Abstract
Polymorphisms in the elaC homolog-2 (ELAC2)/HPC2 gene have been hypothesized to alter the risk of prostate cancer. However, the results of the related published studies remained conflicting. We performed a meta-analysis of 18 studies evaluating the association between ELAC2 Ser217Leu and Ala541Thr polymorphisms and prostate cancer risk. Overall, ELAC2 Leu217 allele was associated with increased prostate cancer risk as compared with the Ser217 allele (odds ratio (OR)=1.13, 95% confidence interval (CI): 1.03–1.24, P=0.019 for heterogeneity), as well as in the heterozygote comparison (OR=1.21, 95% CI: 1.07–1.36, P=0.034 for heterogeneity) and the dominant genetic model (OR=1.20, 95% CI: 1.07–1.35, P=0.025 for heterogeneity). Furthermore, the ELAC2 Thr541 allele was associated with increased prostate cancer risk as compared with the Ala541 allele (OR=1.22, 95% CI: 1.00–0.48, P=0.131 for heterogeneity). In the stratified analyses for Ser217Leu polymorphism, there was significantly increased prostate cancer risk in Asian and Caucasian populations, and studies using sporadic and familial prostate cancer cases. Similar result was found in the Asian population in the stratified analyses for Ala541Thr polymorphism. This meta-analysis showed evidence that ELAC2 Ser217Leu and Ala541Thr polymorphisms were associated with prostate cancer risk, and might be low-penetrance susceptibility markers of prostate cancer.
Keywords: ELAC2/HPC2, polymorphism, risk, meta-analysis
Introduction
Prostate cancer is the most common solid tumor diagnosed and the second leading cause of cancer-related death among American men, with an estimated 192 280 new cases and 27 360 deaths in the United States in 2008.1 To date, the mechanisms that underlie the occurrence and progression of prostate cancer remain largely unknown. Risk factors such as diet, lifestyle and hormones have long been recognized as contributing to the risk of prostate cancer.2, 3, 4 In addition to age and race, family history of prostate cancer is the only other well-established prostate cancer risk factor.2, 5, 6, 7
The elaC homolog-2/hereditary prostate cancer (ELAC2/HPC2) gene at 17p11 is the first candidate gene identified for human prostate cancer on the basis of linkage analysis and positional cloning.8 ELAC2 was thought to serve as a metal-dependent hydrolase and to be potentially involved in DNA inter-strand crosslink repair and mRNA editing.8 Moreover, the human ELAC2 protein was shown to possess 3′-tRNase activity9 and to interact with γ-tubulin,10 a component of the mitotic apparatus, suggesting a possible role for ELAC2 in the regulation of cell-cycle progression.
Mutations in ELAC2 are rare. Sequence analyses of ELAC2 identified two common missense changes, which may function as low-penetrance modifiers of prostate cancer risk. The first variant, Ser217Leu, is located in hydrophilic segment of the protein sequence, and substitution of the hydrophobic leucine residue may alter the protein structure. A second variant, Ala541Thr, is adjacent to a histidine motif and may impair protein function.8, 11 Previously, Camp et al.12 and Severi et al.13 performed two meta-analyses of the association of these two variants in ELAC2 and prostate cancer risk, but presented inconsistent results. Moreover, the number of studies focusing on these two polymorphisms in prostate cancer susceptibility is growing in recent years, but the results are often conflicting.
Hence, to derive a more precise estimation of the association of the Ser217Leu and Ala541Thr variants in ELAC2 and prostate cancer risk, we performed a meta-analysis of all eligible case–control studies.
Methods
Publication search
PubMed was searched using the search terms ‘ELAC2' or ‘HPC2' ‘polymorphism' and ‘prostate cancer'. And the latest search update was 9 December 2009. All published English language papers with available full text matching the eligible criteria were retrieved. Additional studies were identified by a hand search of the references of original studies. Of the studies with overlapping data published by the same investigators, only the most recent or complete study was included in this meta-analysis.
Inclusion and exclusion criteria
For inclusion in the meta-analysis, the identified articles had to provide: (1) information on the evaluation of Ser217Leu or Ala541Thr polymorphisms in ELAC2 and prostate cancer risk, (2) information on using a case–control design and (3) information on containing information about available genotype frequency that can help infer the results in the papers. The major reasons for exclusion of studies were: (1) no control population; (2) no usable data reported and (3) duplicates.
Data extraction
All of the data were extracted independently by two reviewers according to the pre-specified inclusion criteria, and reached a consensus on all the items. For each study, the following characteristics were collected: the first author's last name, year of publication, country of origin, ethnicity, numbers of genotyped cases and controls, source of case groups (familial or sporadic prostate cancer cases), genotyping methods and quality control. Different ethnic descents were categorized as Caucasian, Asian, African or Mixed, which included more than one ethnic descent. For studies including subjects of familial or sporadic prostate cancer cases, data were extracted separately for each group whenever possible.
Statistical analysis
The strength of the association between the Ser217Leu and Ala541Thr polymorphisms and prostate risk was measured by odds ratios (ORs) with 95% confidence intervals (CIs). The statistical significance of the summary OR was determined with the Z-test. For Ser217Leu polymorphism, we first explored the association between allele Leu217 and prostate cancer risk, as well as heterozygote comparison (SL vs SS) and homozygote comparison (LL vs SS) and the dominant genetic model (SL/LL vs SS). For the Ala541Thr polymorphism, we evaluated the same effects. Stratified analyses were also performed by ethnicities and sources of cases.
Heterogeneity assumption was checked by a χ2-based Q-test. A P-value more than 0.05 for the Q-test indicated lack of heterogeneity among the studies, and so the summary OR estimate of each study was calculated by the fixed-effects model (the Mantel–Haenszel method). Otherwise, the random-effects model (DerSimonian and Laird method) was used.14, 15 To explore the reasons of heterogeneity, subgroup analyses were performed by grouping studies that showed similar characteristics, such as ethnicity and source of cases. Inter-study variance (I2) was used to quantify the degree of heterogeneity between studies, and percentage of I2 was used to describe the extent of explained heterogeneity of the characteristics. Potential publication bias was determined using Egger's linear regression test by visual inspection of a funnel plot. All statistical analyses were performed with the Stata software (version 8.2; StataCorp LP, College Station, TX, USA), using two-sided P-values.
Results
There were 18 studies retrieved on the basis of the search criteria for prostate cancer susceptibility related to ELAC2 Ser217Leu and Ala541Thr polymorphisms8, 11, 13, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 The study characteristics are summarized in Table 1. Rokman et al.31 studied both ELAC2 Ser217Leu and Ala541Thr polymorphisms, but there was no available genotype frequency of the Ser217Leu polymorphism in the article, so this study was included when analyzing the association between the Ala541Leu polymorphism and prostate cancer. Therefore, there are 17 studies with 5476 cases and 6794 controls concerning the Ser217Leu polymorphism and 14 studies with 5120 cases and 6209 controls concerning the Ala541Leu polymorphism. For the Ser217Leu polymorphism, there were 9 studies of Caucasian descendents, 4 studies of Asian descendents, 2 of African descendents and 2 of Mixed descendents. For Ala541Thr polymorphism, there were 7 studies of Caucasian descendents, 4 studies of Asian descendents, 1 of African descendents and 2 of Mixed descendents.
Table 1. Main characteristics of selected studies.
| First author | Year | Country | Ethnicity | Case | Control | Genotyping method |
|---|---|---|---|---|---|---|
| Suarez | 2001 | US | Caucasian | 257 | 355 | PCR–RFLP |
| Suzuki | 2002 | Japan | Asian | 81 | 106 | PCR–RFLP |
| Stanford | 2003 | Mixed | Mixed | 551 | 521 | PCR–RFLP |
| Noonan-Wheeler | 2006 | US | Caucasian | 150 | 170 | Prosequencing |
| Robbins | 2008 | US | African | 243 | 296 | Prosequencing |
| Shea | 2002 | Tobago | African | 119 | 223 | PCR–RFLP |
| Xu | 2001 | US | Caucasian | 362 | 182 | PCR–RFLP |
| Yokomizo | 2004 | Japan | Asian | 285 | 233 | PCR–RFLP |
| Meitz | 2002 | UK | Caucasian | 382 | 435 | PCR–RFLP |
| Vesprini | 2001 | Mixed | Mixed | 431 | 1505 | PCR–RFLP |
| Wang | 2001 | US | Caucasian | 444 | 502 | Prosequencing |
| Takahashi | 2003 | Japan | Asian | 98 | 255 | PCR-SSCP |
| Severi | 2003 | Australia | Caucasian | 825 | 732 | PCR–RFLP |
| Fujiwara | 2002 | Japan | Asian | 350 | 356 | PCR–RFLP |
| Adler | 2003 | Canada | Caucasian | 199 | 525 | PCR–RFLP |
| Tavigian | 2001 | Canada | Caucasian | 429 | 148 | Taqman |
| Rebbeck | 2000 | US | Caucasian | 270 | 250 | PCR–RFLP |
| Rokman | 2001 | Finland | Caucasian | 574 | 568 | PCR–RFLP |
Abbreviation: RFLP, restriction fragment length polymorphism.
All the studies used frequency-matched controls to the cases by the age, sex or ethnicity. PCR–restriction fragment length polymorphism assay was adopted in 13 of the 18 studies, and 11 studies mentioned quality control on genotyping, such as randomly repeated assays or validation using directed sequencing. In addition, Rokman et al.31 studied both sporadic and familial prostate cancer risk, but they did not show the data on familial prostate cancer risk, so this study was defined as a study of sporadic prostate cancer risk only. Therefore, 12 studies investigated sporadic prostate cancer risk, four studies8, 25, 26, 28 investigated familial prostate cancer risk and two studies22, 29 investigated both sporadic and familial prostate cancer risk. The distribution of genotypes in the controls was consistent with the Hardy–Weinberg equilibrium in all studies except for two studies.26, 27
The ELAC2 Ser217Leu polymorphism
We observed a wide variation of Leu217 allele frequencies across different ethnicities. The frequency of the Leu217 allele was 1.94% among Asian controls, which was significantly lower than that in Caucasian controls (29.01%, P<0.001).
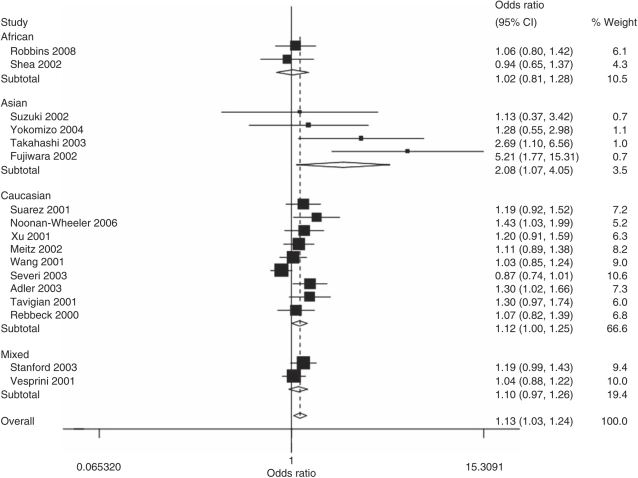
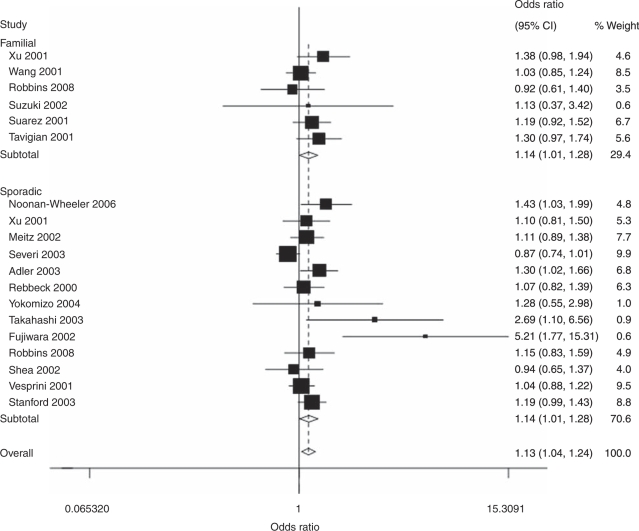
In the overall analysis, the ELAC2 Leu217 allele was associated with increased prostate cancer risk as compared with the Ser217 allele (OR=1.13, 95% CI: 1.03–1.24, P=0.019 for heterogeneity), as well as in heterozygote comparison (OR=1.21, 95% CI: 1.07–1.36, P=0.034 for heterogeneity) and in the dominant genetic model (OR=1.20, 95% CI: 1.07–1.35, P=0.025 for heterogeneity), but not in homozygote comparison (OR=1.11, 95% CI: 0.93, P=0.284 for heterogeneity; Table 2 and Figure 1), and the positive association maintained in some subgroup analyses. Specifically, in the comparison of Leu217 and Ser217, there was significantly increased risk of prostate cancer in Caucasian population (OR=1.12, 95% CI: 1.00–1.25, P=0.045 for heterogeneity), Asian population (OR=2.09, 95% CI: 1.07–4.05, P=0.134 for heterogeneity) and among studies with sporadic prostate cancer (OR=1.14, 95% CI: 1.01–1.28, P=0.007 for heterogeneity) or familial prostate cancer (OR=1.14, 95% CI: 1.01–1.28, P=0.525 for heterogeneity; Table 2 and Figure 2). The same results were observed also in heterozygote comparison and the dominant genetic model.
Table 2. Stratified analyses of the ELAC2 Ser217Leu polymorphism on prostate cancer risk.
| Variables | na | Cases/controls | Leu allele vs Ser allele | SL vs SS | LL vs SS | SL/LL vs SS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb | |||
| Total | 17 | 5476/6794 | 1.13 (1.03–1.24)c | 0.019 | 1.21 (1.07–1.36)c | 0.034 | 1.11 (0.93–1.31) | 0.284 | 1.20 (1.07–1.35)c | 0.025 |
| Ethnicities | ||||||||||
| Caucasian | 9 | 3318/3299 | 1.12 (1.00–1.25)c | 0.045 | 1.18 (1.03–1.36) | 0.097 | 1.15 (0.90–1.46) | 0.110 | 1.18 (1.03–1.37) | 0.054 |
| Asian | 4 | 814/950 | 2.09 (1.07–4.05) | 0.134 | 2.37 (1.26–4.44) | 0.206 | 1.10 (0.17–7.00) | 0.690 | 2.24 (1.17–4.28) | 0.171 |
| African | 2 | 362/519 | 1.02 (0.81–1.28) | 0.619 | 1.17 (0.87–1.58) | 0.290 | 0.65 (0.31–1.34) | 0.542 | 1.11 (0.84–1.46) | 0.387 |
| Mixed | 2 | 982/2026 | 1.10 (0.97–1.26) | 0.286 | 1.09 (0.92–1.29) | 0.407 | 1.22 (0.92–1.62) | 0.363 | 1.11 (0.95–1.31) | 0.316 |
| Sources of cases | ||||||||||
| Sporadic | 13 | 4043/5683 | 1.14 (1.01–1.28)c | 0.007 | 1.25 (1.07–1.47)c | 0.008 | 1.06 (0.88–1.28) | 0.345 | 1.23 (1.05–1.44)c | 0.005 |
| Familial | 6 | 1433/1589 | 1.14 (1.01–1.28) | 0.525 | 1.16 (0.99–1.36) | 0.708 | 1.26 (0.90–1.77) | 0.304 | 1.18 (1.01–1.38) | 0.727 |
Abbreviations: CI, confidence interval; ELAC2, elaC homolog-2; OR, odds ratio.
Number of comparisons.
P-value of Q-test for heterogeneity test.
A random-effects model was used when P-value for heterogeneity test was <0.05; otherwise, a fixed-effects model was used.
Figure 1.
Forest plot of cancer risk associated with the ELAC2 Ser217Leu polymorphism (Leu allele vs Ser allele). The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI. CI, confidence interval; ELAC2, elaC homolog-2; OR, odds ratio.
Figure 2.
A forest plot of cancer risk associated with the ELAC2 Ser217Leu polymorphism (Leu allele vs Ser allele) stratified by source of cases. The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI. CI, confidence interval; ELAC2, elaC homolog-2; OR, odds ratio.
There was significant heterogeneity for the Leu217 versus Ser217 comparison (P=0.019 for heterogeneity), heterozygote comparison (P=0.034 for heterogeneity) and dominant model comparison (P=0.025 for heterogeneity), but not for homozygote comparison (P=0.284 for heterogeneity). Consequently, we assessed the source of heterogeneity for the Leu217 versus Ser217 comparison by ethnicity, source of cases and sample size (subjects >200 in both case and control group). As a result, sample size (χ2=6.22, df=1, P=0.013) but not ethnicity (χ2=6.97, df=3, P=0.073) or source of cases (χ2=0.43, df=1, P=0.513) was found to account for the substantial heterogeneity. In addition, meta-regression analysis indicated that ethnicity could explain 46.3% of the I2, whereas source of cases could explain 43.3% of the I2.
The ELAC2 Ala541Thr polymorphism
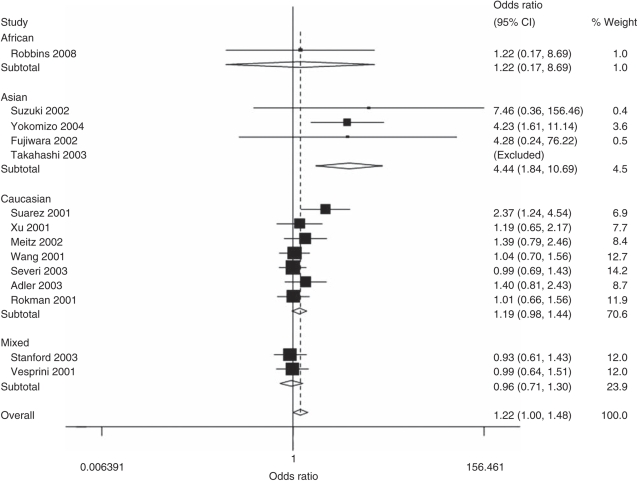
As shown in Table 3 and Figure 3, a statistically significant difference was observed in the comparison of the variant Thr541 allele frequency in control populations between the Asians and Caucasians (0.27 vs 3.64% P<0.001). Overall, the ELAC2 Thr541 allele was associated with increased prostate cancer risk as compared with the Ala541 allele (OR=1.22, 95% CI: 1.00–1.48, P=0.131 for heterogeneity). Moreover, significantly elevated risk was observed among the Asian populations (OR=4.44, 95% CI: 1.84–10.69, P=0.941 for heterogeneity) in comparison of Thr541 and Ala541, as well as in heterozygote comparison (OR=4.27, 95% CI: 1.75–10.40, P=0.928 for heterogeneity) and the dominant genetic model (OR=4.42, 95% CI: 1.82–10.74, P=0.937 for heterogeneity), but not among other populations in any genetic effect models. In addition, there was no significantly increased risk of prostate cancer among studies with sporadic prostate cancer and familial prostate cancer.
Table 3. Stratified analyses of the ELAC2 Ala541Thr polymorphism on prostate cancer risk.
| Variables | na | Cases/controls | Thr allele vs Ala allele | AT vs AA | TT vs AA | AT/TT vs AA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb | |||
| Total | 14 | 5120/6209 | 1.22 (1.00–1.48) | 0.131 | 1.23 (0.97–1.55)c | 0.043 | 1.64 (0.59–4.57) | 0.920 | 1.23 (0.99–1.53) | 0.076 |
| Ethnicities | ||||||||||
| Caucasian | 7 | 3084/3333 | 1.19 (0.98–1.44) | 0.084 | 1.20 (0.91–1.57) | 0.063 | 1.67 (0.44–6.39) | 0.667 | 1.19 (0.94–1.52) | 0.139 |
| Asian | 4 | 805/553 | 4.44 (1.84–10.69) | 0.941 | 4.27 (1.75–10.40) | 0.928 | 2.62 (0.11–64.67) | — | 4.42 (1.82–10.74) | 0.937 |
| African | 1 | 243/296 | 1.22 (0.17–8.69) | — | 1.22 (0.17–8.72) | — | — | — | 1.22 (0.17–8.72) | — |
| Mixed | 2 | 988/2027 | 0.96 (0.71–1.30) | 0.859 | 0.98 (0.72–1.35) | 0.848 | 1.37 (0.22–8.41) | 0.763 | 0.95 (0.70–1.30) | 0.899 |
| Sources of cases | ||||||||||
| Sporadic | 11 | 4134/5246 | 1.14 (0.93–1.38) | 0.276 | 1.12 (0.91–1.37) | 0.294 | 1.64 (0.51–5.31) | 0.992 | 1.13 (0.92–1.38) | 0.275 |
| Familial | 5 | 986/1441 | 1.46 (0.93–2.28) | 0.210 | 1.51 (0.75–3.05)c | 0.035 | 2.31 (0.30–17.59) | 0.263 | 1.50 (0.84–2.68) | 0.094 |
Abbreviations: CI, confidence interval; ELAC2, elaC homolog-2; OR, odds ratio.
Number of comparisons.
P-value of Q-test for heterogeneity test.
A random-effects model was used when P-value for heterogeneity test was <0.05; otherwise, a fixed-effects model was used.
Figure 3.
A forest plot of cancer risk associated with the ELAC2 Ala541Thr polymorphism (Thr allele vs Ala allele). The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI. CI, confidence interval; ELAC2, elaC homolog-2; OR, odds ratio.
There was no significant heterogeneity for the Thr541 versus Ala541 comparison (P=0.131 for heterogeneity), homozygote comparison (P=0.920 for heterogeneity) and the dominant model comparison (P=0.076 for heterogeneity), but for heterozygote comparison (P=0.043 for heterogeneity). Similarly, we assessed the source of heterogeneity for the Thr541 versus Ala541 comparison by ethnicity, source of cases and sample size (subjects >200 in both case and control group). As a result, ethnicity (χ2=10.54, df=3, P=0.014) but not source of cases (χ2=1.09, df=1, P=0.297) or sample size (χ2=0.78, df=1, P=0.378) was found to account for the substantial heterogeneity. In addition, meta-regression analysis indicated that ethnicity could explain 31.5% of the I2, whereas source of cases could explain 22.0% of the I2.
Sensitivity analyses
ELAC2 Ser217Leu
Sensitivity analyses indicated that one independent study by Severi et al.13 was the main origin of the heterogeneity. The heterogeneity was effectively removed after exclusion of this study (Leu217 vs Ser217: P=0.204 for heterogeneity). Although two studies26, 27 did not follow the Hardy–Weinberg equilibrium, the summary ORs were not effectively altered with or without including these two studies. Moreover, no other single study influenced the summary OR qualitatively as indicated by sensitivity analyses.
ELAC2 Ala541Thr
Similarly, sensitivity analyses indicated that no other single study influenced the summary OR qualitatively.
Publication bias
Begg's funnel plot and Egger's test were performed to assess the publication bias. The shape of the funnel plots seemed asymmetrical in allele comparison for both polymorphisms, suggesting the presence of publication bias (Supplementary Figure). Then, Egger's test was used to provide statistical evidence of funnel plot symmetry. As expected, the results have shown an obvious evidence of publication bias (Leu allele vs Ser allele, t=3.21, P=0.006; Thr allele vs Ala allele, t=3.06, P=0.011).
Discussion
The rapid growth of human genetics creates countless opportunities for studies of disease association. Given the number of potentially identifiable genetic markers and the multitude of clinical outcomes to which these may be linked, the testing and validation of statistical hypotheses in genetic epidemiology is a task of unprecedented scale. Meta-analysis provides a quantitative approach for combining the results of various studies with the same topic, and for estimating and explaining their diversity.32
The present meta-analysis, including 18 published case–control studies, explored the association between two polymorphisms in the ELAC2 gene region and prostate cancer risk. We found that both ELAC2 Ser217Leu and Ala541Thr polymorphisms were associated with significant increase in prostate cancer risk. Given the potential roles of ELAC2 in DNA inter-strand crosslink repair, mRNA editing and cell-cycle progression, the ELAC2 polymorphisms may modulate the risk of prostate cancer. Tavtigian et al.8 and Vesprini et al.11 found that the Ser217Leu variant is located in the hydrophilic segment of the ELAC2 protein sequence, and substitution of the hydrophobic leucine residue may alter the protein structure, and the Ala541Thr variant is adjacent to a histidine motif and may impair protein function. Thus, it is reasonable that the Leu217 allele and the Thr541 allele may decrease protein expression leading to impaired ELAC2 function. In our meta-analysis, we found that subjects carrying the Leu217 or Thr541 allele were associated with higher risk of prostate cancer than those with the wild-type allele, which confirmed the hypothesis described above.
We found evidence for the association between the Ser217Leu polymorphism and prostate cancer risk among Caucasians (Leu217 vs Ser217) and Asians (Leu217 vs Ser217) but not among Africans (all genetic models). Moreover, the Ala541Thr polymorphism was associated with increased prostate cancer risk among Asians but not among Caucasians and Africans in all genetic models. It may be a possible reflection of the differences in genetic backgrounds and gene–environment interactions in the etiology. Other factors such as time-lag bias and publication bias may also have a role. In time-lag bias,33 studies with ‘negative' results take longer time to be published, whereas enthusiastic results are published much more quickly. In publication bias,34, 35 small studies with ‘negative' results are never published, whereas equally small studies with similar quality but ‘positive' results would appear in the literature. We examined these possibilities and found that ‘positive' studies were reported more in Leu217 and Thr541 allele carriers' studies (especially in the Asian studies). Furthermore, there is only one published study using African population. Therefore, the observed ethnic differences may be due to chance as studies with small sample size may have insufficient statistical power to detect a slight effect. So, further investigations are warranted to validate the ethnic difference in the effect of these polymorphisms on prostate cancer risk especially in African population.
In this study we also found an association between the Ser217Leu polymorphism and prostate cancer risk among studies using sporadic or familial prostate cancer cases, whereas no significant difference was found in the stratified analysis for the Ala541Thr polymorphism. Different roles of these two polymorphisms in the formation process of sporadic and familial prostate cancer might contribute to this discrepancy.
There are some limitations of this meta-analysis to be acknowledged. First, only published studies were included in the meta-analysis; therefore, publication bias might have occurred and our results may have a substantial risk of being affected by such bias. Second, these results should be interpreted with caution because the control populations were not uniform as in most meta-analyses. Non-differential misclassification bias is possible because the majority of the studies did not consider that some controls might have developed prostate cancer in subsequent years. Third, in some sporadic studies, a small proportion of cases with family history were included,18, 25, 27 because it is not feasible to separate the familial cases from the sporadic cases on the basis of available information. Fourth, our result was based on unadjusted estimates, while a more precise analysis should be conducted adjusted by other factors like age, sex, and smoking and drinking status. Lack of the information for data analysis may cause serious confounding bias. Fifth, lack of the original data of the reviewed studies limited our further evaluation of potential interactions such as gene–gene, gene–environment and different polymorphic loci of the same gene, which may also modulate cancer risk. We minimized the likelihood of bias by developing a detailed protocol before initiating the study, by performing a meticulous search for published studies and by using explicit methods for study selection, data extraction and data analysis. Therefore, our meta-analysis also had some advantages. First, substantial number of cases and controls were pooled from different studies, which significantly increased the statistical power of the analysis. Second, the studies included in our meta-analysis were satisfactory and strictly met our inclusion and criterion.
Conclusions
This meta-analysis showed evidence that the ELAC2 Ser217Leu and Ala541Thr polymorphisms might be associated with prostate cancer risk, which supported the hypothesis that these two polymorphisms may be low-penetrance susceptibility markers of prostate cancer. Further prospective researches with larger numbers of worldwide participants are expected to examine associations between ELAC2 Ser217Leu and Ala541Thr polymorphisms, and prostate cancer risk to make a comprehensive and true conclusion, and other possible confounding risk factors like age, sex, life style and race should also be controlled when important genetic risks for prostate cancer are assessed.
Acknowledgments
This study was supported by the program of key medical department of Jiangsu Province, Department of urology of Jiangsu Province Hospital (XK17 200904).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website (http://www.nature.com/pcan)
Supplementary Material
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Pienta KJ, Esper PS. Is dietary fat a risk factor for prostate cancer. J Natl Cancer Inst. 1993;85:1538–1540. doi: 10.1093/jnci/85.19.1538. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Mukhtar H.Lifestyle as risk factor for cancer: evidence from human studies Cancer Lett(e-pub ahead of print 15 January 2010). [DOI] [PMC free article] [PubMed]
- Imamoto T, Suzuki H, Utsumi T, Endo T, Takano M, Yano M, et al. Association between serum sex hormone levels and prostate cancer: effect of prostate cancer on serum testosterone levels. Future Oncol. 2009;5:1005–1013. doi: 10.2217/fon.09.82. [DOI] [PubMed] [Google Scholar]
- Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian P, Howe GR, Hislop TG, Maisonneuve P. Family history of prostate cancer: a multi-center case–control study in Canada. Int J Cancer. 1997;70:679–681. doi: 10.1002/(sici)1097-0215(19970317)70:6<679::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II: individual countries. BJU Int. 2002;90:174–184. doi: 10.1046/j.1464-410x.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- Tavtigian SV, Simard J, Teng DH, Abtin V, Baumgard M, Beck A, et al. A candidate prostate cancer susceptibility gene at chromosome 17p. Nat Genet. 2001;27:172–180. doi: 10.1038/84808. [DOI] [PubMed] [Google Scholar]
- Takaku H, Minagawa A, Takagi M, Nashimoto M. A candidate prostate cancer susceptibility gene encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 2003;31:2272–2278. doi: 10.1093/nar/gkg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver W, Guevara C, Chen Y, Neuteboom S, Bookstein R, Tavtigian S, et al. The product of the candidate prostate cancer susceptibility gene ELAC2 interacts with the gamma-tubulin complex. Int J Cancer. 2003;104:283–288. doi: 10.1002/ijc.10945. [DOI] [PubMed] [Google Scholar]
- Vesprini D, Nam RK, Trachtenberg J, Jewett MA, Tavtigian SV, Emami M, et al. HPC2 variants and screen-detected prostate cancer. Am J Hum Genet. 2001;68:912–917. doi: 10.1086/319502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp NJ, Tavtigian SV. Meta-analysis of associations of the Ser217Leu and Ala541Thr variants in ELAC2 (HPC2) and prostate cancer. Am J Hum Genet. 2002;71:1475–1478. doi: 10.1086/344516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severi G, Giles GG, Southey MC, Tesoriero A, Tilley W, Neufing P, et al. ELAC2/HPC2 polymorphisms, prostate-specific antigen levels, and prostate cancer. J Natl Cancer Inst. 2003;95:818–824. doi: 10.1093/jnci/95.11.818. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- Adler D, Kanji N, Trpkov K, Fick G, Hughes RM. HPC2/ELAC2 gene variants associated with incident prostate cancer. J Hum Genet. 2003;48:634–638. doi: 10.1007/s10038-003-0091-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Emi M, Nagai H, Nishimura T, Konishi N, Kubota Y, et al. Association of common missense changes in ELAC2 (HPC2) with prostate cancer in a Japanese case–control series. J Hum Genet. 2002;47:641–648. doi: 10.1007/s100380200099. [DOI] [PubMed] [Google Scholar]
- Meitz JC, Edwards SM, Easton DF, Murkin A, Ardern-Jones A, Jackson RA, et al. HPC2/ELAC2 polymorphisms and prostate cancer risk: analysis by age of onset of disease. Br J Cancer. 2002;87:905–908. doi: 10.1038/sj.bjc.6600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan-Wheeler FC, Wu W, Roehl KA, Klim A, Haugen J, Suarez BK, et al. Association of hereditary prostate cancer gene polymorphic variants with sporadic aggressive prostate carcinoma. Prostate. 2006;66:49–56. doi: 10.1002/pros.20320. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Walker AH, Zeigler-Johnson C, Weisburg S, Martin AM, Nathanson KL, et al. Association of HPC2/ELAC2 genotypes and prostate cancer. Am J Hum Genet. 2000;67:1014–1019. doi: 10.1086/303096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert H, Zeigler-Johnson CM, Addya K, Finley MJ, Walker AH, Spangler E, et al. Association of susceptibility alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with prostate cancer severity in European American and African American men. Cancer Epidemiol Biomarkers Prev. 2005;14:949–957. doi: 10.1158/1055-9965.EPI-04-0637. [DOI] [PubMed] [Google Scholar]
- Robbins CM, Hernandez W, Ahaghotu C, Bennett J, Hoke G, Mason T, et al. Association of HPC2/ELAC2 and RNASEL non-synonymous variants with prostate cancer risk in African American familial and sporadic cases. Prostate. 2008;68:1790–1797. doi: 10.1002/pros.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea PR, Ferrell RE, Patrick AL, Kuller LH, Bunker CH. ELAC2 and prostate cancer risk in Afro-Caribbeans of Tobago. Hum Genet. 2002;111:398–400. doi: 10.1007/s00439-002-0816-1. [DOI] [PubMed] [Google Scholar]
- Stanford JL, Sabacan LP, Noonan EA, Iwasaki L, Shu J, Feng Z, et al. Association of HPC2/ELAC2 polymorphisms with risk of prostate cancer in a population-based study. Cancer Epidemiol Biomarkers Prev. 2003;12:876–881. [PubMed] [Google Scholar]
- Suarez BK, Gerhard DS, Lin J, Haberer B, Nguyen L, Kesterson NK, et al. Polymorphisms in the prostate cancer susceptibility gene HPC2/ELAC2 in multiplex families and healthy controls. Cancer Res. 2001;61:4982–4984. [PubMed] [Google Scholar]
- Suzuki K, Ohtake N, Nakata S, Takei T, Matsui H, Ono Y, et al. Association of HPC2/ELAC2 polymorphism with prostate cancer risk in a Japanese population. Anticancer Res. 2002;22:3507–3511. [PubMed] [Google Scholar]
- Takahashi H, Lu W, Watanabe M, Katoh T, Furusato M, Tsukino H, et al. Ser217Leu polymorphism of the HPC2/ELAC2 gene associated with prostatic cancer risk in Japanese men. Int J Cancer. 2003;107:224–228. doi: 10.1002/ijc.11347. [DOI] [PubMed] [Google Scholar]
- Wang L, McDonnell SK, Elkins DA, Slager SL, Christensen E, Marks AF, et al. Role of HPC2/ELAC2 in hereditary prostate cancer. Cancer Res. 2001;61:6494–6499. [PubMed] [Google Scholar]
- Xu J, Zheng SL, Carpten JD, Nupponen NN, Robbins CM, Mestre J, et al. Evaluation of linkage and association of HPC2/ELAC2 in patients with familial or sporadic prostate cancer. Am J Hum Genet. 2001;68:901–911. doi: 10.1086/319513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo A, Koga H, Kinukawa N, Tsukamoto T, Hirao Y, Akaza H, et al. HPC2/ELAC2 polymorphism associated with Japanese sporadic prostate cancer. Prostate. 2004;61:248–252. doi: 10.1002/pros.20107. [DOI] [PubMed] [Google Scholar]
- Rokman A, Ikonen T, Mononen N, Autio V, Matikainen MP, Koivisto PA, et al. ELAC2/HPC2 involvement in hereditary and sporadic prostate cancer. Cancer Res. 2001;61:6038–6041. [PubMed] [Google Scholar]
- Zhuang W, Wu XT, Zhou Y, Liu L, Liu GJ, Wu TX, et al. Interleukin10 -592 promoter polymorphism associated with gastric cancer among Asians: a meta-analysis of epidemiologic studies Dig Dis Sci(e-pub ahead of print 11 August 2009). [DOI] [PubMed]
- Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279:281–286. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Min YI, Meinert CL. Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. JAMA. 1992;267:374–378. [PubMed] [Google Scholar]
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.