endpoints
Secondary endpoints include clinical, laboratory, angiographic, and intravascular ultrasound (IVUS) endpoints. Secondary clinical endpoints comprise:
Death due to cardiac, vascular, non-cardiovascular, and all-cause mortality at 1, 3, 12, and 24 month follow-up;
Myocardial infarction (all; related to target vessel; related to non-target vessel);
Re-PCI or CABG (all; related to target vessel; related to non-target vessel);
New onset of angina pectoris (or increase in angina class according to the Canadian Cardiovascular Society classification);
Stent thrombosis according to the ARC definitions.
Secondary laboratory endpoints include the extent of biomarkers elevation post-PCI and secondary angiographic (QCA) endpoints comprise established quantitative coronary angiographic parameters. In the subpopulation of patients referred for angiographic re-evaluation with or without subsequent re-PCI (i.e., patients with clinically indicated angiographic re-evaluation), a QCA substudy will be performed. Another angiographic secondary endpoint is the angiographic evidence of stent thrombosis as outlined in the ARC definitions.20 In the subpopulation of patients with clinically indicated IVUS examinations, the IVUS recordings will be analysed as previously described.22
Power calculation and statistics
The main outcome parameter is the difference in time to TVF between the two DES after one year, analysed by log-rank test and Cox regression. Statistical significance is set at 5% and power at 80%. Assuming a median time to TVF of 48 months, based on the Endeavor III trial,23 a hazard ratio of 1.35, an accrual time of two years, and an additional follow-up of one year for TVF, 690 patients per group are needed. Eighteen months after the start of the study, an interim analysis for the incidence of TVF on the pooled data will be performed and, if required, a new power analysis will be performed.
Data management
Data entry is performed by the cardiology research team of the Thoraxcentrum Twente. QCA and IVUS analyses are performed in the core lab in Enschede (analysts blinded to clinical information). An independent Clinical Events Committee will adjudicate all events. In patients with clinically driven repeat invasive procedures, all angiograms and IVUS recordings will be evaluated.
Discussion
Endeavor Resolute and Xience V are two examples of newer-generation DES which may overcome the shortcomings of first-generation DES.25 These two DES differ in stent platform, which could have consequences for device flexibility and side branch access.24 In addition, the DES use different coatings, which is reflected in their microscopic appearance.13 Finally, both DES deliver different antiproliferative drugs. Despite marked differences in several DES key components, both stents are commonly expected to further improve the clinical outcome of PCI.25 In the TWENTE study the parameter ‘target vessel failure’ - a clinical endpoint - was chosen to be the primary endpoint. This endpoint reflects both effectiveness and safety aspects of the stents investigated.26
Do we need randomised post-marketing studies in a ‘real world’ scenario?
Obviously, each of the two DES that are compared in the TWENTE study has previously been examined in regulatory trials.8-12 But the study populations of most regulatory trials differ substantially from the patients treated in a ‘real world scenario’. This discrepancy is underlined by the different rates of stent thrombosis in low-risk patients (as included in many regulatory trials) vs. high-risk patients and/or patients treated with DES in off-label scenarios.27 The aforementioned limitations of regulatory trials and the inherent limitations of non-randomised ‘real world’ studies (i.e. registries without control group) recently motivated some ‘real world’ trials.28,29 In the TWENTE study, we adopted very few exclusion criteria in order to reflect the everyday ‘real world’ practice. As part of this practice, patients treated by primary PCI for acute STEMI were not included in the study because this setting is not considered as a standard indication for DES use.
Expected scientific evidence and limitations of the study
To evaluate the weight of the expected scientific evidence of a trial, a scoring system as proposed by Silber et al. can be used.30 According to that scoring system, the TWENTE study may achieve a relatively good score of up to eight. The maximum score of ten points of this scoring system is reserved for double-blinded and multicentre trials.30 The outcome of any single-centre trial is usually received with some reservation, inherent in this study design, because generalisation of the study results is considered to be somewhat limited. Nevertheless, the example of the recently published COMPARE trial shows that single-centre trials can provide very interesting and clinically relevant data.29
Trial registration number
NCT01066650 (www.clinicaltrials.gov); NTR 1256 (www.trialregister.nl)
Acknowledgements
This study is supported in equal shares by research grants from Abbot Vascular and Medtronic Inc., the Netherlands.
References
- 1.Mehilli J, Dibra A, Kastrati A, Pache J, Dirschinger J, Schomig A. Randomized trial of paclitaxel- and sirolimus-eluting stents in small coronary vessels. Eur Heart J. 2006;27:260-6. [DOI] [PubMed] [Google Scholar]
- 2.Vermeersch P, Agostoni P, Verheye S, Van den HP, Convens C, Bruining N, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol. 2006;48:2423-31. [DOI] [PubMed] [Google Scholar]
- 3.Briguori C, Colombo A, Airoldi F, Focaccio A, Iakovou I, Chieffo A, et al. Sirolimus-eluting stent implantation in diabetic patients with multivessel coronary artery disease. Am Heart J. 2005;150:807-13. [DOI] [PubMed] [Google Scholar]
- 4.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937-48. [DOI] [PubMed] [Google Scholar]
- 5.Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198-206. [DOI] [PubMed] [Google Scholar]
- 6.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519-21. [DOI] [PubMed] [Google Scholar]
- 7.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667-78. [DOI] [PubMed] [Google Scholar]
- 8.Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903-13. [DOI] [PubMed] [Google Scholar]
- 9.Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation. 2009;119:680-6. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchida K, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, Zeiher AM, et al. One-year results of a durable polymer everolimus-eluting stent in de novo coronary narrowings (The SPIRIT FIRST Trial). EuroIntervention. 2005;1:266-72. [PubMed] [Google Scholar]
- 11.Meredith IT, Worthley S, Whitbourn R, Walters DL, McClean D, Horrigan M. et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Interv. 2009;2:977-85. [DOI] [PubMed] [Google Scholar]
- 12.Meredith IT, Worthley S, Whitbourn R, Walters D, Popma J, Cutlip D, et al. The next-generation Endeavor Resolute stent: 4-month clinical and angiographic results from the Endeavor Resolute first-in-man trial. EuroIntervention. 2007;3:50-3. [PubMed] [Google Scholar]
- 13.Basalus MW, Ankone MJ, van Houwelingen GK, de Man FH, von Birgelen C. Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. EuroIntervention. 2009;5:157-65. [DOI] [PubMed] [Google Scholar]
- 14.Basalus MW, von Birgelen C. Bench side testing of drug-eluting stent surface and geometry. J Interv Cardiol. 2010;2:159-75. [Google Scholar]
- 15.Hezi-Yamit A, Sullivan C, Wong J, David L, Chen M, Cheng P, et al. Novel high-throughput polymer biocompatibility screening designed for SAR (structure-activity relationship): application for evaluating polymer coatings for cardiovascular drug-eluting stents. Comb Chem High Throughput Screen. 2009;12:664-76. [DOI] [PubMed] [Google Scholar]
- 16.Udipi K, Melder RJ, Chen M, Cheng P, Hezi-Yamit A, Sullivan C, et al. The next generation Endeavor Resolute Stent: role of the BioLinx Polymer System. EuroIntervention. 2007;3:137-9. [PubMed] [Google Scholar]
- 17.Carter AJ, Brodeur A, Collingwood R, Ross S, Gibson L, Wang CA, et al. Experimental efficacy of an everolimus eluting cobalt chromium stent. Cathet Cardiovasc Interv. 2006;68:97-103. [DOI] [PubMed] [Google Scholar]
- 18.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333-42. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903-13. [DOI] [PubMed] [Google Scholar]
- 20.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-51. [DOI] [PubMed] [Google Scholar]
- 21.Vranckx P, Cutlip DE, Mehran R, Kint PP, Silber S, Windecker S, et al. Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention. 2010;5:871-4. [DOI] [PubMed] [Google Scholar]
- 22.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-92. [DOI] [PubMed] [Google Scholar]
- 23.Kandzari DE, Leon MB, Popma JJ, Fitzgerald PJ, O'Shaughnessy C, Ball MW, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48:2440-7. [DOI] [PubMed] [Google Scholar]
- 24.Popma JJ, Mauri L, O'Shaughnessy C, Overlie P, McLaurin B, Almonacid A, et al. Frequency and clinical consequences associated with sidebranch occlusion during stent implantation using zotarolimus-eluting and paclitaxel-eluting coronary stents. Circ Cardiovasc Interv. 2009;2:133-9. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee D, Moliterno DJ. Second-generation drug-eluting stents and the continuous need for rapidly available real-world data. JACC Cardiovasc Interv. 2009;2:1236-9. [DOI] [PubMed] [Google Scholar]
- 26.Fajadet J, Morice MC, Bode C, Barragan P, Serruys PW, Wijns W, et al. Maintenance of long-term clinical benefit with sirolimus-eluting coronary stents: three-year results of the RAVEL trial. Circulation. 2005;111:1040-4. [DOI] [PubMed] [Google Scholar]
- 27.Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946-59. [DOI] [PubMed] [Google Scholar]
- 28.Galloe AM, Thuesen L, Kelbaek H, Thayssen P, Rasmussen K, Hansen PR, et al. Comparison of paclitaxel- and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA. 2008;299:409-16. [DOI] [PubMed] [Google Scholar]
- 29.Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201-9 [DOI] [PubMed] [Google Scholar]
- 30.Silber S. A new and rapid scoring system to assess the scientific evidence from clinical trials. J Interv Cardiol. 2006;19:485-92. [DOI] [PubMed] [Google Scholar]