Abstract
Peptide hydrolases, catalyzing the hydrolysis of 13 dipeptides and 5 tripeptides into their respective amino acids, were studied in small intestinal mucosa and other tissues, in man and in the rat.
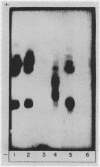
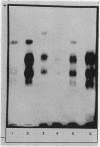
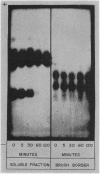
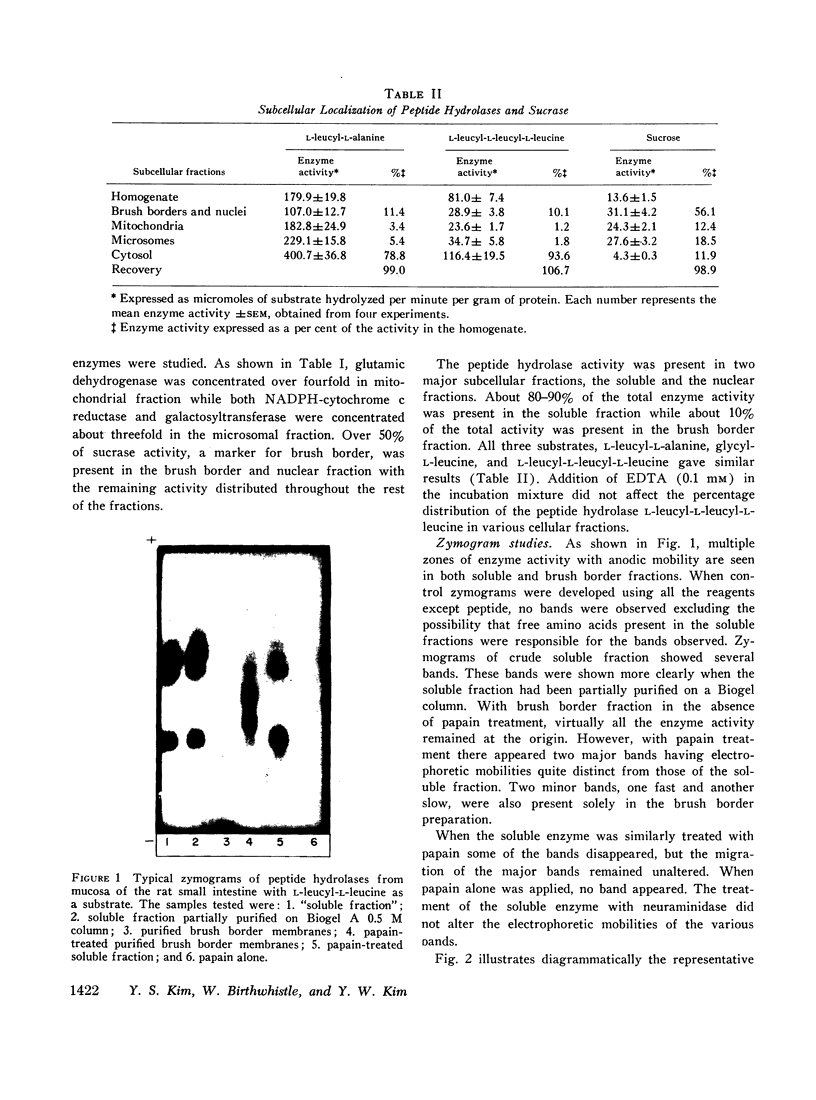
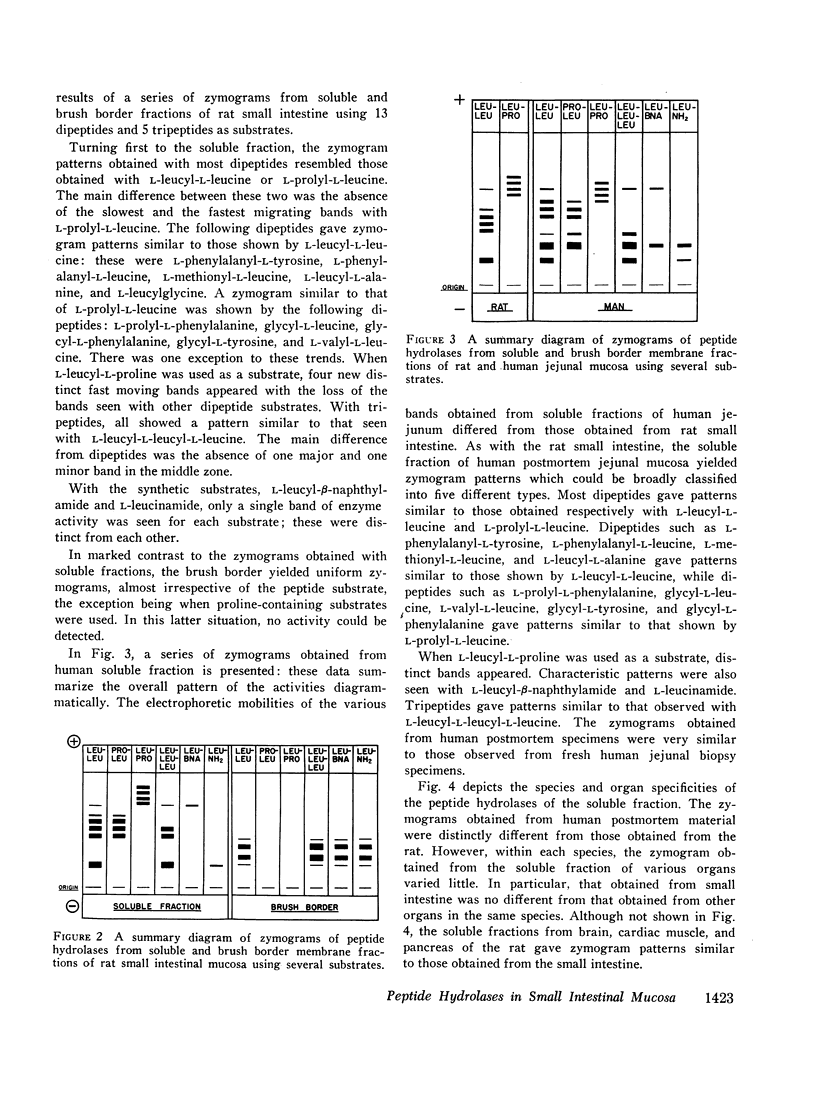
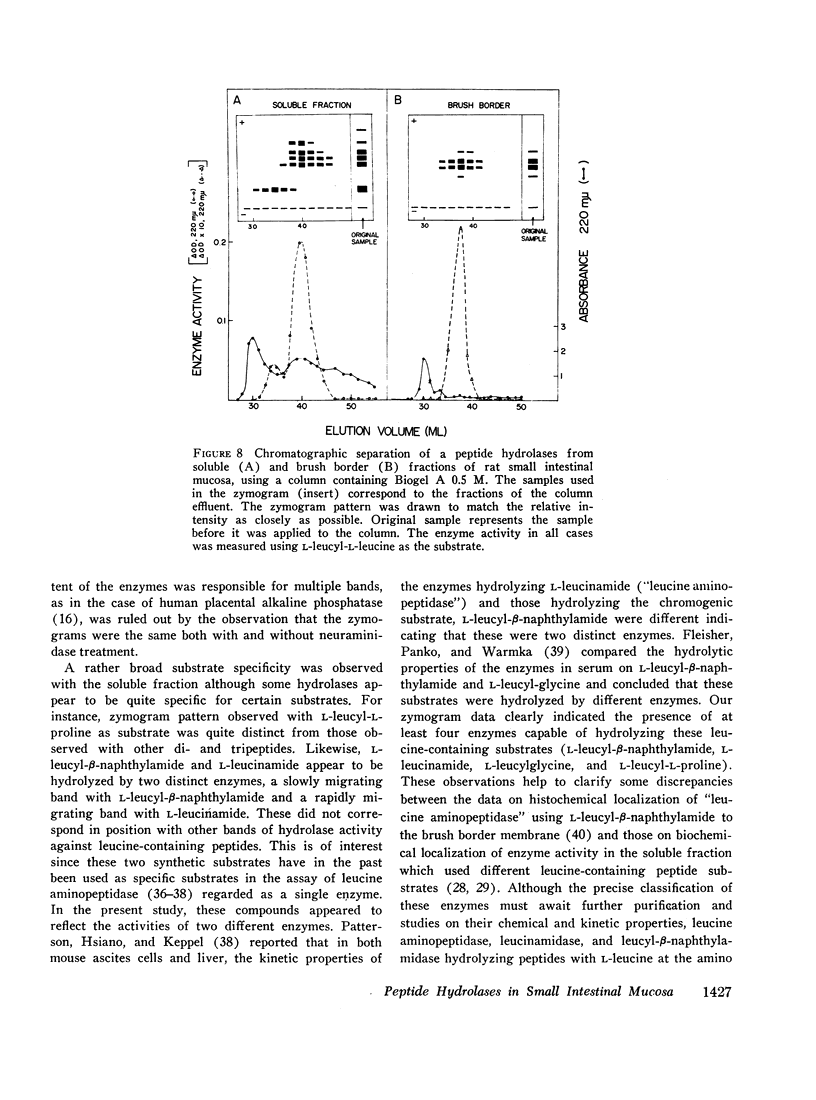
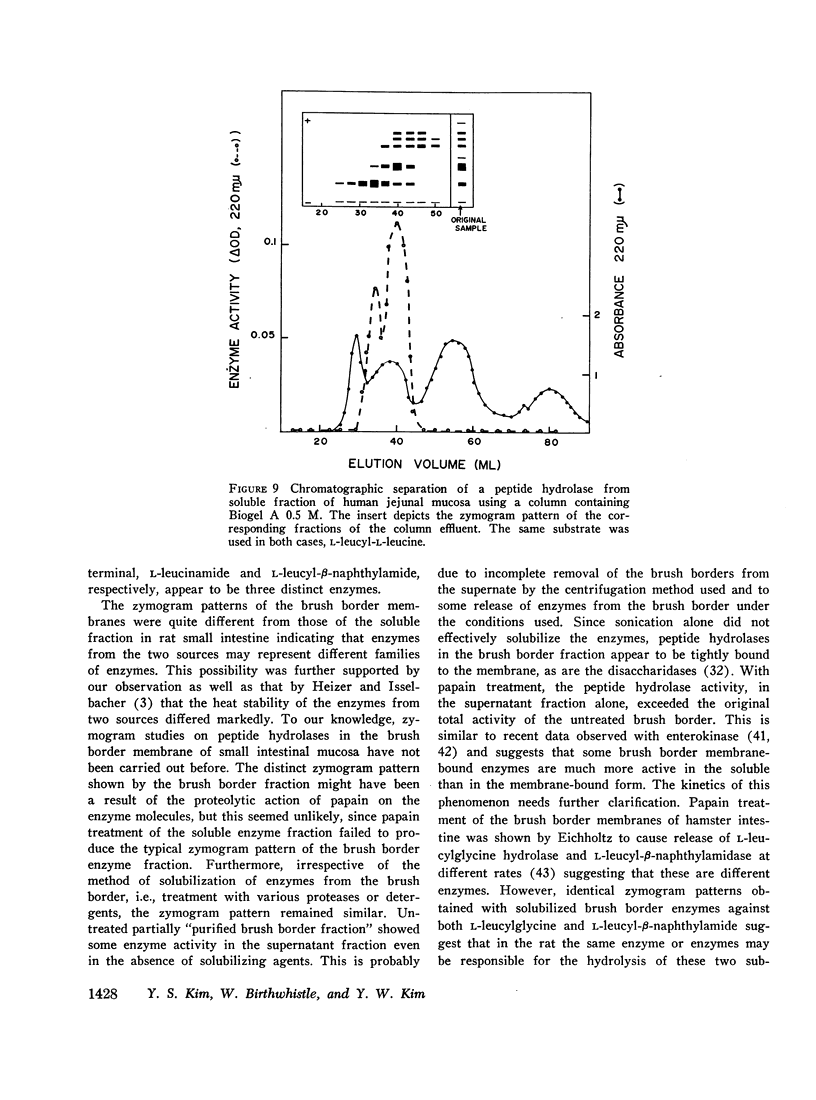
Studies on the subcellular distribution of these enzymes showed enzyme activities in both the soluble and brush border fractions of the rat small intestinal mucosa, the former constituting 80-90% and the latter 10-15% of the total activity. Zymogram studies of peptide hydrolases, in both fractions, yielded multiple bands indicating multiple zones of enzyme activity. With most substrates a rather broad range of enzyme activities was observed in the soluble fraction differing only slightly from substrate to substrate, the exception being when L-leucyl-L-proline was used: this latter led to a zymogram pattern which was quite distinct. The synthetic substrates, L-leucyl-β-naphthylamide and L-leucinamide appeared to be hydrolyzed by two electrophoretically distinct enzymes, different from those hydrolyzing other leucyl-containing peptide substrates.
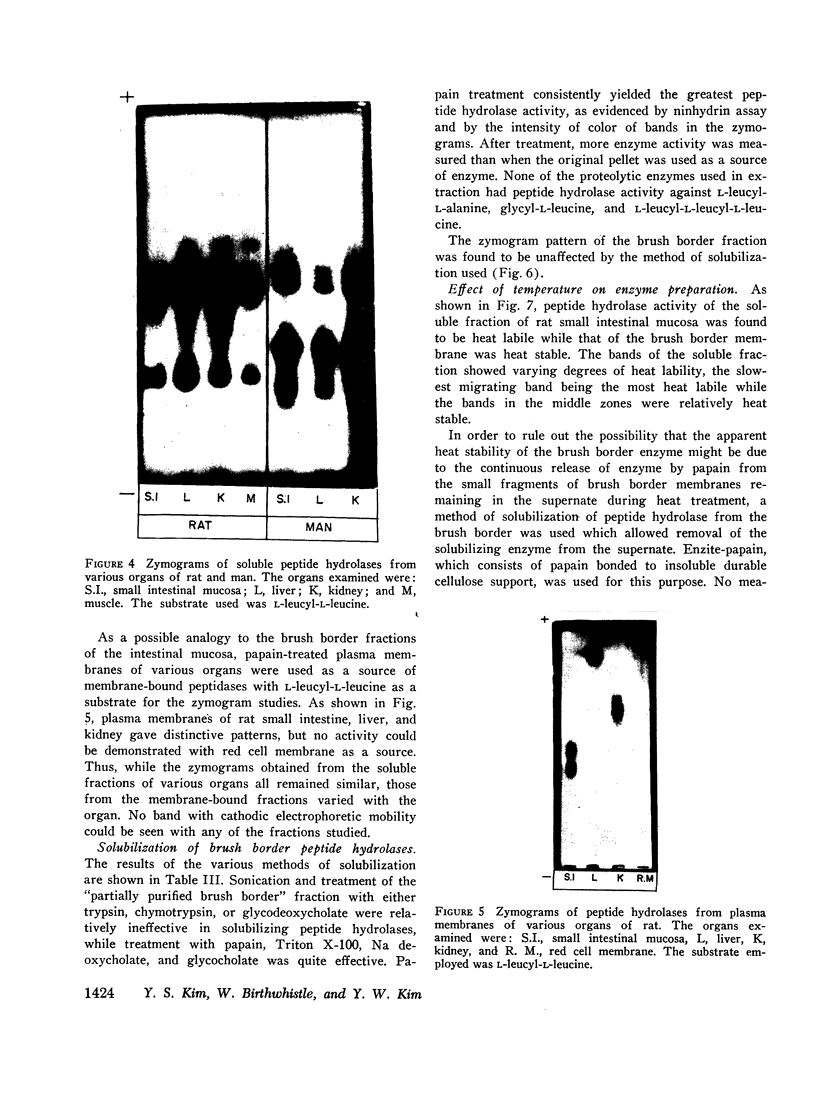
Zymogram patterns of the brush border membrane fraction were quite different from those of the soluble fraction of rat small intestine indicating that enzymes from the two sources may be different. No comparable human data were obtained.
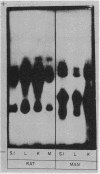
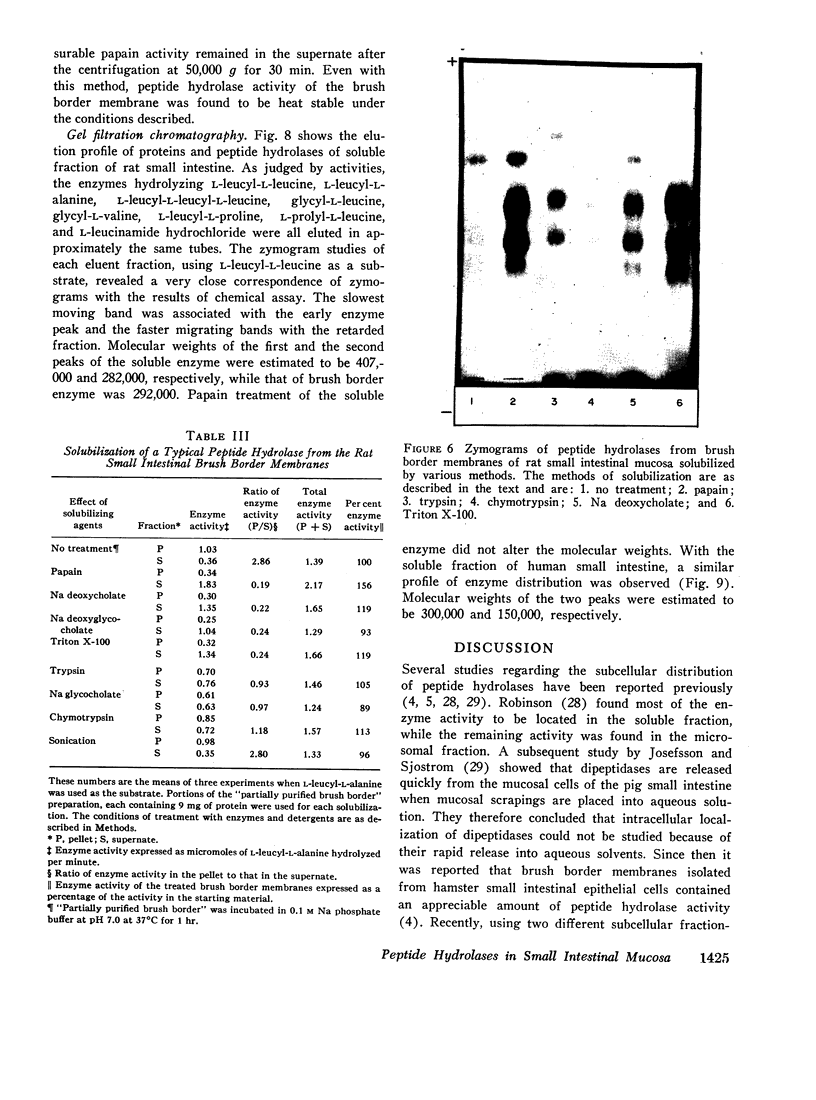
Peptide hydrolases in the soluble fractions of various organs from the same species gave similar zymogram patterns, while those from the plasma membrane-bound fractions of different organs in the same species were peculiar to each organ. From these data, it is suggested that peptide hydrolases in the brush border and the soluble fractions of small intestine are distinct enzymes and may play different roles in cellular function.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., DAHLQVIST A., SEMENZA G. SOLUBILIZATION OF THE HUMAN INTESTINAL DISACCHARIDASES. Biochim Biophys Acta. 1963 Aug 6;73:582–587. doi: 10.1016/0006-3002(63)90329-9. [DOI] [PubMed] [Google Scholar]
- BEAUFAY H., BENDALL D. S., BAUDHUIN P., DE DUVE C. Tissue fractionation studies. 12. Intracellular distribution of some dehydrogenases, alkaline deoxyribonuclease and iron in rat-liver tissue. Biochem J. 1959 Dec;73:623–628. doi: 10.1042/bj0730623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behal F. J., Asserson B., Dawson F., Hardman J. A study of human tissue aminopeptidase components. Arch Biochem Biophys. 1965 Aug;111(2):335–344. doi: 10.1016/0003-9861(65)90194-3. [DOI] [PubMed] [Google Scholar]
- Craft I. L., Geddes D., Hyde C. W., Wise I. J., Matthews D. M. Absorption and malabsorption of glycine and glycine peptides in man. Gut. 1968 Aug;9(4):425–437. doi: 10.1136/gut.9.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dent C. E. Studies on the absorption of proteins: the amino-acid pattern in the portal blood. Biochem J. 1949;44(3):318–335. [PMC free article] [PubMed] [Google Scholar]
- Dolly J. O., Fottrell P. F. Multiple forms of dipeptidases in normal human intestinal mucosa and in mucosa from children with coeliac disease. Clin Chim Acta. 1969 Dec;26(3):555–558. doi: 10.1016/0009-8981(69)90087-4. [DOI] [PubMed] [Google Scholar]
- Eichholz A. Studies on the organization of the brush border in intestinal epithelial cells. V. Subfractionation of enzymatic activities of the microvillus membrane. Biochim Biophys Acta. 1968 Aug;163(1):101–107. doi: 10.1016/0005-2736(68)90037-0. [DOI] [PubMed] [Google Scholar]
- FLEISHER G. A., PANKOW M., WARMKA C. LEUCINE AMINOPEPTIDASE IN HUMAN SERUM: COMPARISON OF HYDROLYSIS OF L-LEUCYLGLYCINE AND L-LEUCYL-BETA-NAPHTHYLAMIDE. Clin Chim Acta. 1964 Mar;9:259–268. doi: 10.1016/0009-8981(64)90105-6. [DOI] [PubMed] [Google Scholar]
- GREEN M. N., TSOU K. C., BRESSLER R., SELIGMAN A. M. The colorimetric determination of leucine aminopeptidase activity with L-leucyl-beta-naphthylamide hydrochloride. Arch Biochem Biophys. 1955 Aug;57(2):458–474. doi: 10.1016/0003-9861(55)90307-6. [DOI] [PubMed] [Google Scholar]
- Ghosh N. K., Goldman S. S., Fishman W. H. Human placental alkaline phosphatase; a sialoprotein. Enzymologia. 1967 Aug 31;33(2):113–124. [PubMed] [Google Scholar]
- Gray G. M., Cooper H. L. Protein digestion and absorption. Gastroenterology. 1971 Oct;61(4):535–544. [PubMed] [Google Scholar]
- Gray G. M., Santiago N. A. Intestinal beta-galactosidases. I. Separation and characterization of three enzymes in normal human intestine. J Clin Invest. 1969 Apr;48(4):716–728. doi: 10.1172/JCI106029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn B., Steiner N., Sumida C., Peters T. J. Intestinal enterokinase. Mechanisms of tts "secretion" into the lumen of the small intestine. Lancet. 1971 Jan 23;1(7691):165–166. doi: 10.1016/s0140-6736(71)91936-2. [DOI] [PubMed] [Google Scholar]
- Heizer W. D., Laster L. Peptide hydrolase activities of the mucosa of human small intestine. J Clin Invest. 1969 Jan;48(1):210–228. doi: 10.1172/JCI105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L., Lindberg T. Intestinal dipeptidases. I. Spectrophotometric determination and characterization of dipeptidase activity in pig intestinal mucosa. Biochim Biophys Acta. 1965 Jul 29;105(1):149–161. doi: 10.1016/s0926-6593(65)80183-7. [DOI] [PubMed] [Google Scholar]
- Josefsson L., Sjöström H. Intestinal dipeptidases. IV. Studies on the release and subcellular distribution of intestinal dipeptidases of the mucosa cells of the pig. Acta Physiol Scand. 1966 May;67(1):27–33. doi: 10.1111/j.1748-1716.1966.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- LONGENECKER J. B., HAUSE N. L. Relationship between plasma amino acids and composition of the ingested protein. Arch Biochem Biophys. 1959 Sep;84:46–59. doi: 10.1016/0003-9861(59)90552-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis W. H., Harris H. Human red cell peptidases. Nature. 1967 Jul 22;215(5099):351–355. doi: 10.1038/215351a0. [DOI] [PubMed] [Google Scholar]
- Lindberg T., Nordén A., Josefsson L. Intestinal dipeptidases. Dipeptidase activities in small intestinal biopsy specimens from a clinical material. Scand J Gastroenterol. 1968;3(2):177–182. doi: 10.3109/00365526809180119. [DOI] [PubMed] [Google Scholar]
- Markert C. L., Møller F. MULTIPLE FORMS OF ENZYMES: TISSUE, ONTOGENETIC, AND SPECIES SPECIFIC PATTERNS. Proc Natl Acad Sci U S A. 1959 May;45(5):753–763. doi: 10.1073/pnas.45.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. M., Craft I. L., Geddes D. M., Wise I. J., Hyde C. W. Absorption of glycine and glycine peptides from the small intestine of the rat. Clin Sci. 1968 Dec;35(3):415–424. [PubMed] [Google Scholar]
- NACHLAS M. M., CRAWFORD D. T., SELIGMAN A. M. The histochemical demonstration of leucine aminopeptidase. J Histochem Cytochem. 1957 May;5(3):264–278. doi: 10.1177/5.3.264. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A. The cellular localization of enterokinase. Biochim Biophys Acta. 1970 Mar 18;198(3):621–622. doi: 10.1016/0005-2744(70)90144-0. [DOI] [PubMed] [Google Scholar]
- PATTERSON E. K., HSIAO S. H., KEPPEL A. STUDIES ON DIPEPTIDASES AND AMINOPEPTIDASES. I. DISTINCTION BETWEEN LEUCINE AMINOPEPTIDASE AND ENZYMES THAT HYDROLYZE L-LEUCYL-BETA-NAPHTHYLAMIDE. J Biol Chem. 1963 Nov;238:3611–3620. [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Peters T. J. The subcellular localization of di- and tri-peptide hydrolase activity in guinea-pig small intestine. Biochem J. 1970 Nov;120(1):195–203. doi: 10.1042/bj1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON G. B. The distribution of peptidases in subcellular fractions from the mucosa of the small intestine of the rat. Biochem J. 1963 Jul;88:162–168. doi: 10.1042/bj0880162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. B., Eichholz A., Crane R. K. Studies on the organization of the brush border in intestinal epithelial cells. IV. Aminopeptidase activity in microvillus membranes of hamster intestinal brush borders. Biochim Biophys Acta. 1967;135(5):959–965. doi: 10.1016/0005-2736(67)90065-x. [DOI] [PubMed] [Google Scholar]
- SEMENZA G., AURICCHIO S., RUBINO A. MULTIPLICITY OF HUMAN INTESTINAL DISACCHARIDASES. I. CHROMATOGRAPHIC SEPARATION OF MALTASES AND OF TWO LACTASES. Biochim Biophys Acta. 1965 Mar 22;96:487–497. doi: 10.1016/0005-2787(65)90565-4. [DOI] [PubMed] [Google Scholar]
- Sadikali F. Dipeptidase deficiency and malabsorption of glycylglycine in disease states. Gut. 1971 Apr;12(4):276–283. doi: 10.1136/gut.12.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe C. Peptide hydrolases in mammalian connective tissue. II. Leucine aminopeptidase. Purification and evidence for subunit structure. Biochemistry. 1969 Mar;8(3):783–794. doi: 10.1021/bi00831a005. [DOI] [PubMed] [Google Scholar]
- Wilfong R. F., Neville D. M., Jr The isolation of a brush border membrane fraction from rat kidney. J Biol Chem. 1970 Nov 25;245(22):6106–6112. [PubMed] [Google Scholar]