Abstract
The cause of of ketotic hypoglycemia, the commonest form of hypoglycemia in childhood, is not known. The present study was undertaken to determine whether the primary defect in this condition is a deficiency of gluconeogenic precursor(s) or an abnormality in the hepatic gluconeogenic enzyme system. Plasma glucose, alanine, and insulin and blood β-hydroxybutyrate (β-OHB), pyruvate, and lactate levels were determined in eight ketotic hypoglycemic children and seven agematched controls maintained on a normal diet and after being fed a provocative hypocaloric low-carbohydrate diet (1200 kcal/1.73 m2, 15% carbohydrate, 17% protein, and 68% fat). On a normal diet, overnight fasting plasma alanine (211±10 μM) and glucose (68±4 mg/100 ml) were significantly lower and blood β-OHB (1.22±0.37 mM) significantly higher in ketotic hypoglycemic children than in controls (alanine, 315±15 μM; glucose, 81±3 mg/100 ml; β-OHB, 0.18±0.08 mM).
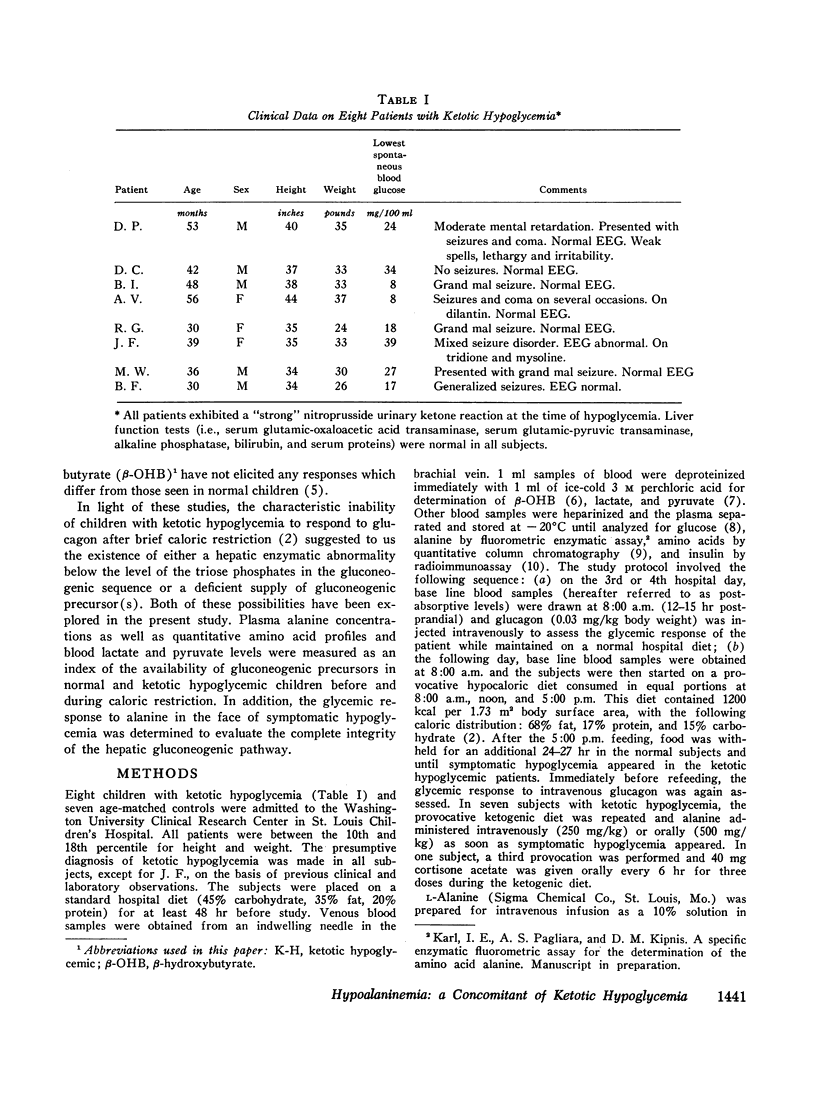
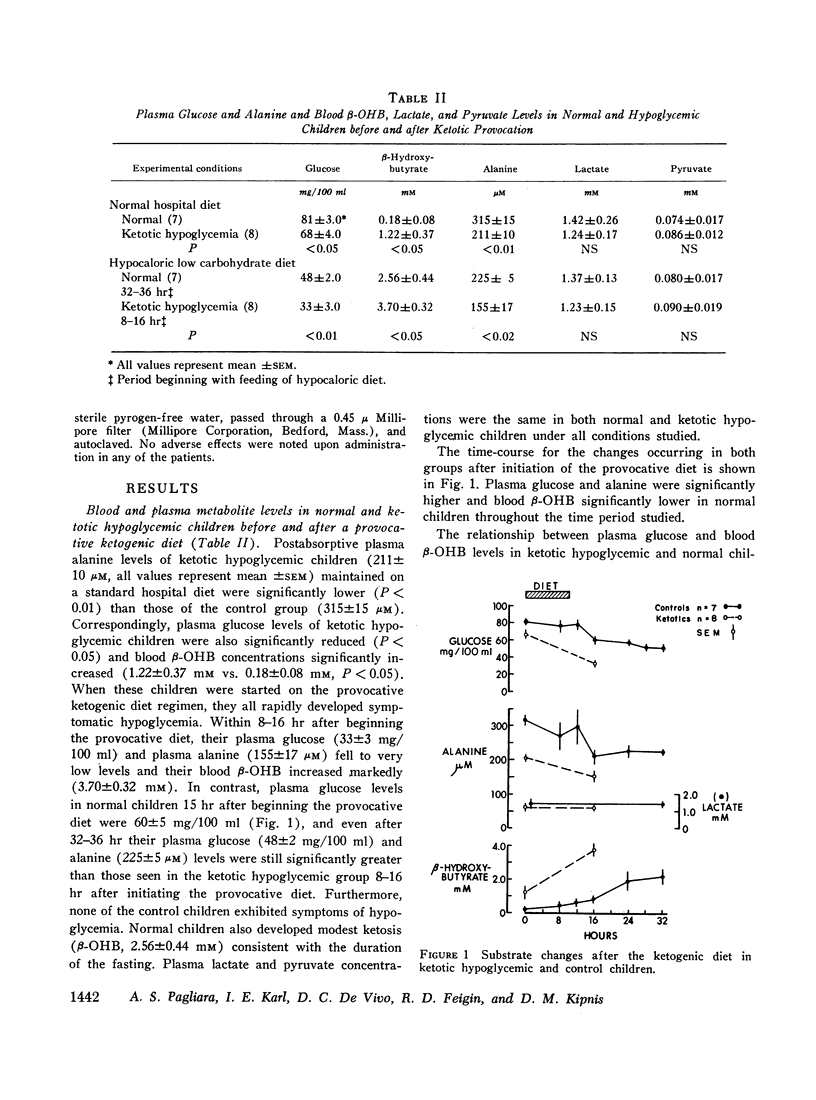
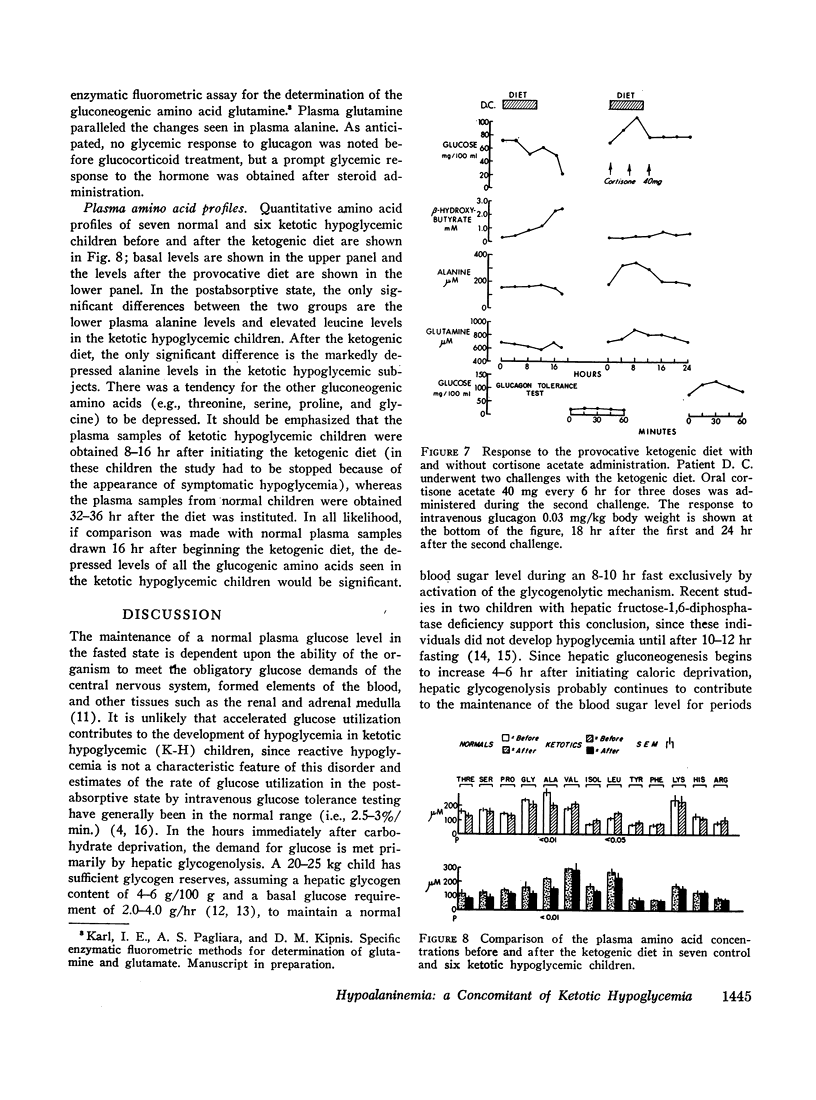
All ketotic hypoglycemic children developed symptomatic hypoglycemia (33±3 mg/100 ml) and ketosis (β-OHB, 3.70±0.32 mM) 8-16 hr after starting the provocative diet and these changes were associated with a further decline in plasma alanine (155±17 μM). Normal children, even after 36 hr on this diet, maintained higher plasma glucose (48±2 mg/100 ml) and alanine (225±5 μM) and lower β-OHB levels (2.56±0.44 mM).
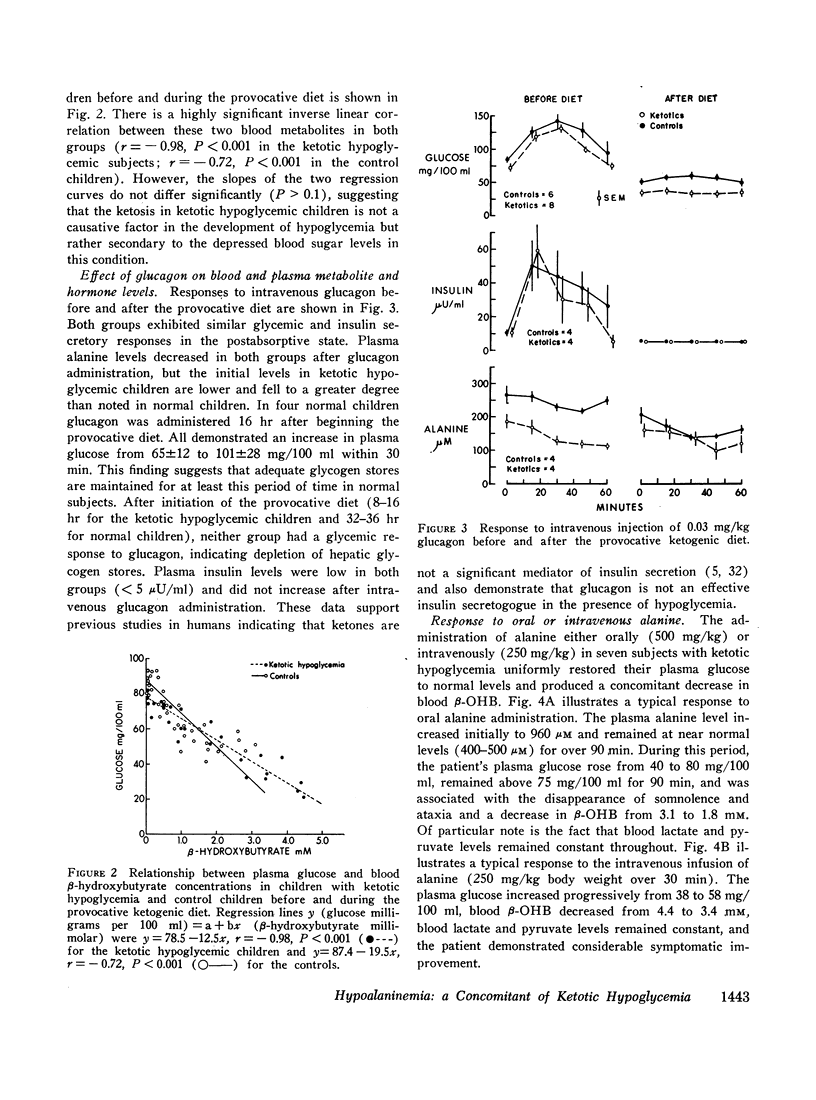
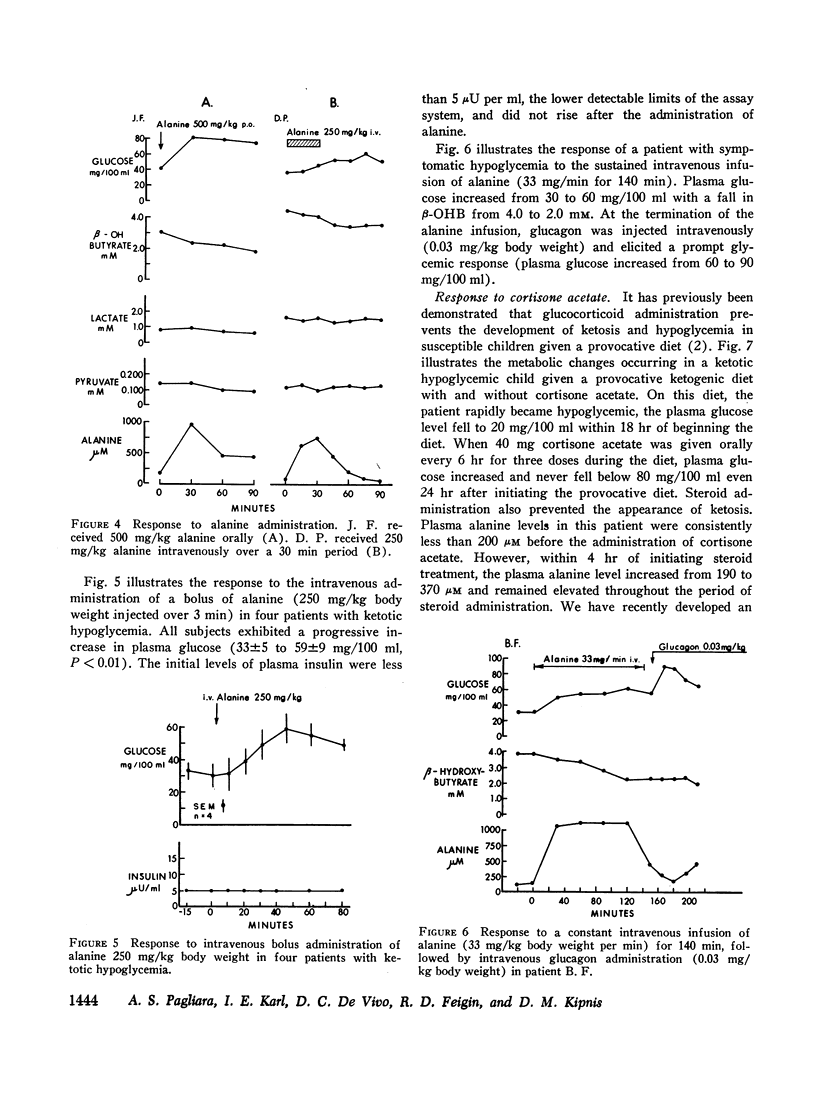
Intravenous infusions of alanine (250 mg/kg) uniformly restored the hypoglycemic plasma glucose levels of ketotic hypoglycemic children to normal. Cortisone acetate (300 mg/m2), given orally in three divided doses during feeding of the provocative diet, produced a 3- to 4-fold increase in plasma alanine within 4-6 hr after beginning steroid therapy and completely prevented the development of hypoglycemia and ketosis. Quantitative amino acid profiles were performed and demonstrated that alanine was the only gluconeogenic amino acid which differed significantly between the two groups. Plasma insulin and blood lactate and pyruvate levels did not differ significantly between normal and ketotic hypoglycemic children under all conditions examined.
These results support the hypothesis that a deficiency in gluconeogenic precursor (e.g., alanine) rather than a defect in the hepatic gluconeogenic enzyme apparatus represents the most likely factor in the pathogenesis of ketotic hypoglycemia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., King K., Schwartz R. Model for the investigation of intractable hypoglycemia: insulin-glucose interrelationships during steady state infusions. Pediatrics. 1968 Jan;41(1):91–105. [PubMed] [Google Scholar]
- Adibi S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968 Jul;25(1):52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- Baker L., Winegrad A. I. Fasting hypoglycaemia and metabolic acidosis associated with deficiency of hepatic fructose-1,6-diphosphatase activity. Lancet. 1970 Jul 4;2(7662):13–16. doi: 10.1016/s0140-6736(70)92474-8. [DOI] [PubMed] [Google Scholar]
- COLLE E., ULSTROM R. A. KETOTIC HYPOGLYCEMIA. J Pediatr. 1964 May;64:632–651. doi: 10.1016/s0022-3476(64)80611-9. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E., Pozefsky T., Cahill G. F., Jr Amino acid metabolism in the regulation of gluconeogenesis in man. Am J Clin Nutr. 1970 Jul;23(7):986–992. doi: 10.1093/ajcn/23.7.986. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J., Huijing F., van de Kamer J. H. A screening method for liver glycogen diseases. Arch Dis Child. 1969 Jun;44(235):311–317. doi: 10.1136/adc.44.235.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunt J. A., McGarry M. E., McCollum A. T., Gould J. B. Studies of children with ketotic hypoglycemia. Yale J Biol Med. 1970 Jun;42(6):420–438. [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- King K. C., Adam P. A., Clemente G. A., Schwartz R. Infants of diabetic mothers: attenuated glucose uptake without hyperinsulinemia during continuous glucose infusion. Pediatrics. 1969 Sep;44(3):381–392. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Loridan L., Senior B. Effects of infusion of ketones in children with ketotic hypoglycemia. J Pediatr. 1970 Jan;76(1):69–74. doi: 10.1016/s0022-3476(70)80132-9. [DOI] [PubMed] [Google Scholar]
- MADISON L. L., MEBANE D., UNGER R. H., LOCHNER A. THE HYPOGLYCEMIC ACTION OF KETONES. II. EVIDENCE FOR A STIMULATORY FEEDBACK OF KETONES ON THE PANCREATIC BETA CELLS. J Clin Invest. 1964 Mar;43:408–415. doi: 10.1172/JCI104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Pozefsky T., Most A. S., Cahill G. F., Jr Muscle and splanchnic glutmine and glutamate metabolism in postabsorptive andstarved man. J Clin Invest. 1971 Apr;50(4):814–817. doi: 10.1172/JCI106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Unger R. H., Soeldner J. S., Cahill G. F., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970 Dec;49(12):2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Goodman A. D. Elevation of plasma glutamate in gout. Its possible role in the pathogenesis of hyperuricemia. N Engl J Med. 1969 Oct 2;281(14):767–770. doi: 10.1056/NEJM196910022811405. [DOI] [PubMed] [Google Scholar]
- Pagliara A., Goodman A. D. Pitfalls in the determination of plasma glutamate. N Engl J Med. 1968 Dec 19;279(25):1402–1402. doi: 10.1056/NEJM196812192792519. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer F., Campbell R. G., Hashim S. A. Experimentally induced hyperketonemia and insulin secretion in the dog. Metabolism. 1970 Apr;19(4):263–270. doi: 10.1016/0026-0495(70)90124-1. [DOI] [PubMed] [Google Scholar]
- Senior B., Loridan L. Gluconeogenesis and insulin in the ketotic variety of childhoofd hypoglycemia and in control children. J Pediatr. 1969 Apr;74(4):529–539. doi: 10.1016/s0022-3476(69)80035-1. [DOI] [PubMed] [Google Scholar]


