Abstract
OBJECTIVE
To evaluate the emerging classes of antihyperglycemic agents that target the incretin pathway, including their therapeutic efficacy and side effect profiles, in order to help identify their place among the treatment options for patients with type 2 diabetes.
QUALITY OF EVIDENCE
MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews were searched. Most evidence is level I and II.
MAIN MESSAGE
Two classes of incretin agents are currently available: glucagonlike peptide 1 (GLP1) receptor agonists and dipeptidyl peptidase 4 (DPP4) inhibitors, both of which lower hyperglycemia considerably without increasing the risk of hypoglycemia. The GLP1 receptor agonists have a greater effect on patients’ glycated hemoglobin A1c levels and cause sustained weight loss, whereas the DPP4 inhibitors are weight-neutral.
CONCLUSION
The GLP1 and DPP4 incretin agents, promising and versatile antihyperglycemic agents, are finding their way into the therapeutic algorithm for treating type 2 diabetes. They can be used in patients not adequately controlled by metformin monotherapy or as initial therapy in those for whom metformin is contraindicated.
Résumé
OBJECTIF
Évaluer les nouvelles classes d’hypoglycémiants qui agissent via la voie des incrétines, y compris leur efficacité thérapeutique et leur profil d’effets indésirables, afin d’aider à établir leur place parmi les options de traitement pour les diabétiques de type 2.
QUALITÉ DES PREUVES
On a consulté MEDLINE, EMBASE et la Cochrane Database of Systematic Reviews. La plupart des preuves étaient de niveaux I et II.
PRINCIPAL MESSAGE
Il existe actuellement deux classes d’incrétines : les agonistes des récepteurs du peptide 1 apparenté au glucagon (GLP1) et les inhibiteurs de la dipeptidyl peptidase (DPP4), qui causent tous deux une réduction importante de l’hyperglycémie sans augmenter le risque d’hypoglycémie. Les agonistes des récepteurs du GLP1 ont un effet plus grand sur l’hémoglobine glycosylée A1c et entraînent une perte de poids durable, alors que les inhibiteurs de la DPP4 sont sans effet sur le poids.
CONCLUSION
Les incrétines des types GLP1 et DPP4 sont des hypoglycémiants versatiles et prometteurs qui s’intègrent progressivement à l’algorithme thérapeutique du traitement du diabète de type 2. Ils peuvent être utiles chez les patients qui ne sont pas adéquatement contrôlés avec la metformine seule ou comme traitement de première intention dans les cas où la metformine est contre-indiquée.
Diabetes is a disease of increasing consequence, affecting an estimated 285 million adults worldwide, of whom approximately 85% to 95% have type 2 diabetes (T2DM).1 Advances over the past few decades have yielded various therapeutic options.2 In the Canadian primary care setting, however, 49% of patients with T2DM failed to achieve the glycated hemoglobin (HbA1c) target levels (≤ 7%) recommended by the Canadian Diabetes Association (CDA),3 with 17% of patients at levels above 8.4%.4 Current approaches thus appear to be inadequate for many patients with T2DM.
Since 2008, Canadian physicians have had access to sitagliptin, an antihyperglycemic drug with a novel mechanism of action: increasing the activity of glucose-regulating hormones called incretins, which stimulate insulin secretion in response to high levels of blood glucose. The fact that incretins are glucose-dependent is key to their unique therapeutic potential, because this offers the possibility of controlling hyperglycemia without placing patients at risk of hypoglycemia.5
The incretin pathway (Figure 1), which represents an important aspect of normal glucose homeostasis, is not the direct target of any traditional antihyperglycemic treatments. However, various new drugs that act on this pathway have been studied extensively in late-phase trials.6 Three of these incretin agents (sitagliptin, saxagliptin, and liraglutide) are now approved in Canada, and others (already in use in Europe or the United States) could reach the Canadian market in the very near future. Here, we examine recent evidence regarding the potential of 4 of the incretin agents (sitagliptin, vildagliptin, exenatide, and liraglutide) to improve routine T2DM management in Canada, as described in the recent CDA treatment guidelines.3 Readers wishing for a broader analysis of the incretin literature, not restricted to the Canadian treatment environment, might wish to consult other relatively recent reviews, such as a series in the American Journal of Medicine in 2009.7–10
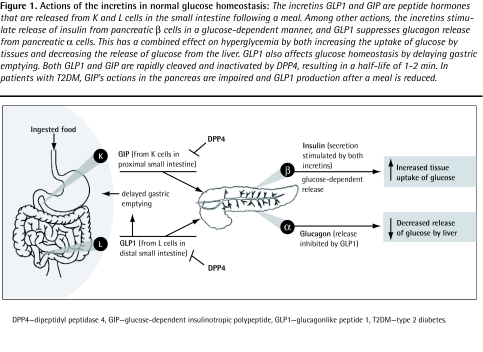
Figure 1.
Actions of the incretins in normal glucose homeostasis: The incretins GLP1 and GIP are peptide hormones that are released from K and L cells in the small intestine following a meal. Among other actions, the incretins stimulate release of insulin from pancreatic β cells in a glucose-dependent manner, and GLP1 suppresses glucagon release from pancreatic α cells. This has a combined effect on hyperglycemia by both increasing the uptake of glucose by tissues and decreasing the release of glucose from the liver. GLP1 also affects glucose homeostasis by delaying gastric emptying. Both GLP1 and GIP are rapidly cleaved and inactivated by DPP4, resulting in a half-life of 1–2 min. In patients with T2DM, GIP’s actions in the pancreas are impaired and GLP1 production after a meal is reduced.
DPP4—dipeptidyl peptidase 4, GIP—glucose-dependent insulinotropic polypeptide, GLP1—glucagonlike peptide 1, T2DM—type 2 diabetes.
Quality of evidence
MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews were searched. Search terms and MeSH headings used included exenatide, liraglutide, vildagliptin, and sitagliptin, in combination with the term clinical. Reference lists in selected articles were reviewed to identify additional relevant information. Randomized controlled trials, meta-analyses, and systematic reviews (level I evidence) were assessed, as were open-label trials and US Food and Drug Administration publications (level II evidence) for “real-world” experience. Level III evidence describing potential rare adverse events was also considered.
Physiologic effects of incretins
The endogenous incretins, namely glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide 1 (GLP1), are peptide hormones secreted from endocrine cells in the small intestine.11 In healthy individuals, both of these molecules activate insulin secretion; GLP1 also inhibits glucagon secretion and slows gastric emptying.11
Levels of evidence
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
A key limitation to therapeutic use of the endogenous incretins is their rapid turnover (1- to 2-minute half-lives), resulting from the action of the circulating enzyme dipeptidyl peptidase 4 (DPP4), which rapidly inactivates GIP and GLP1.11 In addition, GIP’s actions on the pancreas are blunted in patients with T2DM.12 In contrast, the action of GLP1 is essentially intact in patients with T2DM, although this hormone is produced at lower-than-normal levels following meals.12
Promising therapeutic approaches for T2DM therefore include stabilizing endogenous GLP1 using a DPP4 inhibitor (sitagliptin, vildagliptin, or saxagliptin13,14) or supplying an exogenous, more stable molecule with the action of GLP1 (the GLP1 mimetic, exenatide, or the human GLP1 analogue, liraglutide).5
Integrating incretin agents into therapeutic paradigms
As reviewed below, accumulating clinical experience for all of these therapies shows that they can effect considerable improvements in HbA1c levels, combined with sustained beneficial effects on body weight (weight loss with GLP1 receptor agonists and weight neutrality with DPP4 inhibitors). Indeed, it appears that the incretin agents discussed here are at least as effective as commonly used oral antihyperglycemics.15–19 Used in combination with oral agents, the GLP1 receptor agonists (exenatide and liraglutide) might offer glycemic control similar to that of insulin treatment, but with little or no risk of serious hypoglycemia.20–23 Incretin agents might reduce patients’ risk of diabetic complications, including possibly cardiovascular disease (CVD); trials to test this hypothesis are ongoing. Information about these ongoing trials is available from www.clinicaltrials.gov (trial numbers NCT00766857 and NCT00790205).
Key pharmacologic differences among the 4 drugs reviewed here, as well as other clinical considerations, can be found in Table 1.18,20,22–37 The clinical literature supporting the use of the various incretin agents is summarized in Table 2.15,16,18,20–28,31,32–36,38–60
Table 1.
Pharmacology and clinical considerations for the incretin agents
| CONSIDERATIONS |
GLP1 RECEPTOR AGONISTS |
DPP4 INHIBITORS |
||
|---|---|---|---|---|
| EXENATIDE20,22,24–31 | LIRAGLUTIDE18,23,32–34 | VILDAGLIPTIN35,36 | SITAGLIPTIN35,37 | |
| Administration | SC injection | Oral tablet | ||
| Half-life, h | 2.4 | 11–15 | 2.5 | 12–14 |
| Dose | 5 or 10 μg, twice daily | 0.6, 1.2, or 1.8 mg, once daily | 50 mg, twice daily | 100 mg, once daily |
| Origin of active incretins following treatment | Exogenous and endogenous | Endogenous | ||
| Effect on insulin level | Large increase | Moderate increase | ||
| Effect on glucagon level | Moderate decrease | |||
| Mean decrease in HbA1c vs placebo, % | Approximately 0.8 | 0.8–1.6 | Approximately 0.7 | 0.6–1.0 |
| Postprandial hyperglycemia | Moderate decrease | Small decrease | ||
| Gastric emptying | Inhibited | No clinically significant effect | ||
| Body weight | Moderate decrease | Neutral | ||
| Tolerability issues* | Nausea | Upper respiratory tract infection | ||
| Incidence of hypoglycemia | Low rate of hypoglycemia when administered as monotherapy in patients with T2DM; risk might increase when used in combination with sulfonylureas | |||
DPP4—dipeptidyl peptidase 4, GLP1—glucagonlike peptide 1, HbA1c—glycated hemoglobin A1c, SC—subcutaneous, T2DM—type 2 diabetes.
For more complete listings of adverse events, consult the respective product monographs.
Table 2.
Summary of clinical trials involving incretin agents
| INCRETIN AGENT AND COMBINATION | COMPARATOR | N | DURATION, WK | MEAN CHANGE IN HBA1C, %* |
|---|---|---|---|---|
| Sitagliptin | ||||
| • Monotherapy35,38–43 | Placebo | 2389 | 12–24 | −0.44 to −0.66 |
| • Metformin35,40,44 | Placebo | 1437 | 24 | −0.67 to −1.90 |
| • Metformin15,35 | Glipizide | 1172 | 52 | −0.67 |
| • Glimepiride with or without metformin35,45 | Placebo | 441 | 24 | −0.45 |
| • Pioglitazone35,46 | Placebo | 353 | 24 | −0.85 |
| • Metformin47 | Exenatide | 61 | 2 | NA |
| Vildagliptin | ||||
| • Monotherapy35,48–53 | Placebo | 2253 | 12–52 | −0.20 to −0.92 |
| • Metformin54,55 | Placebo | 651 | 12–24 | −0.60 to −0.90 |
| • Insulin56 | Placebo | 296 | 24 | −0.50 |
| • Metformin16 | Pioglitazone | 576 | 24 | −0.88 |
| • Pioglitazone57 | Placebo | 463 | 24 | −1.00 |
| • Various59 | Vildagliptin vs pioglitazone vs vildagliptin and pioglitazone | 607 | 24 | −1.1 |
| Exenatide | ||||
| • Sulfonylurea24 | Placebo | 377 | 30 | −0.86 |
| • Metformin25 | Placebo | 336 | 30 | −0.8 |
| • Metformin and sulfonylurea26 | Placebo | 733 | 30 | −0.75† |
| • TZD with or without metformin27 | Placebo | 233 | 16 | −0.89 |
| • Metformin and sulfonylurea20,21 | Insulin glargine | 689 | 26–32 | −1.36 to −1.16 |
| • Metformin and sulfonylurea22 | Biphasic insulin | 501 | 52 | −1.04 |
| • Monotherapy31 | Placebo | 232 | 24 | −0.9 |
| • Metformin, sulfonylurea, or both28 | None | 314 | 82 | −1.1 |
| • Metformin, sulfonylurea, or both59 | Placebo | 217 | 156 | −1.0 |
| Liraglutide | ||||
| • Monotherapy (LEAD-3)18 | Glimepiride | 746 | 52 | −0.84 to −1.14 |
| • Metformin (LEAD-2)33 | Placebo or glimepiride | 1091 | 26 | −1.0 |
| • Glimepiride (LEAD-1)32 | Placebo or rosiglitazone | 1041 | 26 | −1.1 |
| • Metformin and rosiglitazone (LEAD-4)34 | Placebo | 533 | 26 | −1.5 |
| • Metformin and sulfonylurea (LEAD-5)23 | Insulin glargine | 581 | 26 | −1.33 |
| • Previous oral antihyperglycemics (LEAD-6)60 | Exenatide plus previous oral antihyperglycemics | 464 | 26 | −1.12 |
HbA1c—glycated hemoglobin A1c, LEAD—Liraglutide Effect and Action in Diabetes, NA—not applicable, TZD—thiazolidinedione.
Reported mean change from baseline in patients treated with incretin agents. Where several values are provided (eg, different doses of the study drug), either in a single report or in multiple reports, the range of reported mean values is shown.
Precise value not reported; reduction in HbA1c levels was shown in a figure.
DPP4 inhibitors: sitagliptin and vildagliptin
Oral DPP4 inhibitors increase the availability of endogenous GLP1, thus enhancing glucose-induced insulin secretion and inhibiting glucagon release. These agents have no effect on gastric emptying61,62 and do not affect body weight.35 A comprehensive meta-analysis of trials of once-daily sitagliptin (available in Canada and elsewhere) or twice-daily vildagliptin (marketed in Europe) concluded that these agents were well tolerated,35 although infections, including nasopharyngitis, upper respiratory tract infections, and urinary tract infections, were significantly increased with sitagliptin (relative risk 1.15 compared with placebo [95% confidence interval 1.02 to 1.31; P = .03]). Headache was reported for both drugs but was more common in patients taking vildagliptin.35
As monotherapy or in combination with other anti-hyperglycemic agents, DPP4 inhibitors reduced HbA1c levels to an extent similar to metformin, sulfonylureas,15 and thiazolidinediones (TZDs),16 with an average reduction of 0.6% to 0.7% compared with placebo.35 When added to insulin, vildagliptin reduced HbA1c levels by 0.6%, compared with 0.2% for placebo.56 Although long-term studies are not available comparing DPP4 inhibitors with incretin receptor agonists, a 2-week, randomized, double-blind crossover trial demonstrated significantly increased insulin secretion and tighter control of postmeal hyperglycemia with exenatide, relative to sitagliptin (P < .0001).47
GLP1 receptor agonists: exenatide and liraglutide
As peptide-based therapies, the GLP1 receptor agonists require subcutaneous administration. Thus far, exenatide has been approved for use in the United States, Europe, and elsewhere; liraglutide is now approved in the United States, Canada, and Europe. Both have been examined in multiple randomized clinical trials, in which they were used as monotherapy or as add-on therapies.
The GLP1 receptor agonists are well tolerated, and trials to date have identified no common safety concerns,18,20,22,23–28,32–34 although administration of exenatide is dose-dependently associated with nausea.29,63 Liraglutide is also associated with nausea, a side effect that does not appear to be dose dependent and which declines in frequency within 4 weeks of treatment.18,60
It has also been suggested, largely on the basis of studies in rodents, that liraglutide treatment might be associated with increased risk of C-cell adenoma or carcinoma, cancers affecting the calcitonin-producing cells of the thyroid. Indeed, mice and rats treated with liraglutide might experience acute or chronic calcitonin overexpression and C-cell hyperplasia, followed in some cases by C-cell adenoma or (at the highest doses) carcinoma. However, these effects seem to be restricted to rodents, in which normal C-cells carry functional GLP1 receptors. No calcitonin up-regulation or C-cell abnormalities have been seen in nonhuman primates treated with liraglutide. Furthermore, a calcitonin screening program, carried out in 5000 individuals, found no evidence that liraglutide stimulates human C-cells. To date, there have been no clear cases of C-cell cancers in patients receiving liraglutide.
There have been isolated reports of adverse events with these drugs, notably postmarketing reports of acute pancreatitis in patients taking exenatide. In the liraglutide development program, incidence of pancreatitis is in line with expected rates in the T2DM population.64
One potentially important distinction between the 2 agents relates to their routes of excretion. Exenatide is primarily excreted by the kidneys; although dose adjustment is not necessary for patients with moderate renal impairment (creatinine clearance 30 to 80 mL/min), it might be required for those with more severe kidney disease.65 Clearance of liraglutide, by contrast, is independent of the kidneys and the creatinine clearance rate.66
The available clinical studies offer important insights into the place of these new agents among the therapeutic options for T2DM care, as discussed below.
Exenatide
Exenatide is a synthetic version of a Gila monster salivary peptide that has 53% sequence identity with human GLP1.67 It has a half-life of 2.4 hours after subcutaneous injection in humans, with peak action achieved after 2 to 3 hours.30 Studies showed that when administered twice daily, immediately before breakfast and dinner, exenatide lowered glucose for 5 to 7 hours, predominantly affecting glycemic excursion at those meals. Postlunch hyperglycemia and fasting blood glucose levels also declined significantly (P < .05).63,68
Exenatide’s efficacy as monotherapy (5- and 10-μg twice-daily dosing) was explored in 1 study, which found that HbA1c levels declined steadily during the first 12 weeks of treatment and remained stable at 0.7% and 0.9% below baseline, respectively for the 2 different dosages.31 Patients experienced dose-dependent weight loss (2.8 and 3.1 kg, respectively) and improved systolic and diastolic blood pressure. In other studies, in which exenatide (10 μg twice daily) was combined with either metformin or a sulfonylurea, similar improvements in HbA1c levels occurred, with stable values of 0.8% to 0.9% below baseline.24,25 In the exenatide-sulfonylurea study, 41% of patients achieved HbA1c levels below 7.0%.25 Both combinations also caused progressive weight loss.24,25
Exenatide has also been studied in more complex regimens, as an add-on to metformin plus either a sulfonylurea or a TZD. In combination with metformin and a sulfonylurea, exenatide (10 μg twice daily) led to a mean placebo-adjusted reduction in HbA1c levels of 1.0%, with 34% of patients achieving HbA1c levels below 7.0% after 30 weeks.26 Again, significant weight loss of 1.6 kg occurred (P ≤ .01).26 In this multidrug regimen, patients treated with exenatide experienced changes in HbA1c levels similar to those observed in patients receiving once-daily insulin glargine20 or twice-daily biphasic insulin,22 but with weight loss instead of the weight gain associated with insulin treatments.20,22 Likewise, when combined with metformin plus a TZD, exenatide reduced HbA1c levels by an average of 0.9%, with a 1.5-kg weight reduction.27
In a longer-term, open-label extension trial of exenatide used as an add-on to sulfonylureas or metformin, patients experienced a mean reduction in HbA1c levels of 1.1% by 82 weeks, with half achieving HbA1c levels below 7.0%. Mean weight loss from baseline was 4.4 kg. Four severe cases of hypoglycemia were reported, all associated with sulfonylurea use.28 Decreased HbA1c levels and weight loss were sustained after 3 years.
In addition to the twice-daily formulation discussed above, a longer-acting exenatide formulation has been devised for once-weekly dosing. Extended-release exenatide appears to offer improved glycemic control relative to twice-daily exenatide, with similar body-weight benefits.69 More clinical findings are expected for extended-release exenatide (registered with www.clinicaltrials.gov as NCT00308139 and NCT00877890), as well as for albiglutide (registered with www.clinicaltrials.gov as NCT00849017 and NCT00838916), a novel GLP1 receptor agonist that might also offer good glycemic control with infrequent dosing.70
Liraglutide
Liraglutide is a GLP1 analogue with 97% sequence identity to the human hormone. Liraglutide contains a single amino acid substitution relative to endogenous GLP1 and is linked to a fatty acid chain, resulting in slow absorption into circulation, increased reversible albumin binding, and reduced susceptibility to DPP4. These effects extend liraglutide’s benefits, increasing its plasma half-life to 11 to 15 hours,71,72 with maximal concentration after 8 to 12 hours.73 Injected once daily, at any time of day, irrespective of meals, liraglutide reduced fasting blood glucose and glycemic excursions associated with all meals.18
Most recently, a series of 6 phase 3 studies (the Liraglutide Effect and Action in Diabetes [LEAD] studies) tested the use of liraglutide in different therapeutic regimens. The LEAD studies examined glycemic and weight effects and the safety of once-daily liraglutide (alone or in combination with other treatments), compared with placebo or active comparators.
The year-long LEAD-3 study explored liraglutide’s use as possible monotherapy; LEAD-3 patients discontinued previous oral antihyperglycemics and received either the sulfonylurea glimepiride or liraglutide, the latter at 1 of 2 doses (1.2 or 1.8 mg). The mean reduction in HbA1c levels with liraglutide was dose-dependent (−0.84% [SD 1.23%] vs −1.14% [SD 1.24%], respectively), but liraglutide at either dose was superior to glimepiride.18 Half of patients administered the higher dose of liraglutide achieved HbA1c levels below 7.0%.18 Within 16 weeks of treatment, mean body-weight reduction compared with glimepiride was 3.2 kg and 3.6 kg with liraglutide, depending on dose. Both the HbA1c levels and weight reductions were sustained throughout the 52-week study.18
In LEAD-1, LEAD-2, and LEAD-4, researchers tested the use of liraglutide combined with glimepiride, metformin, or metformin and rosiglitazone, respectively. These combination regimens reduced mean HbA1c levels by more than 1% over 26 weeks.32–34 Liraglutide had consistent beneficial effects on body weight, relative to the comparable regimen without liraglutide. For instance, in patients receiving liraglutide and metformin, mean weight was reduced by 1.8 to 2.8 kg, depending on liraglutide dose.33 In addition, as seen across the LEAD studies, treatment with liraglutide led to consistent improvements relative to baseline in systolic blood pressure.74 Further, β-cell function also increased with liraglutide treatment,75 as has been observed in studies of sitagliptin.76,77 Incidence of hypoglycemia was low with liraglutide, both in monotherapy18 and in combination regimens.
In LEAD-5, once-daily liraglutide was compared directly with insulin glargine in patients receiving concomitant metformin and glimepiride.23 Liraglutide led to significantly lower HbA1c levels compared with glargine (P = .0015). As is commonly observed following transition to insulin,78 patients starting glargine gained weight. Conversely, those administered liraglutide lost weight, with a difference of 3.5 kg at study’s end.23 These data,23 along with a similar study of exenatide versus insulin glargine in patients using metformin and a sulfonylurea,20 suggest that the GLP1 receptor agonists represent attractive alternatives to insulin treatment in patients for whom oral combination therapy does not work.
The final LEAD study, LEAD-6, offers a head-to-head comparison between the 2 GLP1 receptor agonists.60 In this study, liraglutide and exenatide both significantly reduced HbA1c levels relative to baseline. However, the extent of this reduction was significantly greater for liraglutide (P < .0001). Treatment-associated nausea declined with time for both study arms but persisted longer in patients treated with exenatide. Hypoglycemia was also less frequent in patients treated with liraglutide.
Analysis across the available LEAD studies shows a consistent improvement in HbA1c levels with liraglutide (1.0% to 1.6%), and a very low incidence of hypoglycemic episodes. In addition, liraglutide treatment was associated with sustained weight loss, systolic blood pressure reduction, and improved β-cell function.18,23,32–34
Conclusion
With accumulating data establishing the glycemic control and other benefits of the incretin agents, it is now possible to envision how these new drugs might eventually be incorporated into routine care of T2DM.
The 4 agents reviewed in Table 118,20,22–37 are similar with respect to their low risk of hypoglycemia, but some salient differences have emerged among them. The DPP4 inhibitors control glucose parameters with comparable efficacy to other antihyperglycemics, without associated weight gain or hypoglycemia.35 (The DPP4 inhibitor saxagliptin received approval from Health Canada during the final stages of revision of this review. Concomitant use of saxagliptin and metformin has been explored in patients failing metformin monotherapy14 and those naïve to pharmacologic treatment.79)
Compared with the DPP4 inhibitors, the GLP1 receptor agonists might yield greater reductions in HbA1c levels.47 Indeed, when used as part of multidrug regimens, both exenatide and liraglutide appear to be at least as effective as insulin therapy. Because insulin (unlike the incretin agents and other antihyperglycemics) has no absolute ceiling dose,3 there is no theoretical limit to its efficacy. In practice, however, insulin dosing is constrained by the danger of serious hypoglycemia, a concern that does not apply when the incretin agents are used in monotherapy. Studies in the postmarketing setting will be required to determine whether insulin or the GLP1 receptor agonists offer patients superior glycemic control in routine use.
In addition to their efficacy in controlling hyperglycemia, the GLP1 receptor agonists offer the advantage of weight loss (not seen with the DPP4 inhibitors) and they delay gastric emptying, a desirable effect for controlling postmeal glycemia, but one that can be associated with nausea in some patients. For both exenatide and liraglutide, nausea is generally transient, although it apparently persists longer in patients taking exenatide.60 Patients taking either of these agents should therefore be advised to eat small, frequent meals, particularly during the early weeks of treatment. Finally, for patients with renal impairment, the reduced clearance of exenatide could represent an additional consideration in choosing between the GLP1 receptor agonists.66
For many newly diagnosed patients, the CDA-recommended target of HbA1c levels of 7.0% or lower might be achievable within 6 months using monotherapy with sitagliptin,38 vildagliptin,48 exenatide,31 or liraglutide.18 Indeed, it appears the more ambitious target of HbA1c levels of 6.5% or lower (appropriate for certain patients, according to the CDA, to reduce risk of diabetic nephropathy) is also achievable within this time frame for at least one-third of such patients.18,31,39
The CDA stopped short of recommending this latter target for most patients, recognizing the findings from the recent ACCORD (Action to Control Cardiovascular Risk in Diabetes) study,80 which examined the effects of targeting HbA1c levels of 6.0% or lower in patients with T2DM and a history of, or risk factors for, CVD. The ACCORD study showed increased mortality among patients undergoing intensive treatment to control HbA1c levels; data from this trial also suggested that intensive control could increase risk of severe hypoglycemia, while failing to yield significant reduction in CVD outcomes. However, these findings should be compared with those in 2 other large trials—ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation)81 and VADT (Veterans Affairs Diabetes Trial)82—in which intensive glycemic control had no evident effect on mortality. It is likely that the safety of intensive glycemic control will depend on the strategies used to achieve it.82 Using the incretin agents,18,31 it might be possible to attain a high level of glycemic control while avoiding the hypoglycemia risk seen in the ACCORD study.80
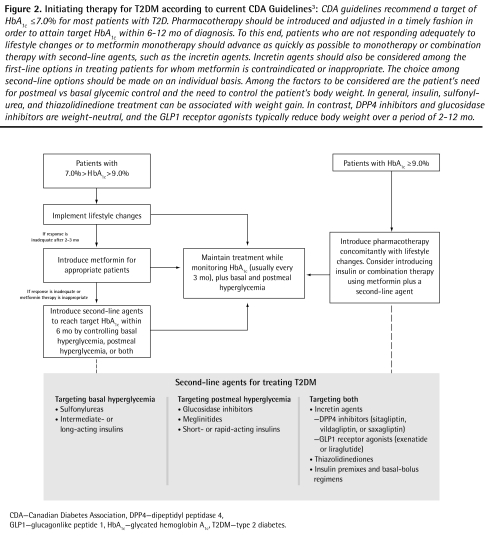
Figure 2 identifies the place of the incretin agents in treatment initiation, as described in the 2008 CDA treatment guidelines.3 These guidelines emphasize that intervention must be aggressive and rapid, using monotherapy or combination therapy with agents from different classes to achieve the target HbA1c levels within 6 to 12 months of diagnosis.3
Figure 2.
Initiating therapy for T2DM according to current CDA Guidelines3: CDA guidelines recommend a target of HbA1c ≤ 7.0% for most patients with T2D. Pharmacotherapy should be introduced and adjusted in a timely fashion in order to attain target HbA1c within 6–12 mo of diagnosis. To this end, patients who are not responding adequately to lifestyle changes or to metformin monotherapy should advance as quickly as possible to monotherapy or combination therapy with second-line agents, such as the incretin agents. Incretin agents should also be considered among the first-line options in treating patients for whom metformin is contraindicated or inappropriate. The choice among second-line options should be made on an individual basis. Among the factors to be considered are the patient’s need for postmeal vs basal glycemic control and the need to control the patient’s body weight. In general, insulin, sulfonylurea, and thiazolidinedione treatment can be associated with weight gain. In contrast, DPP4 inhibitors and glucosidase inhibitors are weight-neutral, and the GLP1 receptor agonists typically reduce body weight over a period of 2–12 mo.
CDA—Canadian Diabetes Association, DPP4—dipeptidyl peptidase 4, GLP1—glucagonlike peptide 1, HbA1c—glycated hemoglobin A1c, T2DM—type 2 diabetes.
Lifestyle changes represent a first, key step toward improved glycemic control in patients with moderately elevated HbA1c levels. However, the guidelines recommend that physicians wait only 2 to 3 months before introducing pharmacotherapy in patients who do not respond adequately. For most patients with T2DM, metformin is an appropriate and cost-effective first-line oral agent; it is expected that other treatments will need to be added as the disease progresses.3 Sitagliptin is approved for use as an add-on to metformin,3 and saxagliptin as an add-on to metformin or a sulfonylurea. Second-line status (after metformin monotherapy) is shared with insulin and all other oral agents,3 consistent with the consensus position of the European Association for the Study of Diabetes and the American Diabetes Association.83 Second-line status is expected to be extended to the GLP1 receptor agonists as they become available.84
Incretin agents or other second-line treatments should be introduced in a timely fashion, to avoid unnecessary delays in achieving target HbA1c levels. They might also be used as first-line treatments in patients for whom metformin is inappropriate. Recent data, notably reports demonstrating the efficacy of the GLP1 receptor agonists in monotherapy,18,31 might justify early use of incretin agents in a wider variety of patients than is recommended under the current guidelines.
The GLP1 receptor agonists offer additional benefits with respect to weight loss; however, the incretin agents are all versatile therapies that can be used effectively alone or in combination with established antihyperglycemics, helping patients achieve robust and sustainable reductions in HbA1c levels without increasing their risk of hypoglycemia.
Acknowledgments
We thank John Ashkenas, PhD, of SCRIPT in Toronto, Ont, for his editorial assistance and Novo Nordisk Canada Inc for supporting our independent work on this project.
EDITOR’S KEY POINTS
Accumulating evidence and clinical experience for glucagonlike peptide 1 (GLP1) receptor agonists and dipeptidyl peptidase 4 (DPP4) inhibitors shows that they can effect considerable improvements in glycated hemoglobin (HbA1c) levels as well as sustained beneficial effects on body weight.
The DPP4 inhibitors control glucose parameters with comparable efficacy to other antihyperglycemic agents, without associated weight gain or hypoglycemia.
Compared with the DPP4 inhibitors, GLP1 receptor agonists might yield greater reductions in HbA1c levels. When used as part of multidrug regimens, GLP1 agonists appear to be at least as effective as insulin therapy. The GLP1 receptor agonists offer the advantage of weight loss; they also delay gastric emptying, which is a desirable effect for controlling postmeal glycemia but one that can be associated with nausea in some patients.
Incretin agents are finding their way into the therapeutic algorithm for treating type 2 diabetes. They can be used as second-line agents in patients not adequately controlled by metformin monotherapy or as initial therapy in those for whom metformin is contraindicated.
POINTS DE REPÈRE DU RÉDACTEUR
De plus en plus de preuves et d’expériences clinique montrent que les agonistes des récepteurs du peptide 1 apparenté au glucagon (GLP1) et les inhibiteurs de la dipeptidyl peptidase (DPP4) peuvent améliorer considérablement les niveaux d’hémoglobine glycosylée (HbA1c) et avoir des effets bénéfiques durables sur le poids corporel.
Les inhibiteurs de la DPP4 contrôlent les paramètres du glucose avec une efficacité comparable à celle des autres hypoglycémiants, sans gain de poids ni hypoglycémie associés.
En comparaison des inhibiteurs de la DDP4, les agonistes des récepteurs du GLP1 pourraient causer une plus forte réduction des niveaux d’HbA1c. En association avec d’autres médicaments, les agonistes du GLP1 semblent au moins aussi efficaces que l’insulinothérapie; ils ont l’avantage de réduire le poids, en plus de retarder la vidange gastrique, un effet intéressant pour contrôler la glycémie postprandiale, mais qui peut causer des nausées chez certains patients.
Les incrétines prennent lentement leur place dans l’algorithme thérapeutique du diabète de type 2. Ils peuvent être utilisés comme agents de seconde intention chez des patients non adéquatement contrôlés par une monothérapie à la metformine ou comme thérapie initiale lorsque la metformine est contre-indiquée.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Dr Ross and Dr Ekoé contributed to the literature review and to preparing the article for publication.
Competing interests
Dr Ross has received honoraria from Novo Nordisk, Eli Lilly, and Merck for continuing medical education lectures. Dr Ekoé is a member of the Novo Nordisk Advisory Board and has received honoraria for lectures from Novo Nordisk.
References
- 1.International Diabetes Federation . IDF diabetes atlas. 4th ed. Brussels, Belgium: International Diabetes Federation; 2009. Available from: www.diabetesatlas.org. Accessed 2010 May 31. [PubMed] [Google Scholar]
- 2.National Diabetes Education Program . Diabetes medications supplement. Working together to manage diabetes. Bethesda, MD: National Diabetes Education Program; 2007. Available from: http://ndep.nih.gov/media/Drug_tables_supplement.pdf. Accessed 2010 May 31. [Google Scholar]
- 3.Canadian Diabetes Association Clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(Suppl 1):S1–201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Harris SB, Ekoé JM, Zdanowicz Y, Webster-Bogaert S. Glycemic control and morbidity in the Canadian primary care setting (results of the Diabetes in Canada Evaluation study) Diabetes Res Clin Pract. 2005;70(1):90–7. doi: 10.1016/j.diabres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Chia CW, Egan JM. Incretin-based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2008;93(10):3703–16. doi: 10.1210/jc.2007-2109. Epub 2008 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med. 2009;122(6 Suppl):S11–24. doi: 10.1016/j.amjmed.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med. 2009;122(6 Suppl):S37–50. doi: 10.1016/j.amjmed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Mudaliar S, Henry RR. Incretin therapies: effects beyond glycemic control. Am J Med. 2009;122(6 Suppl):S25–36. doi: 10.1016/j.amjmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Nauck MA. Unraveling the science of incretin biology. Am J Med. 2009;122(6 Suppl):S3–10. doi: 10.1016/j.amjmed.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Vilsbøll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–66. doi: 10.1007/s00125-004-1342-6. Epub 2004 Feb 13. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh CHS. Incretin-based therapies for type 2 diabetes. Can J Diabetes. 2008;32(2):131–9. doi: 10.1016/S1499-2671(08)22010-1. [DOI] [PubMed] [Google Scholar]
- 13.Augeri DJ, Robl JA, Betebenner DA, Magnin DR, Khanna A, Robertson JG, et al. Discovery and preclinical profile of saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48(15):5025–37. doi: 10.1021/jm050261p. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Hissa MN, Garber AJ, Luiz Gross J, Yuyan Duan R, Ravichandran S, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32(9):1649–55. doi: 10.2337/dc08-1984. Epub 2009 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9(2):194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 16.Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008;10(1):82–90. doi: 10.1111/j.1463-1326.2007.00820.x. Epub 2007 Nov 22. [DOI] [PubMed] [Google Scholar]
- 17.Pinelli NR, Cha R, Brown MB, Jaber LA. Addition of thiazolidinedione or exenatide to oral agents in type 2 diabetes: a meta-analysis. Ann Pharmacother. 2008;42(11):1541–51. doi: 10.1345/aph.1L198. Epub 2008 Oct 28. [DOI] [PubMed] [Google Scholar]
- 18.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–81. doi: 10.1016/S0140-6736(08)61246-5. Epub 2008 Sep 24. [DOI] [PubMed] [Google Scholar]
- 19.Garber AJ, Henry R, Ratner R. Monotherapy with liraglutide, a once-daily human GLP-1 analog, provides sustained reductions in A1c, FPG, and weight compared with glimepiride in type 2 diabetes: LEAD-3 Mono 2-year results. Diabetes. 2009;58(Suppl 1):A42–3. [Google Scholar]
- 20.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–69. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 21.Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover non-inferiority trial. Clin Ther. 2007;29(11):2333–48. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(2):259–67. doi: 10.1007/s00125-006-0510-2. Epub 2006 Dec 8. [DOI] [PubMed] [Google Scholar]
- 23.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–55. doi: 10.1007/s00125-009-1472-y. Epub 2009 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 26.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 27.Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146(7):477–85. doi: 10.7326/0003-4819-146-7-200704030-00003. Erratum in: Ann Intern Med 2007;146(12):896. [DOI] [PubMed] [Google Scholar]
- 28.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1c, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8(4):436–47. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 29.Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151(1–3):123–9. doi: 10.1016/j.regpep.2008.07.003. Epub 2008 Jul 16. [DOI] [PubMed] [Google Scholar]
- 30.Amylin Pharmaceuticals Byetta (exenatide) injection. [prescribing information]. San Diego, CA: Amylin Pharmaceuticals; 2009. Available from: http://pi.lilly.com/us/byetta-pi.pdf. Accessed 2009 Aug 1.
- 31.Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30(8):1448–60. doi: 10.1016/j.clinthera.2008.08.006. Erratum in: Clin Ther 2008;30(10):1937. [DOI] [PubMed] [Google Scholar]
- 32.Marre M, Shaw J, Brandel M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26(3):268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 Study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. Epub 2008 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human GLP-1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met+TZD) Diabetes Care. 2009;32(7):1224–30. doi: 10.2337/dc08-2124. Epub 2009 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;(2):CD006739. doi: 10.1002/14651858.CD006739.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Midlands Therapeutics Review & Advisory Committee . Vildagliptin: summary of product characteristics. Staffordshire, UK: Midlands Therapeutics Review & Advisory Committee; 2008. Available from: www.keele.ac.uk/schools/pharm/MTRAC/ProductInfo/verdicts/V/Vildagliptin.pdf. Accessed 2009 Feb 1. [Google Scholar]
- 37.Merck Frosst Canada Ltd Januvia. [product monograph]. Ottawa, ON: Health Canada Drug Product Database; 2010. Available from: http://webprod.hc-sc.gc.ca/dpd-bdpp/dispatch-repartition.do?lang=eng. Accessed 2009 Feb 1.
- 38.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–7. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79(2):291–8. doi: 10.1016/j.diabres.2007.08.021. Epub 2007 Oct 22. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30(8):1979–87. doi: 10.2337/dc07-0627. Epub 2007 May 7. Erratum in: Diabetes Care 2008;31(8):1713. [DOI] [PubMed] [Google Scholar]
- 41.Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP, et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23(6):1329–39. doi: 10.1185/030079907X188152. Epub 2007 Apr 30. [DOI] [PubMed] [Google Scholar]
- 42.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49(11):2564–71. doi: 10.1007/s00125-006-0416-z. Epub 2006 Sep 26. [DOI] [PubMed] [Google Scholar]
- 43.Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61(1):171–80. doi: 10.1111/j.1742-1241.2006.01246.x. Epub 2006 Dec 5. [DOI] [PubMed] [Google Scholar]
- 44.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, Sitagliptin Study 020 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29(12):2638–43. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 45.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–45. doi: 10.1111/j.1463-1326.2007.00744.x. Epub 2007 Jun 26. [DOI] [PubMed] [Google Scholar]
- 46.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P, Sitagliptin Study 019 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–68. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24(10):2943–52. doi: 10.1185/03007990802418851. Epub 2008 Sep 10. [DOI] [PubMed] [Google Scholar]
- 48.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76(1):132–8. doi: 10.1016/j.diabres.2006.12.009. Epub 2007 Jan 12. [DOI] [PubMed] [Google Scholar]
- 49.Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007;39(3):218–23. doi: 10.1055/s-2007-970422. [DOI] [PubMed] [Google Scholar]
- 50.Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83(2):233–40. doi: 10.1016/j.diabres.2008.10.006. Epub 2008 Dec 31. [DOI] [PubMed] [Google Scholar]
- 51.Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38(6):423–8. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- 52.Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7(6):692–8. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 53.Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, et al. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab. 2008;10(8):675–82. doi: 10.1111/j.1463-1326.2008.00850.x. Epub 2007 Nov 22. [DOI] [PubMed] [Google Scholar]
- 54.Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27(12):2874–80. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 55.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30(4):890–5. doi: 10.2337/dc06-1732. Epub 2007 Feb 2. [DOI] [PubMed] [Google Scholar]
- 56.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50(6):1148–55. doi: 10.1007/s00125-007-0633-0. Epub 2007 Mar 27. [DOI] [PubMed] [Google Scholar]
- 57.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9(2):166–74. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 58.Rosenstock J, Kim SW, Baron MA, Camisasca RP, Cressier F, Couturier A, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9(2):175–85. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 59.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275–86. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 60.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. Epub 2009 Jun 8. [DOI] [PubMed] [Google Scholar]
- 61.Ahrén B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007;30(6):1344–50. doi: 10.2337/dc07-0233. Epub 2007 Mar 2. [DOI] [PubMed] [Google Scholar]
- 62.Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47(11):1663–70. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 63.Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62(2):173–81. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 64.Noel RA, Braun DK, Patterson R, Bloomgren G. Increased risk of acute pancreatitis observed in patients with type 2 diabetes. Pharmacoepidemiol Drug Saf. 2008;17(Suppl 1):S254–5. [Google Scholar]
- 65.US Food and Drug Administration . Byetta (exenatide)—renal failure. Silver Spring, MD: US Food and Drug Administration; 2009. Available from: www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm188703.htm. Accessed 2009 May 14. [Google Scholar]
- 66.Jacobsen L, Hindsberger C, Robson R, Zdravkovic M. Pharmacokinetics of the long-acting human GLP-1 analogue liraglutide in subjects with renal impairment. Diabetologia. 2007;50(Suppl.1):S352. [Google Scholar]
- 67.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402–5. [PubMed] [Google Scholar]
- 68.Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26(8):2370–7. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- 69.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–50. doi: 10.1016/S0140-6736(08)61206-4. Epub 2008 Sep 7. [DOI] [PubMed] [Google Scholar]
- 70.Rosenstock J, Reusch J, Bush M, Yang F, Stewart M, Albiglutide Study Group The potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32(10):1880–6. doi: 10.2337/dc09-0366. Epub 2009 Jul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43(9):1664–9. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 72.Degn KB, Juhl CB, Sturis J, Brock B, Chandramouli V, Rungby J, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and α- and β-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53(5):1187–94. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 73.Elbrønd B, Jakobsen G, Larsen S, Agersø H, Jensen LB, Rolan P, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25(8):1398–404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 74.Colagiuri S, Frid A, Zdravkovic M, Le-Thi TD, Vaag A. The once-daily human GLP-1 analog liraglutide reduces systolic blood pressure in patients with type 2 diabetes. Diabetes. 2008;57(Suppl 1):A164–5. [Google Scholar]
- 75.Matthews D, Marre M, Le-Thi TD, Zdravkovic M, Simó R. Liraglutide, a once-daily human GLP-1 analog, significantly improves beta-cell function in subjects with type 2 diabetes. Diabetes. 2008;57(Suppl 1):A150–1. [Google Scholar]
- 76.Riche DM, East HE, Riche KD. Impact of sitagliptin on markers of β-cell function: a meta-analysis. Am J Med Sci. 2009;337(5):321–8. doi: 10.1097/MAJ.0b013e31818eb721. [DOI] [PubMed] [Google Scholar]
- 77.Aaboe K, Vilsboll T, Knop FK, Deacon CF, Holst JJ, Madsbad S, et al. Twelve weeks treatment with the DPP-4 inhibitor sitagliptin improves the insulin-secreting capacity of the β-cells in subjects with type 2 diabetes mellitus—a randomized trial. Diabetes. 2009;58(Suppl 1):A163. [Google Scholar]
- 78.Davies M, Lavalle-Gonzalez F, Storms F, Gomis R. Initiation of insulin glargine therapy in type 2 diabetes subjects suboptimally controlled on oral antidiabetic agents: results from the AT.LANTUS trial. Diabetes Obes Metab. 2008;10(5):387–99. doi: 10.1111/j.1463-1326.2008.00873.x. Epub 2008 Mar 18. [DOI] [PubMed] [Google Scholar]
- 79.Jadzinsky M, Pfutzner A, Paz-Pacheco E, Xu Z, Allen E, Chen R, et al. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11(6):611–22. doi: 10.1111/j.1463-1326.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 80.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. Epub 2008 Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. Epub 2008 Jun 8. [DOI] [PubMed] [Google Scholar]
- 82.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. Epub 2008 Dec 17. Erratum in: N Engl J Med 2009;361(10):1024–5, 1028. [DOI] [PubMed] [Google Scholar]
- 83.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29(8):1963–72. doi: 10.2337/dc06-9912. Erratum in: Diabetes Care 2006;49(11):2816–8. [DOI] [PubMed] [Google Scholar]
- 84.Hanna A, Zinman B, Gerstein H, Knudsen LB, Baggio L, Yale JF. Glucagon-like peptide-1 based therapies. Endocrinol Sci Update. 2008;218(025):1–6. Available from: www.endocrinologyupdate.ca/crus/218-025%20English%20(6%20pages).pdf. Accessed 2010 May 31. [Google Scholar]