Abstract
Background
Abnormal hedonic behavior is a key feature of many psychiatric disorders. Several paradigms measure reward-seeking behavior in rodents, but each has limitations. We describe a novel approach for monitoring reward-seeking behavior in rodents: sniffing of estrus female urine by male mice, along with number of ultrasonic vocalizations (USVs) emitted during the test.
Methods
The female urine sniffing test (FUST) was designed to monitor reward-seeking activity in rodents together with tests of helplessness and sweet solution preference. USVs and dopamine release from the nucleus accumbens (NAc) were recorded. Sniffing activity was measured in 1) manipulation-naive C57BL/6J and 129S1/SVImJ mice and Wistar-Kyoto rats; 2) stressed mice;3) two groups of mice that underwent the learned helplessness paradigm—one untreated, and one treated with the SSRI citalopram; and 4) GluR6 knockout mice, known to display lithium-responsive, mania-related behaviors.
Results
Males from all three strains spent significantly longer sniffing female urine than sniffing water. Males emitted USVs and showed significantly elevated NAc dopamine levels while sniffing urine. Foot-shock stress significantly reduced female urine sniffing time. Compared with mice that did not undergo the LH paradigm, LH males spent less time sniffing female urine, and citalopram treatment alleviated this reduction. Compared with their wildtype littermates, GluR6KO males sniffed female urine longer and showed enhanced saccharin preference.
Conclusions
In rodents, sniffing female urine is a preferred activity accompanied by biological changes previously linked to reward-seeking activities. The FUST is sensitive to behavioral and genetic manipulation and to relevant drug treatment.
Keywords: Behavior, microdialysis, odor, reward-seeking, rodents, sex
Hedonic behavior—also known as reward-seeking or pleasure-seeking behavior—and its underlying biological basis have been extensively investigated in human and laboratory-based animal models (1). Anhedonia is defined as a “sharp, pervasive impairment of the capacity to experience pleasure or to respond affectively to the anticipation of pleasure” (2, p. 349); anhedonic behavior is the objective (outward) display associated with this subjective feeling. Notably, abnormal hedonic behavior is a key feature of many common psychiatric diseases, including major depressive disorder (3). In contrast, the clinical hallmark of bipolar disorder is the presence of manic symptoms, which enhance hedonic drive and motivation (for a review, see Hasler et al. [4]). The need for paradigms that assess reward-seeking behavior in animal models is essential for exploring mechanisms involved in these diseases and for developing promising treatment strategies (5).
It is important to emphasize that the terms “hedonic” and “reward-seeking” behavior have complex meanings and are sometimes used interchangeably in the literature. In this study, we defined reward-seeking behavior as “the ability to elicit an approach behavior, similar to incentive motivation (an incentive is defined as any stimulus that activates an approach behavior). … Liking and positive affect are intrinsically rewarding and are measurable by approach, consummatory responses, or affective reactions” (6, p.15–16).
Traditionally, reward-seeking behavior in laboratory animals has been studied using three appetitive strategies: operant paradigms, brain stimulation reward techniques, and nonoperant paradigms (7). These include conditioned place-preference (CPP) (8–10), intracranial self-stimulation (11), and measuring an animal's preference for sweetened fluids (sucrose or saccharin solutions) versus water (8,12,13). Although widely used, each of these reward-seeking paradigms has limitations. For instance, most operant paradigms depend heavily on learning and memory and motor activity abilities (7). Similarly, the use of brain stimulation reward techniques requires surgery and sometimes extensive training periods that exceed the recovery time from the surgery; they also include measures that are sensitive to altered ability to perform (11). Reduced sucrose could result from low body weight rather than stress responses (14), and consumption can also be influenced by altered taste sensation or appetite induced by genetic manipulation or pharmacologic treatment rather than direct changes in reward-seeking state.
For most mammals, chemosensory cues related to sexual behavior are a primary mechanism for social communication. This mode of communication not only transmits information, it can even directly alter physiology and behavior (15). The urine of mice and rats, for instance, contains substantial quantities of lipocalin-family proteins, which are thought to be responsible for the binding and release of pheromones of low molecular weight, thus playing a role in individual identity and scent mark ownership (16). In addition, several studies have shown that adult mice emit ultrasonic vocalizations (USVs) with frequencies ranging from 30 to 70 kHz (17) in the presence of females or when exposed to urinary pheromones (18–20). Indeed, past studies have used pheromones as a rewarding cue in operant paradigms (for instance, in the CPP) (6). Thus, assays of appetitive response to pheromones may be useful for assessing spontaneous reward-seeking behaviors without relying on components of taste, activity, or learning/memory.
Here, we present an alternative novel approach for evaluating rodent models of mood disorders. The female urine sniffing test (FUST) is a nonoperant test for measuring reward-seeking behavior in rodents based on interest in pheromonal odors from the opposite sex. Specifically, we measured the duration of female urine sniffing and USVs emitted by male rodents during exposure to female urine collected during the estrus phase of the female's estrus cycle. We also conducted microdialysis to measure dopamine levels in the nucleus accumbens (NAc) of manipulation-naive mice. NAc dopamine levels are increased by chemical reward stimuli such as cocaine and amphetamine, and dopamine in the NAc modulates reward behavior (21,22).
To assess the sensitivity and utility of this paradigm, we tested it in different rodent strains (C57BL/6J mice, 129S1/SVImJ mice, and Wistar-Kyoto rats) and in several preclinical experimental paradigms previously established for the study of mood disorders. These included 1) the learned helplessness (LH) paradigm for detecting the “negative spectrum” of reward-seeking behavior (23); 2) chronic treatment of mice that underwent the LH paradigm with citalopram—a selective serotonin reuptake inhibitor (SSRI) antidepressant—to evaluate putative SSRI reversal of this behavior; and 3) GluR6 knockout (KO) mice to observe the “positive spectrum” of reward-seeking behavior. We previously reported that mice of this phenotype display a collection of behavioral phenocopies similar to the symptoms of mania, such as hyperactivity, elevated risk-taking behaviors, aggression, and aggravated response to psychostimulants. These behaviors can be partially alleviated by treatment with the mood stabilizer lithium (24).
Methods and Materials
Animals
C57BL/6J and 129S1/SVImJ mice (male, 20–25 g) were obtained from Jackson Laboratories (Bar Harbor, Maine). Wistar-Kyoto rats (male, 300–350 g) were obtained from Taconic Farms (Hudson, New York). GluR6KO mice and their wildtype (WT) littermates were bred in-house as previously described (24).
All animals were singly housed 1 week before the FUST in a conventional vivarium maintained at a constant temperature (22° ± 1°C) on a 12 hour light–dark cycle (light: 6 am–6 pm) with food and water ad libitum. Behavioral tests were conducted in a separate room between 10 am and 3 pm. All experimental procedures were approved by the Animal Care and Use Committee of the National Institute of Mental Health and followed National Institutes of Health guidelines.
The FUST as a Measure of Reward-Seeking Activity
The basic test procedure was adapted from the olfactory habituation–dishabituation test (see Wrenn et al. for a detailed description [25]), modified to assess reward-seeking behavior. One hour before the test, rodents were habituated to a sterile cotton-tipped applicator inserted into their home cage. For the test, rodents were transferred to a dimly-lit room (∼3 lux lighting). The test had three phases: 1) one exposure (3 min) to the cotton tip dipped in sterile water, during which sniffing duration was measured; 2) an interval of 45 min during which no cotton tip was presented to the animal; and 3) one exposure (3 min) to a cotton tip applicator infused with fresh urine collected from females of the same strain in estrus (Figure 1), during which sniffing duration was measured. All measurements of sniffing behavior in the FUST were conducted by two independent observers. Interrater reliability for scoring the behaviors ranged from r = .989 to r = 1.00 (p < .01). We also conducted a series of preliminary experiments to determine whether estrus female urine would have a different effect on reward-seeking behavior compared with other novel odors (see Figure S1 in Supplement 1 for additional details). Details regarding the assessment of the vaginal estrus condition of each female mouse are also described in Supplement 1.
Figure 1.

Illustration of the female urine sniffing test (FUST). A male C57BL/6J mouse sniffs a cotton-tipped applicator dipped into urine from a female mouse in estrus to assess reward-seeking behavior in rodents. The behavior counted as sniffing duration only when the mouse or rat nose was adjacent to the cotton-tipped applicator (as seen in the figure) and the researcher could detect that the rodent was sniffing.
Vocalization Measurements
USVs were recorded during the FUST in manipulation-naive mice (C57BL/6J; 129S1/SVImJ; GluR6KO vs. WT and LH vs. non–learned helplessness [NLH] mice; n = 10–14). An ultrasound Microphone (Avisoft UltraSoundGate condenser microphone capsule CM16, Avisoft Bioacoustics, Berlin, Germany) sensitive to frequencies of 10–180 kHz was installed approximately 20 cm above the home cage to measure any USVs emitted during the FUST. USVs were recorded using Avisoft Recorder software (Version 3.2) as previously described (26). Briefly, settings included a sampling rate at 250 kHz, 16-bit format. Recordings were transferred to Avisoft SASLab Pro (Version 4.4) for acoustical analysis, and a fast Fourier transformation (FFT) was conducted. Parameters analyzed included number of USVs, duration of calls, and qualitative assessment of frequency and amplitude at the maximum of the spectrum.
Surgery and Microdialysis
Surgery and microdialysis were conducted as previously described (27,28) (see Methods in Supplement 1 for details).
The LH Paradigm and Locomotor Field Test
The LH paradigm was conducted as previously described (29) (see Methods in Supplement 1 for details). Although the locomotor activity component of the FUST paradigm is modest, we also conducted the open field test as previously described (30) to assess whether treatment with citalopram and saline affected locomotor activity differently. We found no significant difference between the two treatment groups [t (17) = .834; p = .416].
Stress Paradigms
The Induction phase of the LH paradigm described earlier was used to subject manipulation-naive mice (C57BL/6J; n = 13) to severe stress (inescapable, unpredictable, and uncontrollable repeated foot-shocks over a 2-hour period). FUST behavior was assessed 24 hours before and 24 hours after the LH Induction phase.
Citalopram Treatment
129S1/SVImJ mice (n = 8) that had undergone the LH paradigm were anesthetized by inhalation of 3% isoflurane in oxygen, and Alzet osmotic minipumps (model 1002, Alzet Osmotic Pumps, Cupertino, California) were placed subcutaneously. Pumps were filled with sterile saline or citalopram (2 μmol/L) continuously infused into the third ventricle for 14 days (Alzet Brain Kit 3, Alzet Osmotic Pumps). Animals recovered for 12 days before subsequent testing.
Saccharin Preference Test
To compare the reward-seeking behavior of GluR6KO mice in the FUST to a widely used nonoperant appetitive paradigm, we conducted the saccharin preference test in GluR6KO mice and their WT controls (see Methods in Supplement 1 for details).
Data Analysis
Statistical analysis was performed using GraphPad Prism Version 4 (GraphPad Software, La Jolla, California). Sniffing duration for manipulation-naive rodents was analyzed by paired t tests for each strain. USV duration was analyzed by one-sample t test compared with zero (no USVs were emitted during water exposure). Differences in dopamine levels were analyzed by one-way repeated-measures analysis of variance (ANOVA). Sniffing duration of C57BL/6J mice before and after the stress paradigm was analyzed by two-way ANOVA, with stress condition (before and after) as a repeated measure. Two-way repeated-measures ANOVA was used to assess the following: sniffing duration in the LH paradigm as well as number of USVs; sniffing duration between treated and nontreated LH mice; and sniffing duration as well as number of USVs in GluR6KO mice and their WT controls. GluR6KO mice and their WT controls emitted USVs only when female urine was introduced; therefore, differences in the duration of USVs and peak call frequencies were analyzed separately by independent t tests. Saccharin preference between GluRKO mice and their WT controls at different concentrations were measured by two-way ANOVA.
Data are reported as mean ± SEM. Significance was evaluated at p < .05, two-tailed.
Results
FUST and Water Sniffing in Males of Three Rodent Strains
The FUST was conducted in male mice (129S1/SVImJ and C57BL/6J) and inbred Wistar-Kyoto rats (Figure 2). Male rodents of all three strains sniffed female urine significantly longer than water [C57BL/6J mice: t (13) = 8.28; p < .01; 129S1/SVImJ: t (10) = 4.41; p < .01; Wistar-Kyoto rats: t (9) = 7.97; p < .01].
Figure 2.
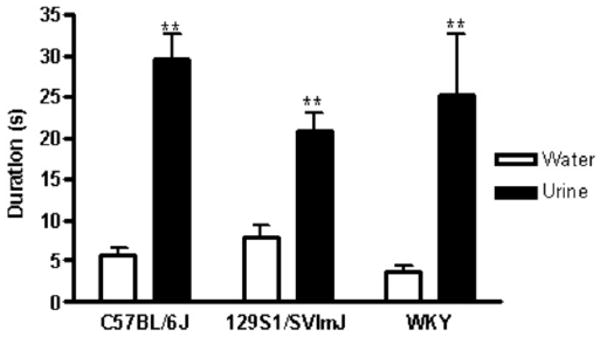
Cumulative time spent sniffing for male C57BL/6J and 129S1/SVImJ mice and Wistar-Kyoto (WKY) rats exposed to female urinary pheromone odors. Different strains of manipulation-naive male mice (C57BL/6J; 129S1/SVImJ) and rats (WKY) underwent the female urine sniffing test (FUST) paradigm. Male mice and rats were introduced for 3 min to a cotton-tipped applicator dipped in water (as a control). After 45 min, they were introduced for 3 min to a cotton-tipped applicator dipped into estrus female urine from the same strain. All male rodents spent significantly more time sniffing estrus female urine than water during the 3-min FUST (**p<.01; n = 10 – 14).
Vocalizations During the FUST
USVs during the FUST were monitored in a subset of mice (Figure 3). Males emitted USVs only when female urine was present [C57BL/6J: t (13) = 2.27; p < .05; 129s1/SVImJ: t (11) = 2.25; p < .05].
Figure 3.
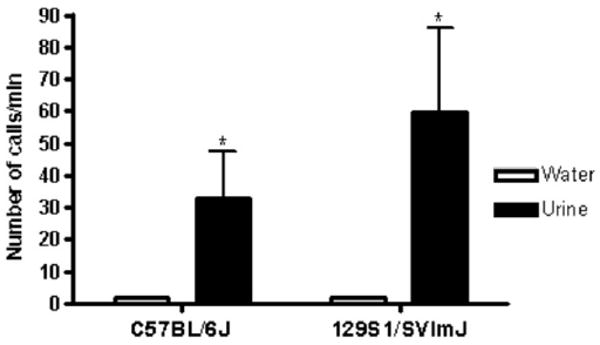
Ultrasonic vocalizations (USVs) in male C57BL/6J and 129S1/SVImJ mice sniffing water or female urine. Different strains of manipulation-naive male mice (C57BL/6J; 129S1/SVImJ) underwent the female urine sniffing test paradigm. Male mice and rats were introduced for 3 min to a cotton-tipped applicator dipped in water (as a control). After 45 min, they were introduced for 3 min to a cotton-tipped applicator dipped into estrus female urine from the same strain. All male mice displayed significantly more USVs during exposure to estrus female urine than water (*p < .05; n = 12–14).
Changes in NAc Extracellular Dopamine Levels During the FUST
Dopamine release in the NAc was measured in a subset of manipulation-naive 129S1/SVImJ mice during the following periods: baseline (three samples per animal), water exposure (two samples per animal), postwater exposure (two samples per animal), female urine exposure (two samples per animal), post–female urine exposure after 4 min (two samples per animal), and post–female urine exposure after 8 min (two samples per animal); an average for each time point was calculated (Figure 4). Sample means within each period were subjected to one-way repeated-measure ANOVA, and type of exposure was found to influence dopamine levels significantly [F(5,20) = 4.924, p = .0063]. Post hoc tests revealed that dopamine levels were significantly higher in urine, and post–urine I and II periods than during baseline, water, or postwater exposure periods (Bonfer-roni's Multiple Comparison Test: baseline vs. urine, t = 8.805, p < .001; baseline vs. post–urine I, t = 6.676, p < .01; water vs. post–urine II, t = 3.644, p < .05; water vs. urine, t = 8.921, p < .001; water vs. post–urine I, t = 6.793, p < .01; water vs. post–urine II, t = 3.17, p < .05; postwater vs. urine, t = 8.737, p < .001; postwater vs. post–urine I, t = 5.733, p < .01; postwater vs. posturine, t = 3.032, p < .05).
Figure 4.
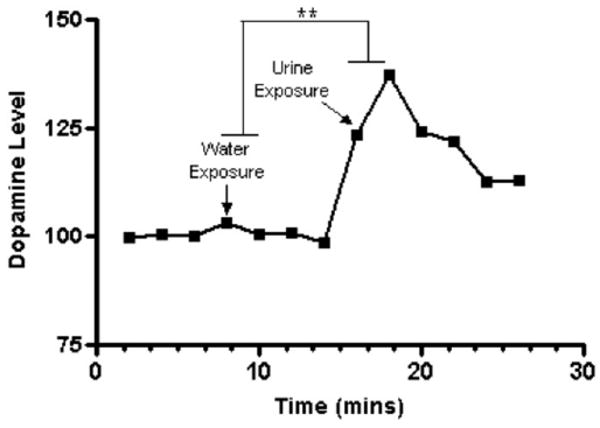
Dopamine levels in 129S1/SVImJ mice during the female urine sniffing test (FUST). 129S1/SVImJ male mice underwent microdialysis surgeries and then underwent the FUST paradigm. Male mice and rats were introduced for 3 min to a cotton-tipped applicator dipped in water (as a control). After 45 min, they were introduced for 3 min to a cotton-tipped applicator dipped into estrus female urine from the same strain. Extracellular NAc dopamine samples were collected during the entire paradigm. During the FUST, 129S1/SVImJ male mice displayed significantly elevated levels of extracellular dopamine released in the NAc after exposure to estrus female urine but not water (**p < .01; n = 5). NAc, nucleus accumbens.
Effects of Foot-Shock Stress on the FUST
The FUST was conducted in male C57BL/6J mice before and after foot-shocks (administered during the Induction phase of the LH paradigm; Figure 5). Significant effects were found for odor source. Mice spent more time sniffing urine than water [F (1,24) = 93.78; p < .01]. Significant effects were also found for stress; mice sniffed the cotton tip more before the stress manipulation than after it [F (1,24) = 9.59; p < .01]. The interaction between the two was also significant. Although foot-shock stress did not significantly alter time spent sniffing water, it significantly reduced time spent sniffing female urine [F (1,24) = 8.55; p < .01].
Figure 5.
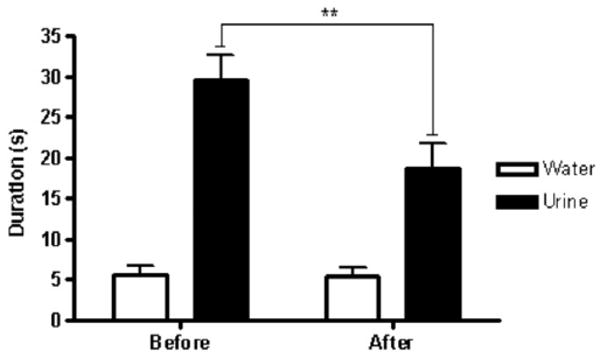
Time spent sniffing by C57BL/6J mice before and after foot-shock stress. C57BL/6J male mice underwent a foot-shock stress paradigm. The female urine sniffing test (FUST) paradigm was conducted twice: once before stress induction and once afterward. Male mice and rats were introduced for 3 min to a cotton-tipped applicator dipped in water (as a control). After 45 min, they were introduced for 3 min to a cotton-tipped applicator dipped into estrus female urine from the same strain. After foot-shock stress exposure, C57BL/6J male mice had significantly decreased mean sniffing duration of estrus female urine but not water in the FUST (**p< .01; n = 13).
Sniffing and Vocalizations in Animals That Underwent the Learned Helplessness Paradigm
The FUST, in conjunction with USV recording, was performed in male C57BL/6J mice after the third part of the paradigm. Both NLH and LH animals spent more time sniffing urine than water [urine vs. water: F(1,38) = 35.55; p < .01, Figure 6A]. Overall, NLH mice sniffed more than LH mice [F(1,38) = 50.75; p < .05]. The interaction showed that LH males sniffed urine significantly less than NLH males; there were no significant differences between NLH and LH mice in duration of water sniffing [F (1,38) = 6.67; p < .05]. When we measured the number of USVs in the LH paradigm, there were significant differences only for odor source [water vs. urine; F(1,12) = 7.95; p < .05; Figure 6B]. No significant differences were found for helplessness conditions [NLH vs. LH; F (1,12) = .52, p = .49] or their interaction [F (1,12) = .52, p = .49].
Figure 6.
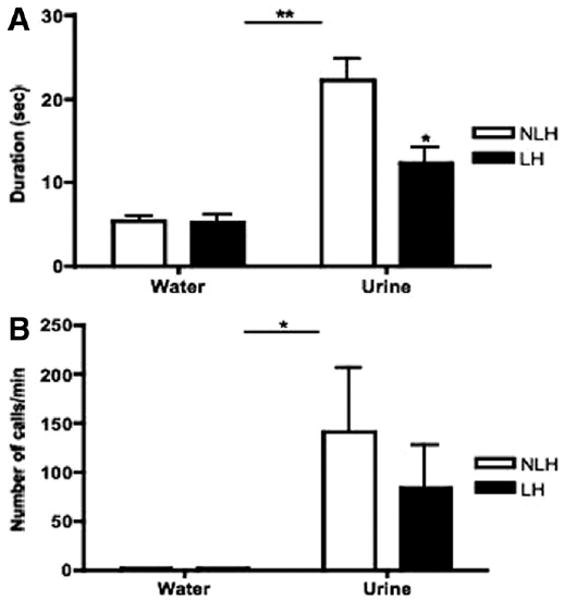
(A) After undergoing the learned helplessness (LH) paradigm, both LH and non-LH (NLH) male C57BL/6J mice underwent the female urine sniffing test (FUST) paradigm. Both the LH and NLH male C57BL/6J mice spent significantly more time sniffing estrus female urine than water (**p < .01). NLH C57BL/6J mice spent significantly longer sniffing the estrus female urine than the LH mice (*p < .05; n = 8–12). No significant differences were found between these groups for time spent sniffing the water-dipped applicator. (B) The LH and NLH C57BL/6J mice displayed significantly more ultrasonic vocalizations during exposure to urine than water in the FUST paradigm (*p < .05; n = 7).
Effect of Chronic Citalopram Treatment on Sniffing in LH Mice
A separate cohort of 129S1/SVImJ mice underwent helplessness induction and then received saline or citalopram treatment 12 days before the FUST. Both citalopram- and saline-treated mice spent more time sniffing urine than water [water vs. urine: F(1,13) = 137.59; p < .01; Figure 7]. Overall, mice treated with citalopram spent more time sniffing in both of the conditions than mice treated with saline [citalopram vs. saline: F(1,13) = 9.20; p < .05]. The interactions showed that although there were no significant differences between citalopram- and saline-treated mice in time spent sniffing water, citalopram-treated mice spent more time sniffing urine than saline-treated mice [F(1,13) = 5.88; p < .05].
Figure 7.
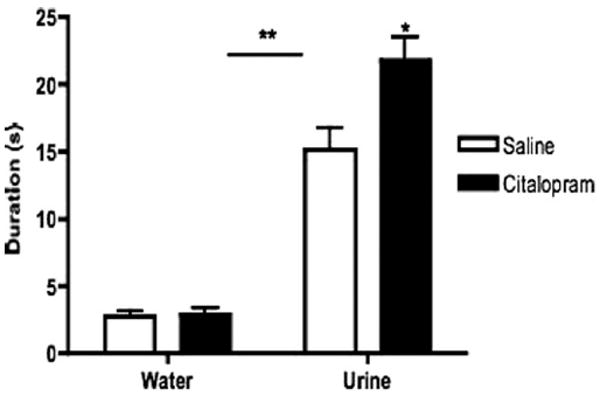
Time spent sniffing female urinary pheromone odors in learned helplessness (LH) 129S1/SVImJ mice treated with saline or citalopram. LH 129S1/SVImJ male mice were treated with the selective serotonin reuptake inhibitor citalopram or saline for 14 days and then underwent the female urine sniffing test (FUST) paradigm. LH 129S1/SVImJ male mice treated with either citalopram or saline both spent significantly more time sniffing estrus female urine than water (**p < .01). However, LH 129S1/SVImJ mice treated with citalopram spent significantly longer sniffing the urine-dipped applicator than the saline-treated LH mice in this paradigm (*p < .05; n = 7–8). No significant differences were found between these two groups for time spent sniffing the water-dipped applicator.
Outcome of Saccharin Preference Test, Sniffing Behavior, and Vocalizations During the FUST in GluR6KO Mice
Male GluR6KO mice and their WT littermates underwent the saccharin preference test (Figure 8). In addition, another cohort of GluR6KO and WT male mice underwent the FUST (Figure 9A), as well as USV recording during the FUST (Figure 9B–9D). GluR6KO mice showed a significantly higher preference for saccharin solution than water compared with their WT controls [genotype: F(1,113) = 5.26; p < .05]. In addition, there was a significant effect for saccharin concentration; as saccharin concentrations rose, the preference of mice from both strains increased accordingly [F (5,113) = 14.05; p < .01]. Scheffe post hoc tests revealed that this difference was significant at the low concentration (.05% saccharin; p < .05). No significant effect was found for the interaction between genotype and saccharin concentration [F (5,113) = 1.52, p = .19].
Figure 8.
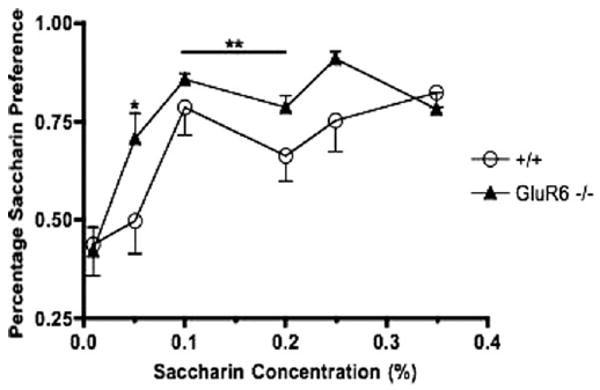
Male GluR6 knockout (KO) mice and their wild-type (WT) littermates underwent the saccharin preference test. Animals were given one bottle of tap water and one of saccharin diluted in tap water to a final concentration ranging from .005% to .35% overnight, and the percentage of saccharin preference per day was calculated. GluR6 KO male mice displayed a significantly higher preference for saccharin solution than water compared to WT mice across the range of saccharin concentrations (**p < .01; *p < .05; n = 13).
Figure 9.
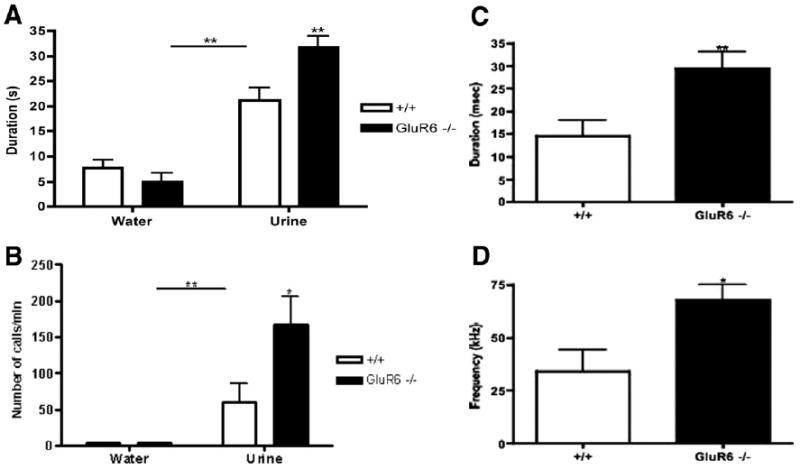
(A) GluR6 knockout (KO) and wildtype (WT) male mice spent significantly more time sniffing estrus female urine than water in the female urine sniffing test (FUST) paradigm (**p < .01). Genotype did not affect sniffing per se; however, whereas GluR6KO mice spent significantly less time sniffing water than their WT controls, they spent significantly more time sniffing estrus female urine than the WT mice (**p< .01; n = 11). (B) GluR6KO and WT mice displayed significantly more ultrasonic vocalizations (USVs) during exposure to estrus female urine than water in the FUST paradigm (**p < .01; n = 12). There were no significant differences between the groups in number of USVs after exposure to the water. GluR6KO mice exhibited more USVs during the FUST than their WT controls (*p < .05). (C) GluR6KO mice that underwent the FUST paradigm displayed longer bouts of USVs than their WT controls during exposure to estrus female urine (**p < .01; n = 12). (D) GluR6KO mice displayed a significantly higher peak call frequency during bouts of USVs than WT mice during exposure to estrus female urine (*p < .05; n = 12).
Both GluR6KO and WT males spent more time sniffing urine than water in the FUST paradigm [water vs. urine: F(1,20) = 78.65; p < .01; Figure 9A]. Genotype had no significant effect on the sniffing per se [genotype: F(1,20) = 4.26, p = .052]; however, a significant interaction revealed that GluR6KO mice sniffed the female urine more and spent less time sniffing water than their WT controls [F (1,20) = 8.56; p < .01; Figure 9A].
Similarly, vocalizations were significantly influenced by genotype and odor source [water vs. urine; F(1,22) = 24.04; p < .01; genotype (GluR6KO vs. WT); F(1,22) = 5.41; p < .05; interaction F(1.22) = 5.41; p < .05; Figure 9B]. GluR6KO mice emitted more USVs than their WT controls, the duration of these USVs was longer [t (22) = 2.87; p< .01; Figure 9C], and the frequency of the USVs was higher [t (22) = 2.74; p < .05; Figure 9D].
Discussion
We designed and validated a novel, simple test to assess changes in reward-seeking behavior in rodent models of mood disorders. The FUST described here has two components: 1) presenting an ordinary, natural, sexual incentive stimulus and 2) observing rodent behavioral response to the stimulus. To the best of our knowledge, this is the first study to demonstrate that interest in pheromones from the opposite sex (31) can provide a quantitative, nonoperant measure of reward-seeking behavior in rodent models of mood disorders.
The FUST was derived from two previous areas of investigation: using estrus urine or bedding as a sexual incentive stimulus (31,32) and the olfactory habituation–dishabituation test (25,33–37). Echoing the rodent response to sexually receptive females, estrus urine is known to elicit behavioral displays similar to reward incentive stimuli, including shortened approaching time to stimulus, increased number of approaches, and longer time spent with the approached stimulus (for a review, see Martinez-Garcia et al.) (38). Given that the hormonal factors and neural circuitry that control those elements are similar (39), we hypothesized that sexual incentive motivation could be used to evaluate reward-seeking behavior under conditions relevant to the study of mood disorders. Although we cannot discount the putative contribution of the novelty of estrus female urine in the reward-seeking behavior observed in this study, our preliminary data show that it is not novelty per se but rather the sexual reward–preference component that contributes to the reward-seeking behavior observed here (Methods in Supplement 1).
In this study, the time male rodents spent sniffing female estrus urine was used to assess reward-seeking behavior. Given that male mice vocalize in the ultrasonic range in the presence of females and when exposed to female urinary pheromones (17), we also measured USVs during this procedure to validate further these behavioral observations. Our results indicate that both mice and rats found a cotton-tipped applicator dipped in female urine more rewarding than one dipped in water, as previously described (35–37). All rodent strains in this study spent more time sniffing the female urine and emitted USVs only when female urine was introduced.
USVs in adult mice have not been well characterized, and much remains unknown regarding whether particular calls reflect positive or negative affective states (40). In male mice, USVs are primarily reported in reproductive contexts (41); the USVs elicited by female urine are emitted in frequencies ranging from 30 to 110 Hz (42). In rats, USVs are thought to facilitate sexual behavior (43) and help keep females in near proximity (44). Typically, sexually receptive females and estrus bedding elicit 50-Hz calls, believed to reflect positive affect in rats (43). These findings support the notion that male USVs may play a key role in facilitating mounting and/or intromission in rodents (45). In addition, several studies have found that systemic or NAc administration of amphetamine increased 50 -Hz USVs in rats (46,47) and 30-Hz USVs in mice (45). Here, we similarly showed that the odor of female urine elicited USVs in rodents, suggesting that it has physiologic incentive properties that may reflect those of sexually receptive females and psychostimulants.
We also measured dopamine levels in the NAc of manipulation-naive mice (129S1/SVImJ) during the FUST. The NAc and its dopaminergic inputs from the ventral tegmental area are key anatomic substrates for various rewards, including food, sex, and social interactions (48,49). In rodents, consuming natural rewards (such as food, sugar, or sugar derivatives) or receiving chemical rewards (such as psychostimulants) activates NAc neurons and increases dopamine levels in the NAc. Previous studies found that encounters with sexually receptive females or estrus urine or bedding caused dopamine release in the NAc of male rodents (50). Consistent with the literature, we found that mice exposed to female urine exhibited significantly elevated dopamine levels compared with baseline and to levels exhibited during water exposure. This suggests that these rodents found the female urine to be a rewarding cue, and their responsiveness to this cue was mediated via a prominent reward network.
Finally, we assessed the ability of the FUST to differentiate between animals at opposing ends of the reward-seeking spectrum. As a model of depression, we used the well-validated LH paradigm (51) in which roughly 50% to 80% of mice develop failure to escape—also known as learned helplessness—after uncontrollable and inescapable foot-shocks. This failure to escape persists for weeks and can be alleviated by repeated or chronic—but not acute—treatment with antidepressants (52). We used the LH paradigm to detect the sensitivity of the FUST and validate its usefulness and found that animals that underwent this paradigm spent less time sniffing estrus urine but not water (Figure 5), suggesting that the FUST is sensitive to the influence of stressors. Similarly, LH mice (manipulation-naive C57BL/6J) spent less time sniffing estrus urine, but not water, than NLH mice (Figure 6A). The LH mice they also tended to emit fewer USVs, although this finding did not reach statistical significance. Notably, chronic treatment with citalopram significantly increased the time that LH mice spent sniffing the estrus urine, although it did not affect time spent sniffing water (Figure 7). Other studies have similarly reported that stress dampens reward-seeking behavior in rodents and that these effects can be mitigated by treatment with antidepressants (53). Taken together, these data illustrate that the FUST is sensitive to changes in the well-established LH paradigm.
GluR6KO mice, a rodent model of mania, were used to evaluate the FUST further. These mice display behavioral phenocopies similar to manic symptoms, including hyperactivity, elevated risk-taking behaviors, aggression, and aggravated response to psychostimulants; these behaviors can be partially alleviated by treatment with lithium (24). For comparison purposes, we first assessed the reward-seeking behavior of GluR6 mice in a widely used nonoperant paradigm: saccharin preference. GluR6KO mice exhibited increased preference for saccharin solutions across different concentrations than their WT controls (Figure 8), suggesting they did indeed display upregulated reward-seeking behavior. In the FUST, GluR6KO mice sniffed the urine-dipped applicator longer than their WT controls, whereas time spent sniffing the water-dipped applicator showed no genotype differences (Figure 9A). In addition, GluR6KO mice emitted more USVs during exposure to female urine, and these USVs were distinct from those emitted by WT mice (longer duration and higher average peak frequency; Figure 9B–9D). Taken together, the data further support the notion that GluR6 KO might be a hyper-reward-seeking state and indicate that the FUST is sensitive to upward changes in animal models of mania.
This novel paradigm is, however, associated with its own limitations. The most obvious is that genetic manipulation or certain drugs might cause olfactory system dysfunction (32). Several studies have shown that olfactory bulbectomy abolished mounting behavior in male mice (32) and reduced the time that male mice spent sniffing estrus odors (54). In addition, mice could have a dysfunctional gonadal hormone system, making them insensitive to the FUST. Furthermore, because the FUST paradigm has a social behavior component, the preference for estrus female odor might also be related to social behavior and not just sexual behavior (55). Another key issue is that the use of volatile stimuli and the sensitivity of the rodent olfactory system prevented us from presenting two odors at the same time. Therefore, as in the olfactory habituation–dishabituation test, two olfactory choices—in this case water and female estrus urine—were presented sequentially rather than simultaneously. Finally, this model is designed for male rodents exclusively; additional experiments should be conducted in an attempt to adapt the FUST paradigm to females. Future studies should also evaluate the effects of mood-stabilizing drugs on female estrus urine sniffing preference; for instance, recent work in this area examined the effects of mood stabilizers on the saccharin preference test (56).
Taking these caveats into account, we propose that measuring spontaneous sniffing and vocalization responses to urinary pheromones of the opposite sex may prove to be a valuable addition to the armamentarium of assays that measure appetitive behaviors in rodent models of reward-seeking behavior. Multiple tasks that evaluate different aspects of reward sensitivity in rodents are likely to provide insights into the multiple neurobiological and behavioral processes mediating mood disorders (57).
Summary
The FUST is a simple, unique, nonoperant paradigm for measuring reward-seeking behavior in rodents. Notably, it is effective in both rats and various strains of mice and is sensitive to both ends of the reward-seeking spectrum, to the influence of stressors, and to psychopharmacologic manipulation. The FUST is both theoretically relevant and treatment-sensitive. Using the FUST in conjunction with other tests of reward-seeking behavior will enable us to study generalized components of behaviors that model the symptoms of mood disorders and other neuropsychiatric illnesses.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest. Dr. Manji is now with Johnson and Johnson Pharmaceutical Research and Development (Titusville, NJ), and Dr. Maria Luisa Scattoni is now with the Instituto Superiore di Sanita (Rome, Italy). Ioline Henter provided invaluable editorial assistance.
Supplementary material cited in this article is available online.
References
- 1.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 2.Klein DF. Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 4.Hasler G, Drevets WC, Gould TD, Gottesman MHK., II Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Gould TD, Einat H. Animal models of bipolar disorder and mood stabilizer efficacy: A critical need for improvement. Neurosci Biobehav Rev. 2007;31:825–831. doi: 10.1016/j.neubiorev.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paredes RG. Evaluating the neurobiology of sexual reward. Ilar J. 2009;50:15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Crawley JN. What's Wrong with My Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice. Hoboken, NJ: Wiley-Liss; 2007. [Google Scholar]
- 8.Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 9.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 10.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 11.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 12.Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: Greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- 13.Harris RBS, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav. 1997;63:91–100. doi: 10.1016/s0031-9384(97)00425-3. [DOI] [PubMed] [Google Scholar]
- 14.Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: Validity as a model of depression. Physiol Behav. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- 15.Kelliher KR, Wersinger SR. Olfactory regulation of the sexual behavior and reproductive physiology of the laboratory mouse: Effects and neural mechanisms. Ilar J. 2009;50:28–42. doi: 10.1093/ilar.50.1.28. [DOI] [PubMed] [Google Scholar]
- 16.Beynon RJ, Hurst JL. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides. 2004;25:1553–1563. doi: 10.1016/j.peptides.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- 18.Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: Primary role in mouse chemosensory gender recognition. Physiol Behav. 1982;29:315–327. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]
- 19.Sipos ML, Kerchner M, Nyby JG. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus) Behav Neural Biol. 1992;58:138–143. doi: 10.1016/0163-1047(92)90375-e. [DOI] [PubMed] [Google Scholar]
- 20.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 24.Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: Methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- 26.Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, et al. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez L, Stanley BG, Hoebel BG. A small, removable microdialysis probe. Life Sci. 1986;39:2629–2637. doi: 10.1016/0024-3205(86)90119-0. [DOI] [PubMed] [Google Scholar]
- 28.Paredes D, Granholm AC, Bickford PC. Effects of NGF and BDNF on baseline glutamate and dopamine release in the hippocampal formation of the adult rat. Brain Res. 2007;1141:56–64. doi: 10.1016/j.brainres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci U S A. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agmo A, Pfaff DW. Research on the neurobiology of sexual behavior at the turn of the millennium. Behav Brain Res. 1999;105:1–4. doi: 10.1016/s0166-4328(99)00078-9. [DOI] [PubMed] [Google Scholar]
- 32.Hull EM, Dominguez JM. Getting his act together: Roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Gregg B, Thiessen DD. A simple method of olfactory discrimination of urines for the Mongolian gerbil, Meriones unguiculatus. Physiol Behav. 1981;26:1133–1136. doi: 10.1016/0031-9384(81)90221-3. [DOI] [PubMed] [Google Scholar]
- 34.Luo AH, Cannon EH, Wekesa KS, Lyman RF, Vandenbergh JG, Anholt RR. Impaired olfactory behavior in mice deficient in the alpha subunit of G(o) Brain Res. 2002;941:62–71. doi: 10.1016/s0006-8993(02)02566-0. [DOI] [PubMed] [Google Scholar]
- 35.Chadman KK, Gong S, Scattoni ML, Botluck SE, Gandhy SU, Heintz N, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C Knockin mice. Austism Rev. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, et al. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Garcia F, Martinez-Ricos J, Agustin-Pavon C, Martinez-Hernandez J, Novejarque A, Lanuza E. Refining the dual olfactory hypothesis: Pheromone reward and odour experience. Behav Brain Res. 2009;200:277–286. doi: 10.1016/j.bbr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 41.Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. New York: CRC Press; 2001. pp. 3–18. [Google Scholar]
- 42.Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntosh TK, Barfield RJ, Geyer LA. Ultrasonic vocalisations facilitate sexual behaviour of female rats. Nature. 1978;272:163–164. doi: 10.1038/272163a0. [DOI] [PubMed] [Google Scholar]
- 44.Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS ONE. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: A quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66:639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- 48.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 49.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 50.Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after d-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henn FA, Edwards E, Muneyyirci J. Animal models of depression. Clin Neurosci. 1993;1:152–156. [Google Scholar]
- 52.Gambarana C, Scheggi S, Tagliamonte A, Tolu P, De Montis MG. Animal models for the study of antidepressant activity. Brain Res Brain Res Protoc. 2001;7:11–20. doi: 10.1016/s1385-299x(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 53.Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol Behav. 2008;93:467–473. doi: 10.1016/j.physbeh.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O'Carroll AM, Young WS. Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Flaisher-Grinberg S, Overgaard S, Einat H. Attenuation of high sweet solution preference by mood stabilizers: A possible mouse model for the increased reward-seeking domain of mania. J Neurosci Methods. 2009;177:44–50. doi: 10.1016/j.jneumeth.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–714. doi: 10.1016/j.neubiorev.2005.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


