Abstract
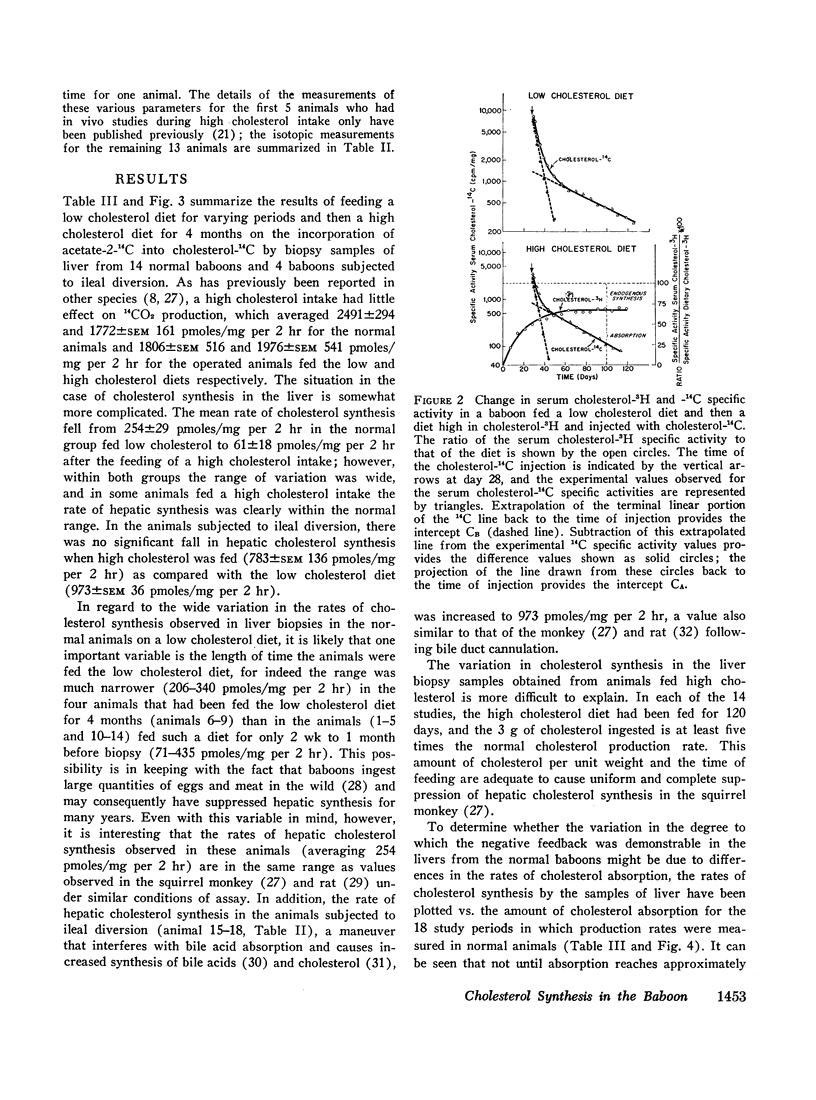
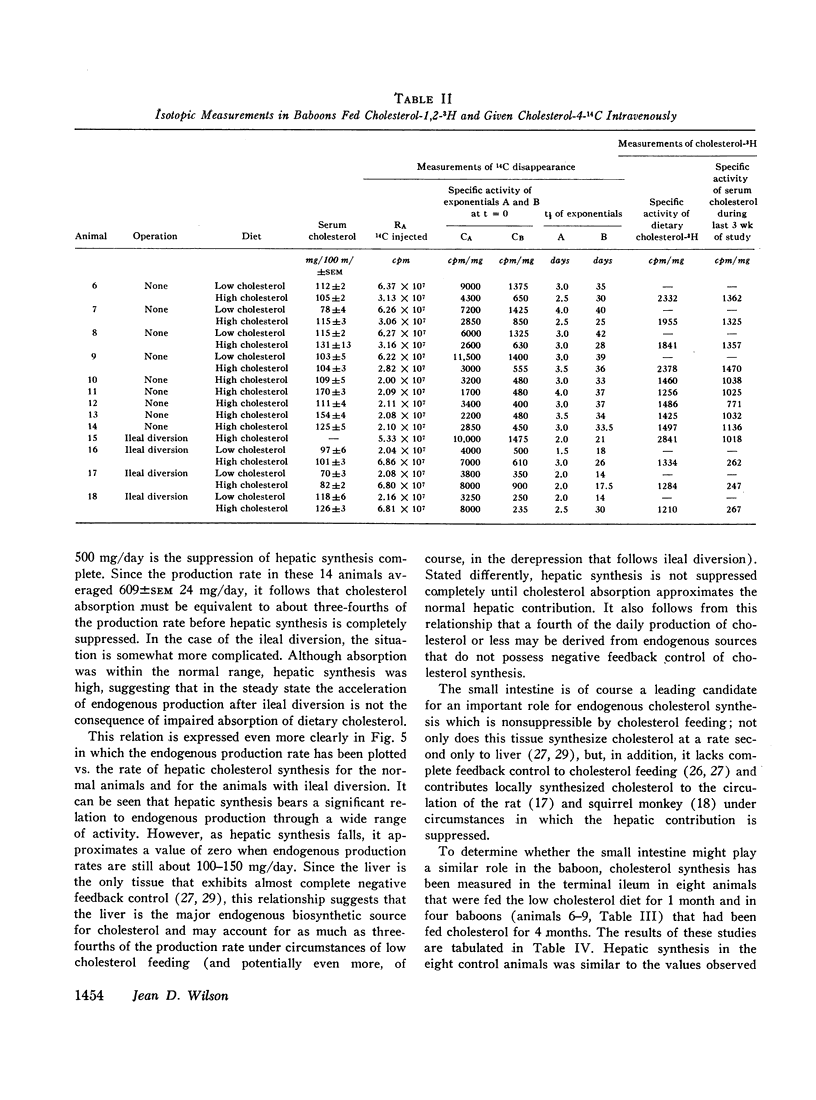
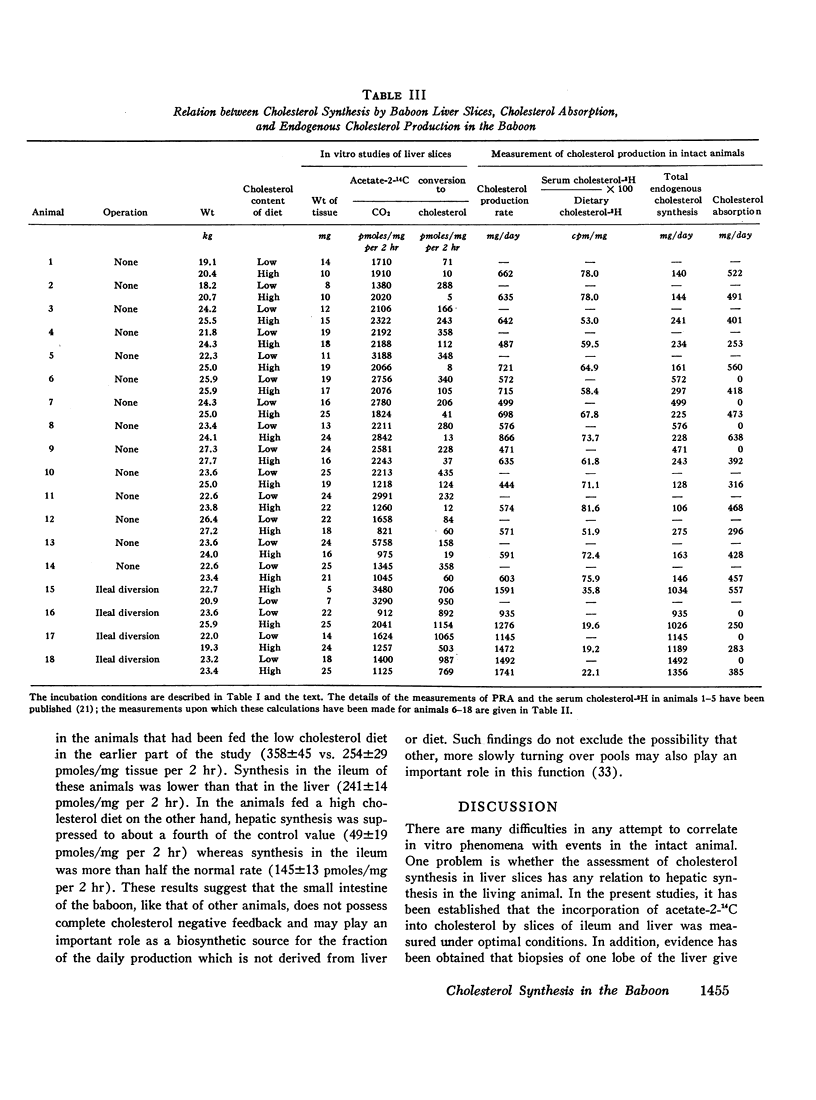
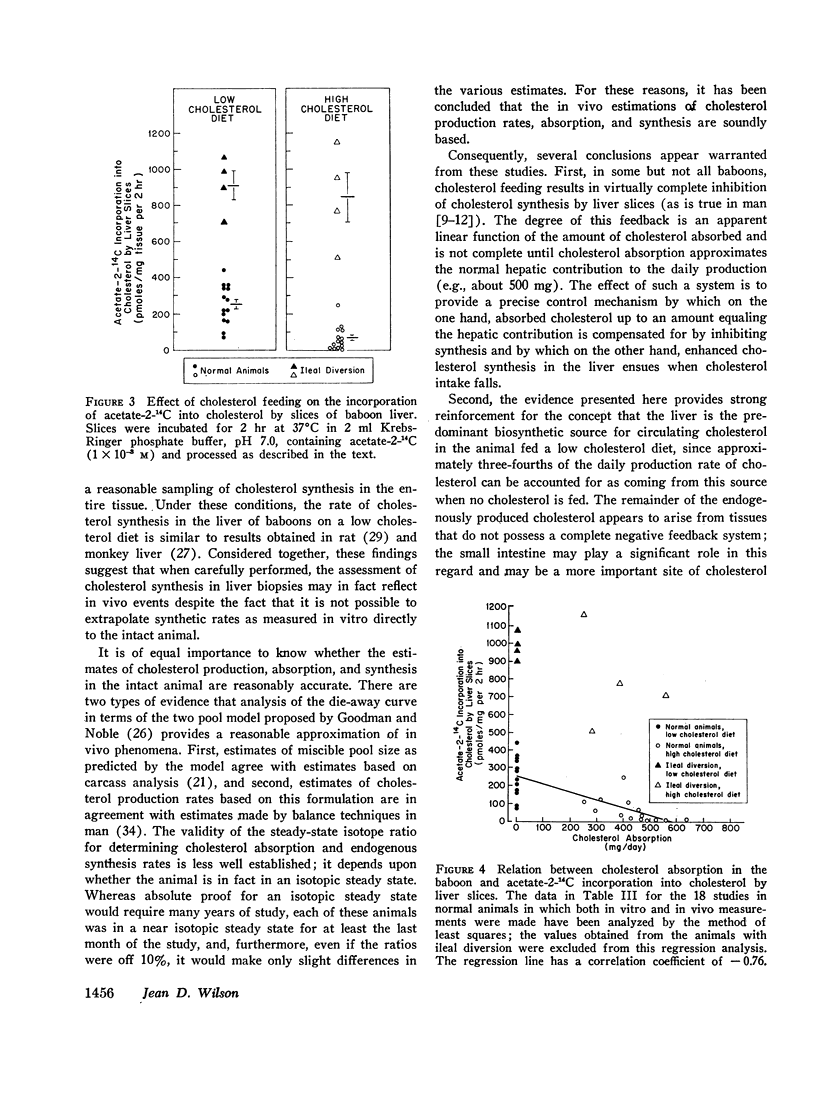
To determine the relation between cholesterol absorption, total endogenous cholesterol synthesis, and hepatic cholesterol synthesis in a primate, cholesterol synthesis has been studied in biopsies of liver and ileum from normal baboons fed varying amounts of cholesterol and in biopsies of liver from baboons that had been subjected to ileal diversion. In addition, total cholesterol production rates, cholesterol absorption, and total endogenous cholesterol synthesis have been measured in these animals by a double isotope technique in which the animals were given a single injection of cholesterol-4-14C and fed constant amounts of cholesterol-1,2-3H for 4 months. From these studies, it has been concluded that on a low cholesterol intake cholesterol synthesis in the liver accounts for about three-fourths of total endogenous cholesterol production. The feeding of cholesterol produces complete inhibition of hepatic synthesis in the normal animal only when absorption approximates the amount synthesized by the liver when no cholesterol is fed, e.g., 400-500 mg/day. Finally, the intestine, which does not possess complete negative feedback control of cholesterol synthesis when cholesterol is fed, may be a significant site of nonhepatic cholesterol synthesis in these animals.
In studies of four baboons subjected to ileal diversion, it was concluded that the regulation of cholesterol synthesis is distinctly different when the enterohepatic circulation is interrupted. These animals did not exhibit negative feedback of hepatic cholesterol synthesis when cholesterol was fed, despite the fact that cholesterol absorption approximated that of normal animals fed similar diets. The inference has been drawn that bile acids may be involved directly or indirectly in the regulation of hepatic cholesterol synthesis in this species or that the ileum itself may modulate the hepatic negative feedback.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHATTATHIRY E. P., SHIPERSTEIN M. D. FEEDBACK CONTROL OF CHOLESTEROL SYNTHESIS IN MAN. J Clin Invest. 1963 Oct;42:1613–1618. doi: 10.1172/JCI104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H., Frantz I. D., Jr, Gebhard R. L., Moore R. B. Ileal bypass versus ileal excision: effect on cholesterol synthesis and whole blood cholesterol concentrations in the rabbit. Preliminary report. Surg Clin North Am. 1967 Dec;47(6):1353–1362. doi: 10.1016/s0039-6109(16)38385-2. [DOI] [PubMed] [Google Scholar]
- Buchwald H., Varco R. L. Ileal bypass in patients with hypercholesterolemia and atherosclerosis. Preliminary report on therapeutic potential. JAMA. 1966 May 16;196(7):627–630. [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKLES N. E., TAYLOR C. B., CAMPBELL D. J., GOULD R. G. The origin of plasma cholesterol and the rates of equilibration of liver, plasma, and erythrocyte cholesterol. J Lab Clin Med. 1955 Sep;46(3):359–371. [PubMed] [Google Scholar]
- Fujiwara T., Hirono H., Arakawa T. Idiopathic hypercholesterolemia: demonstration of an impaired feedback control of cholesterol synthesis in vivo. Tohoku J Exp Med. 1965 Nov 25;87(2):155–167. doi: 10.1620/tjem.87.155. [DOI] [PubMed] [Google Scholar]
- GOULD R. G. Lipid metabolism and atherosclerosis. Am J Med. 1951 Aug;11(2):209–227. doi: 10.1016/0002-9343(51)90107-6. [DOI] [PubMed] [Google Scholar]
- GOULD R. G., TAYLOR C. B., HAGERMAN J. S., WARNER I., CAMPBELL D. J. Cholesterol metabolism. I. Effect of dietary cholesterol on the synthesis of cholesterol in dog tissue in vitro. J Biol Chem. 1953 Apr;201(2):519–528. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P. Turnover of plasma cholesterol in man. J Clin Invest. 1968 Feb;47(2):231–241. doi: 10.1172/JCI105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969 May;10(3):304–315. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969 Jan;10(1):91–107. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- HOTTA S., CHAIKOFF I. L. The role of the liver in the turnover of plasma cholesterol. Arch Biochem Biophys. 1955 May;56(1):28–37. doi: 10.1016/0003-9861(55)90330-1. [DOI] [PubMed] [Google Scholar]
- KAPLAN J. A., COX G. E., TAYLOR C. B. CHOLESTEROL METABOLISM IN MAN. STUDIES ON ABSORPTION. Arch Pathol. 1963 Oct;76:359–368. [PubMed] [Google Scholar]
- LINDSEY C. A., Jr, WILSON J. D. EVIDENCE FOR A CONTRIBUTION BY THE INTESTINAL WALL TO THE SERUM CHOLESTEROL OF THE RAT. J Lipid Res. 1965 Apr;6:173–181. [PubMed] [Google Scholar]
- MORRIS M. D., CHAIKOFF I. L., FELTS J. M., ABRAHAM S., FANSAH N. O. The origin of serum cholesterol in the rat; diet versus synthesis. J Biol Chem. 1957 Feb;224(2):1039–1045. [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res. 1971 Mar;12(2):233–247. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Strong J. P., McGill H. C., Jr Diet and experimental atherosclerosis in baboons. Am J Pathol. 1967 Apr;50(4):669–690. [PMC free article] [PubMed] [Google Scholar]
- TAYLOR C. B., PATTON D., YOGI N., COX G. E. Diet as source of serum cholesterol in man. Proc Soc Exp Biol Med. 1960 Apr;103:768–772. doi: 10.3181/00379727-103-25664. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., SHEPPARD H., CHAIKOFF I. L. Cholesterol synthesis by liver. III. Its regulation by ingested cholesterol. J Biol Chem. 1953 Mar;201(1):137–141. [PubMed] [Google Scholar]
- Taylor C. B., Mikkelson B., Anderson J. A., Forman D. T., Choi S. S. Human serum cholesterol synthesis. Exp Mol Pathol. 1965 Oct;4(5):480–488. doi: 10.1016/0014-4800(65)90012-2. [DOI] [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Failure of bile acids to control hepatic cholesterogenesis: evidence for endogenous cholesterol feedback. J Clin Invest. 1969 Dec;48(12):2398–2408. doi: 10.1172/JCI106206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. Biosynthetic origin of serum cholesterol in the squirrel monkey: evidence for a contribution by the intestinal wall. J Clin Invest. 1968 Jan;47(1):175–187. doi: 10.1172/JCI105707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Lindsey C. A., Jr Studies on the influence of dietary cholesterol on cholesterol metabolism in the isotopic steady state in man. J Clin Invest. 1965 Nov;44(11):1805–1814. doi: 10.1172/JCI105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. The measurement of the exchangeable pools of cholesterol in the baboon. J Clin Invest. 1970 Apr;49(4):655–665. doi: 10.1172/JCI106277. [DOI] [PMC free article] [PubMed] [Google Scholar]


