Abstract
The Ca2+/cAMP response element binding protein CREB mediates transcription of genes essential for the development and function of the central nervous system. Here we investigated the ability of caffeine to stimulate CREB-dependent gene transcription in primary cultures of developing mouse cortical neurons. Using the CREB-dependent reporter gene CRE-luciferase we show that stimulation of CREB activity by caffeine exhibits a bell-shaped dose-response curve. Maximal stimulation occurred at 10 mM caffeine, which is known to release Ca2+ from ryanodine sensitive internal stores. In our immature neuronal cultures, 10 mM caffeine was more effective at stimulating CREB activity than depolarization with high extracellular KCl (50 mM). Quantitative real-time PCR analysis demonstrated that transcripts derived from endogenous CREB target genes, such as the gene encoding brain derived neurotrophic factor BDNF, are increased following caffeine treatment. The dose response curves of CREB target genes to caffeine exhibited gene-specificity, highlighting the importance of promoter structure in shaping genomic responses to Ca2+ signaling. In the presence of a weak depolarizing stimulus (10 mM KCl), concentrations of caffeine relevant for premature infants undergoing caffeine treatment increased CRE-luciferase activity and Bdnf transcript levels. The ability of caffeine to enhance activity-dependent Bdnf expression may contribute to the neurological benefit observed in infants receiving caffeine treatment.
Keywords: CREB, caffeine, cortical neurons, calcium, activity-dependent gene expression, ryanodine receptor
Caffeine, which is present in coffee, tea, soft drinks and chocolate, is the most commonly used psychostimulant in the world [1]. Caffeine exerts multiple effects on cells. At the low doses of caffeine achieved by dietary intake (1–10 μM), the primary effect of caffeine in the central nervous system is believed to be inhibition of adenosine receptors and subsequent modulation of neurotransmitter release [2–3]. But higher doses of caffeine can also 1) block GABA-A receptors, reducing the inhibitory input in functional neuronal networks, 2) inhibit phosphodiesterase activity leading to increased cellular cAMP levels and 3) release Ca2+ from intracellular ryanodine sensitive stores stimulating Ca2+ signaling in numerous cell types including neurons. [4].
Caffeine is one of the most commonly prescribed drugs in pediatric emergency rooms. Clinically, caffeine is utilized for the treatment of premature infants with apnea [5–6]. In newborns, the half-life of caffeine slows from the 2–5 hours reported in adults to approximately 80 hours in full-term infants and over 100 hours in premature infants [7–9]. Due to their reduced metabolism of caffeine, premature infants receiving caffeine treatment accumulate significantly higher concentrations of plasma caffeine than those observed in adults due to dietary caffeine intake. A study in 1996 monitoring caffeine levels in the serum of 59 premature infants, however, reported a mean serum caffeine concentration of 29.9 mg/L (154 μM) with a high concentration of 93.3 mg/L observed (480 μM) [10]. More recent studies have reported serum concentrations ranging from 19–80 mg/L (98–412 μM) [9] or 11–33 mg/L (57–170 μM) [11]. Interestingly, infants treated with caffeine are less likely to exhibit neurodevelopmental deficits [12–13], suggesting that caffeine treatment exerts a positive influence on developing neurons.
The Ca2+/cAMP response element binding protein CREB mediates transcription of genes critical for development and function of the nervous system [14]. CREB-mediated transcription of the Bdnf gene, which encodes brain-derived neurotrophic factor, promotes neuron survival, neurite outgrowth and synaptic plasticity [15]. In this study we directly test the ability of caffeine to regulate CREB activity in developing cortical neurons.
Materials and Methods
Cell culture and transfection
Cortical neuron cultures were prepared from mouse brains on embryonic day 15.5 (E15.5) as previously described [16]. Neurons were plated onto p35 dishes or 24-well dishes coated with 15 μg/ml polyornithine (Sigma) and 2 μg/ml laminin (Invitrogen) and cultured in Neurobasal media supplemented with 2% B27, 1mM glutamine and penicillin/streptomycin (Invitrogen). Neurons were transiently transfected 3–5 DIV using calcium phosphate as previously described [17].
Luciferase assays
Plasmids used for luciferase assays were as follows: pCRE-Luc PathDetect cis-reporter plasmid (Stratagene) and TK Renilla (Promega). Two days following transfection, cells were stimulated with either 50 mM KCl, 10 mM KCl with or without caffeine as indicated. Following stimulation, the medium was aspirated, cells rinsed once with PBS and then harvested for luciferase assays using the Dual Luciferase Assay kit (Promega). Data presented are the mean of quadruplicate samples with standard error indicated. Each experiment was repeated at least three times with similar results using independent cortical neuron preparations. Students paired t-test was used to determine statistical significance of results (p < 0.05) with n = 4.
RNA analysis
RNA was isolated from cortical neurons using Trizol (Sigma). mRNA was reverse transcribed using oligo dT primers and qScript cDNA Supermix (Quanta Biosciences). Real Time PCR was conducted on a LightCycler 480 (Roche) using SYBR Green I (Roche) master mix. Results were normalized to actin amplification. PCR primers used were as follows: mouse BDNF exon I: GGACAGCAAAGCCACAATGTTCCA and TTGCCTTGTCCGTGGACGTTTACT; mouse BDNF exon IV: ACCAGGTGAGAAGAGTGATGACCA and AGTTGCCTTGTCCGTGGACGTTTA; mouse full-length trkB: TTTCCTTGCCGAGTGCTACAACCT and TGAAAGTCCTTGCGTGCATTGTCG; actin: TGTGATGGTGGGAATGGGTCAGAA and TGTGGTGCCAGATCTTCTCCATGT. Statistical analysis was performed using student paired t-test with n=4.
Results
Caffeine regulates CREB-dependent reporter gene expression
Increases in intracellular Ca2+ activate CREB, which stimulates gene transcription via binding to Ca2+/cAMP response element CRE in the promoter of CREB-dependent genes [18]. We assayed the ability of caffeine to stimulate CREB activity using a CREB-dependent reporter gene consisting of four tandem CREs (Stratagene). Primary cortical neurons isolated from E15.5 mouse embryos were transiently transfected with the CRE-luciferase reporter construct, along with the TK-Renilla luciferase control vector for normalization. Two days after transfection, neurons were treated with a range of caffeine concentrations from 100 μM to 30 mM for 5 hours prior to harvesting for luciferase assays. Low doses of caffeine (0.1–0.25 mM) failed to stimulate CRE luciferase activity (Figure 1A). Treatment of neurons with 0.5 mM caffeine stimulated CRE-luciferase activity approximately 50% compared to unstimulated neurons (1.5 ± 0.2; p=0.02). Caffeine concentrations ranging from 1 mM to 10 mM continued to increase CRE-luciferase activity in a dose-dependent manner, with 1, 2, 5, or 10 mM caffeine stimulated CRE-luciferase activity 3.3, 6.8, 43 and 69-fold, respectively, compared to untreated neurons. Doses of caffeine higher than 10 mM became increasingly less effective at stimulating CRE-luciferase. Treatment of neurons with 20 mM and 30 mM caffeine resulted in 44 and 5.4-fold stimulation, respectively.
Figure 1. Caffeine stimulates CRE-dependent transcription.
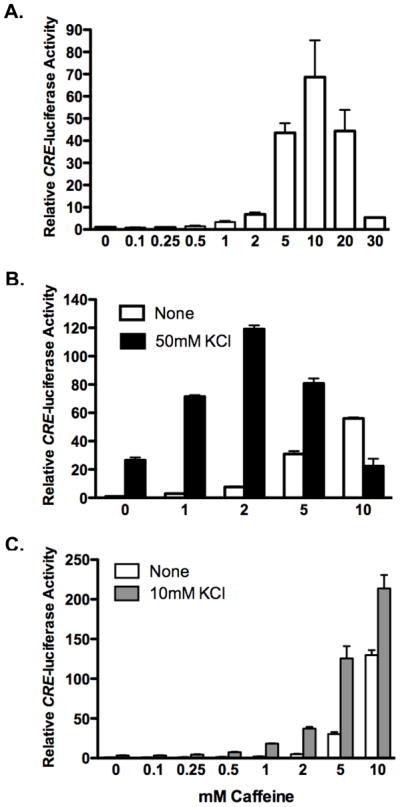
Cortical neurons were transfected with CRE-luciferase to assay CREB activity and TK Renilla luciferase for normalization. Two days after transfection, neurons were stimulated with increasing concentrations of caffeine alone (A) or in combination with depolarization triggered by the addition of either 50 mM (B) or 10 mM (C) extracellular KCl. Following 5 hours of stimulation, neurons were harvested for dual-luciferase assays. Data shown is plotted relative to untreated neurons. Bars are the average of three independent transfections with standard error indicated.
Neuronal depolarization, triggered by the addition of high extracellular KCl, is routinely used as an in vitro model to study activity-dependent CREB-mediated gene transcription. Ca2+ influx via voltage dependent Ca2+ channels following depolarization, leads to CREB activation [19]. In order to compare the effectiveness of caffeine stimulation to that of depolarization-mediated CREB activation, cortical neurons were stimulated with caffeine (1 – 10 mM) in the presence or absence of 50 mM KCl for five hours. As observed previously, increasing concentrations of caffeine up to 10 mM increasingly stimulated CRE-luciferase activity. Treatment of neurons with 10 mM caffeine provided a more robust stimulation of CRE-luciferase activity than treatment with 50 mM KCl depolarizing stimulus typically used to study CREB activation in neuronal cultures (Figure 1B). In the presence of 50 mM KCl, caffeine concentrations up through 5 mM enhanced depolarization-stimulated CRE-luciferase activity, 2.6, 4.6 and 2.8-fold, respectively. Co-stimulation of neurons with 10 mM caffeine and 50 mM KCl was less effective at increasing CRE-luciferase activity than caffeine treatment alone. Thus, in the presence of 50 mM KCl the bell-shaped caffeine response curve is shifted to the left.
We next investigated the interaction of caffeine with a weaker depolarizing stimulus of 10 mM KCl on CREB activation. In contrast to 50 mM KCl which stimulated CRE-luciferase 27-fold, treatment of neurons with 10 mM KCl resulted in a 3-fold increase is CRE-luciferase activity (Figure 1C). Cortical neurons were co-treated with a 10 mM KCl and increasing doses of caffeine for 5 hours prior to harvesting for luciferase assays. Co-stimulation with caffeine concentrations of 0.1 mM caffeine did not enhance CRE-luciferase activity due to depolarization. Lower doses (0.01–0.05 mM caffeine) were also tested and found not to stimulate CRE-luciferase activity (data not shown). In contrast, treatment of sub-maximally depolarized neurons with 0.25, 0.5, 1, 2, 5, or 10 mM caffeine increased CRE-luciferase activity 1.4, 2.3, 5.5, 12, 38 and 65-fold, respectively, relative to neurons stimulated with 10 mM KCl alone. At all concentrations of caffeine from 0.25 to 10 mM caffeine, co-stimulation with 10 mM KCl increased CRE-luciferase activity beyond the level achieved by caffeine alone (Figure 1C).
Caffeine increases endogenous CREB-dependent gene transcript levels
We next tested whether caffeine could increase expression of endogenous CREB-dependent genes c-fos and Bdnf. Cortical neurons were stimulated with increasing doses of caffeine from 1 mM to 10 mM in the presence or absence of 10 mM KCl for 5 hours prior to isolation of RNA for real-time PCR analysis. Gene-specific primer pairs were employed for PCR amplification to quantify c-fos and Bdnf transcripts. Primers for the Bdnf gene were designed to specifically detect either Bdnf promoter I or promoter IV derived transcripts, since both promoters are stimulated by Ca2+ via CREB activation [20–24]. Amplification of actin was conducted for normalization. Exposure of cortical neurons to caffeine increased c-fos and Bdnf transcript levels. Interestingly, however, the caffeine concentration yielding the greatest increase in transcript levels exhibited promoter specificity. For c-fos, stimulation of transcript levels increased at each concentration tested through 10 mM (Figure 2A). In contrast, Bdnf promoter I transcripts peaked at 2 mM caffeine, whereas Bdnf promoter IV transcripts peaked at 5 mM caffeine (Figures 2B-C). Stimulation of neurons with 1, 2, 5 and 10 mM caffeine increased c-fos transcript levels 5.8, 11, 25 and 70- fold respectively; Bdnf promoter I transcript levels increased 6.2, 7.2, 9.2, and 5.1-fold respectively; and Bdnf promoter IV transcripts increased 3.4, 5.7, 11, and 10-fold respectively, compared to untreated neurons.
Figure 2. Caffeine stimulates expression of endogenous CREB-dependent genes.
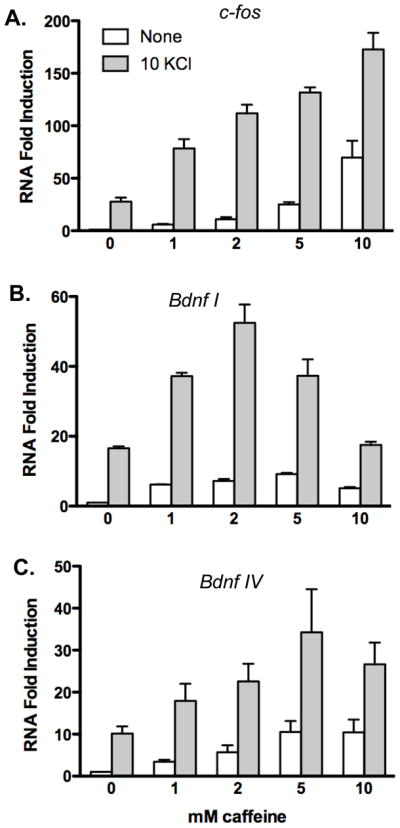
Cortical neurons were stimulated with caffeine in the presence or absence of 10 mM KCl and harvested for RNA after 5 hours of stimulation. Real-time PCR analysis was conducted to determine the relative expression levels of A) c-fos, B) Bdnf promoter I or C) Bdnf promoter IV transcripts. RNA samples are normalized to actin amplification. Data are plotted relative to unstimulated neurons. Data shown are average of three independent experiments with standard error indicated.
Depolarization of neurons by addition of 10 mM KCl stimulated c-fos 27-fold compared to unstimulated neurons. In the presence 10 mM KCl, treatment with caffeine enhanced c-fos transcript levels an additional 2.9, 4.1, 4.9, and 6.4-fold respectively, compared to neurons treated with 10 mM KCl alone. Depolarization with 10 mM KCl increased Bdnf promoter I and promoter IV transcripts 17 and 10-fold, respectively. In the presence of 10 mM KCl, inclusion of caffeine at 1, 2 or 5 mM stimulated Bdnf promoter I transcripts 2.2, 3.1 and 2.2-fold compared to KCl treated neurons. Inclusion of 10 mM caffeine failed to further stimulate of Bdnf promoter I transcripts in neurons depolarized with 10 mM KCl. Quantification of Bdnf promoter IV transcript levels showed a 1.8, 2.3, 3.4, and 2.7-fold enhancement, respectively, by co-stimulation of neurons with 1, 2, 5 and 10 mM caffeine in the presence of depolarization by 10 mM KCl.
Clinically relevant doses of caffeine stimulate BDNF transcripts following weak depolarization
Caffeine treatment of premature infants can result in caffeine serum concentrations of 100–500 μM [9–11]. Given that caffeine has been reported to yield neurological benefits to these infants [12], we tested the ability of these lower doses of caffeine to increase activity-dependent Bdnf gene expression. Cortical neurons were treated with either 250 or 500 μM caffeine in the presence of 10 mM KCl stimulation for five hours prior to harvesting RNA for RT-PCR analysis. Analysis of Bdnf promoter I and Bdnf promoter IV revealed that both 250 μM and 500 μM caffeine enhance transcript levels beyond those observed in the absence of caffeine (Figure 3). Bdnf promoter I transcripts were stimulated 1.3-fold (p = 0.003) and 2-fold, whereas Bdnf promoter IV transcripts were increased 1.2-fold (p = 0.039) and 1.9-fold by 250 μM and 500 μM caffeine in the presence of KCl, compared to KCl treated alone. Since the gene encoding the BDNF receptor TrkB is also a target of CREB [16], we examined the ability of caffeine to stimulate TrkB transcript levels. Primers were designed to specifically detect full-length catalytically active TrkB receptor, which mediates BDNF signaling to promote neuron survival and development. TrkB transcripts were stimulated 2.1-fold by inclusion of 500 μM caffeine. Although we observed a trend for increased expression 250 μM caffeine, it did not achieve statistical significance.
Figure 3. Clinically relevant caffeine doses enhance Bdnf expression.
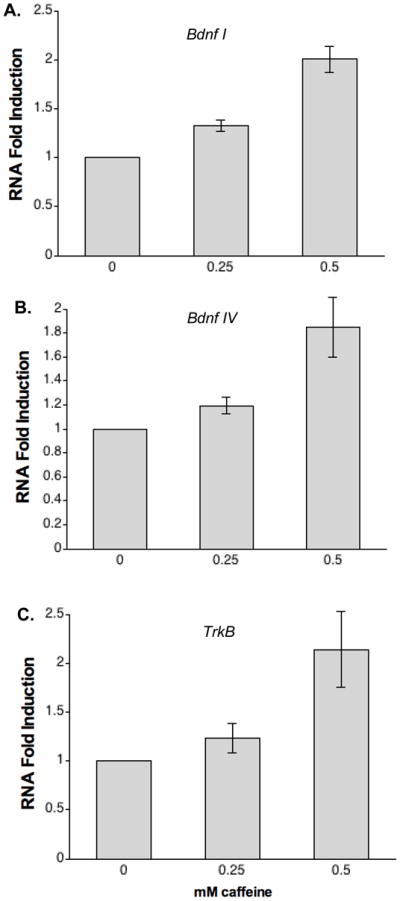
Cortical neurons were stimulated with either 0.25 mM or 0.5 mM caffeine in the presence of 10 mM extracellular KCl. RNA was purified and subjected to real-time PCR analysis to quantify Bdnf promoter I, Bdnf promoter IV and TrkB transcript levels. Data plotted relative to neurons treated with 10 mM KCl alone. Data shown are average of quadruplicates with standard error indicated. Stimulation of Bdnf transcripts was statistically significant at both 250 μM and 500 μM caffeine. Increased expression of TrkB transcripts was statistically significant at 500 μM caffeine as assessed by student paired t-test (p < 0.05).
Discussion
Previous studies administering caffeine in rodents have found that acute treatment with high doses of caffeine (75 mg/kg) stimulated c-fos expression in the brain [25–26]. In contrast, lower doses aimed at mimicking dietary intake in humans (10mg/kg) did not increase c-fos immunostaining. Since c-fos transcription can be stimulated by CREB activation, we sought to test the ability of caffeine to trigger CREB-mediated transcription in neurons. In cultured cortical neurons, we were unable to detect significant increases in CREB activity at caffeine concentrations between 10 μM- 100 μM after 5 hours of stimulation (Figure 1 and data not shown). Higher doses of caffeine stimulated CREB-dependent transcription. The response curve of CREB activation to caffeine was biphasic. The concentrations of caffeine required for CREB activation mediate release of Ca2+ from ryanodine sensitive stores. The bimodal response of CREB activation due to increasing concentrations of caffeine is characteristic of Ca2+-induced Ca2+ release (CICR) from intracellular stores [27–28]. At low concentrations, Ca2+ can stimulate release of Ca2+ from intracellular stores via ryanodine receptors, which are subsequently inhibited by increasing Ca2+concentrations. Our observation that the level of CREB activity peaks at a lower concentration of caffeine (2 mM vs. 10 mM) in the presence of a strong depolarizing stimulus known to elevate intracellular Ca2+, in consistent with CICR.
During our analysis of CREB activation by caffeine, we routinely observed that 10 mM caffeine invokes a more robust increase in CRE-luciferase than depolarization with high KCl routinely used to assess Ca2+-stimulated CREB activation. This finding suggests that intracellular release of Ca2+ via ryanodine receptors is a more effective mechanism to activate CREB-dependent gene transcription in developing cortical neurons. Interestingly, co-stimulation of neurons with 10 mM caffeine and 50 mM KCl failed to induce increased CRE-luciferase activity compared to depolarization alone and remained lower than caffeine treatment alone. Higher concentrations of caffeine (20–30 mM) blocked activation of CREB by depolarization (Supplementary Figure 1). Application of 20 mM caffeine to hippocampal slices has previously been reported to reduce the amplitude of Ca2+ transients triggered by action potentials [29]. These findings are consistent with a requirement for intracellular release in mediating CREB-dependent changes in gene expression due to neuronal depolarization. An important role for release of Ca2+ from intracellular stores has previously been implicated in CREB-mediated gene transcription due to neuronal synaptic activity. Release of Ca2+ from the ryanodine receptor has been shown to be important for long-term potentiation, the molecular correlate of learning and memory formation [30–31]. Depletion of intracellular stores with cyclopiazonic acid (CPA) or inhibition of ryanodine receptors using inhibitory concentrations of ryanodine, disrupt CREB phosphorylation and activation due to synaptic activity and depolarization [32–33]. Although the inhibition of CREB activity at higher caffeine concentrations is consistent with the loss of RyR signaling, inhibition of inositol 1,4,5 triphosphate receptor opening may also contribute to the observed reduction of CREB activity since high doses of caffeine can also inhibit IP3 receptors in neurons [34–35].
Clinically, caffeine is important for the treatment of premature infants with apnea. Using a CREB-dependent reporter gene and real-time PCR analysis of the endogenous CREB-dependent genes we demonstrate that clinically relevant doses of caffeine (250 – 500 μM) can cooperate with depolarization to enhance CREB function in developing neurons. Increased expression of BDNF and its high affinity receptor TrkB enhances BDNF signaling, thus promoting the survival and maturation of developing neurons. Caffeine mediated enhancement of activity-dependent Bdnf and TrkB gene expression may contribute to the neurodevelopmental benefits observed in infants receiving caffeine treatment. The dose-dependency of Bdnf and TrkB stimulation may also explain the observed trend toward higher doses of caffeine exhibiting greater advantage reported by Steer et al. [36]. Increased BDNF signaling, which promotes neurite outgrowth, may also contribute to increased dendritic length of pyramidal neurons observed in neonatal rats treated with caffeine [37].
Supplementary Material
Cortical neurons transiently transfected with CRE-luciferase were treated with 50 mM KCl in the presence or absence of caffeine as indicated for 5 hours prior to harvesting for luciferase assays. Data shown are average of quadruplicate samples plotted relative to untreated neurons. All samples were normalized using TK Renilla expression. Data shown is the average of triplicates with standard error is indicated.
Acknowledgments
We thank C. Roby for technical assistance with cortical neuron preparations and B. Krueger for valuable discussions. T. K. was funded as a BIRCWH Scholar for the Maryland’s Organized Research Effort in Women’s Health funded by NICHD/ORWH/NIDDK grant K12HD43489. S. Connolly was supported by the National Institute of Health Grant NS058464.
Abbreviations
- BDNF
brain derived neurotrophic factor
- cAMP
3,5-cyclic adenosine monophosphate
- CICR
Ca2+-induced Ca2+ release
- CPA
cyclopiazonic acid
- CRE
cAMP response element
- CREB
cAMP response element binding protein
- DMSO
dimethyl sulfoxide
- IP3
inositol 1,4,5 triphosphate
- PBS
physiologic saline solution
- PCR
polymerase chain reaction
- RyR
ryanodine receptor
- TrkB
tropomyosin-related kinase B
- TK
thymidine kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–3. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 3.Ferre S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–79. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura H. The potential of caffeine for functional modification from cortical synapses to neuron networks in the brain. Curr Neuropharmacol. 2005;3:309–16. doi: 10.2174/157015905774322543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson DK. On the caffeination of prematurity. N Engl J Med. 2007;357:1967–8. doi: 10.1056/NEJMe078200. [DOI] [PubMed] [Google Scholar]
- 6.Bancalari E. Caffeine for apnea of prematurity. N Engl J Med. 2006;354:2179–81. doi: 10.1056/NEJMe068028. [DOI] [PubMed] [Google Scholar]
- 7.Aranda JV, Gorman W, Bergsteinsson H, Gunn T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J Pediatr. 1977;90:467–72. doi: 10.1016/s0022-3476(77)80718-x. [DOI] [PubMed] [Google Scholar]
- 8.Parsons WD, Neims AH. Prolonged half-life of caffeine in healthy tem newborn infants. J Pediatr. 1981;98:640–1. doi: 10.1016/s0022-3476(81)80784-6. [DOI] [PubMed] [Google Scholar]
- 9.Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit. 2008;30:709–16. doi: 10.1097/FTD.0b013e3181898b6f. [DOI] [PubMed] [Google Scholar]
- 10.Lee TC, Charles BG, Steer PA, Flenady VJ. Saliva as a valid alternative to serum in monitoring intravenous caffeine treatment for apnea of prematurity. Ther Drug Monit. 1996;18:288–93. doi: 10.1097/00007691-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Leon AE, Michienzi K, Ma CX, Hutchison AA. Serum caffeine concentrations in preterm neonates. Am J Perinatol. 2007;24:39–47. doi: 10.1055/s-2006-958163. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 13.Davis PG, Schmidt B, Roberts RS, Doyle LW, Asztalos E, Haslam R, Sinha S, Tin W. Caffeine for Apnea of Prematurity trial: benefits may vary in subgroups. J Pediatr. 2010;156:382–7. doi: 10.1016/j.jpeds.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 14.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 15.Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1–14. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingsbury TJ, Murray PD, Bambrick LL, Krueger BK. Ca(2+)-dependent regulation of TrkB expression in neurons. J Biol Chem. 2003;278:40744–48. doi: 10.1074/jbc.M303082200. [DOI] [PubMed] [Google Scholar]
- 17.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–36. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–31. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–9. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 20.Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–82. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 21.Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–40. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 22.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–26. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 23.Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002;277:35920–31. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- 24.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk TJ. Mouse and rat BDNF gene structure and expression revisited. Neurosci Res. 2007;85:525–35. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett HJ, Semba K. Immunohistochemical localization of caffeine-induced c-Fos protein expression in the rat brain. J Comp Neurol. 1998;401:89–108. doi: 10.1002/(sici)1096-9861(19981109)401:1<89::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Deurveilher S, Lo H, Murphy JA, Burns J, Semba K. Differential c-Fos immunoreactivity in arousal-promoting cell groups following systemic administration of caffeine in rats. J Comp Neurol. 2006;498:667–89. doi: 10.1002/cne.21084. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Tripathy A, Pasek DA, Meissner G. Potential for pharmacology of ryanodine receptor/calcium release channels. Ann N Y Acad Sci. 1998;853:130–48. doi: 10.1111/j.1749-6632.1998.tb08262.x. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi N, Xu L, Pasek DA, Evans KE, Chen SR, Meissner G. Calmodulin regulation and identification of calmodulin binding region of type-3 ryanodine receptor calcium release channel. Biochemistry. 2005;44:15074–15081. doi: 10.1021/bi051251t. [DOI] [PubMed] [Google Scholar]
- 29.Sandler VM, Barbara JG. Calcium-induced calcium release contributes to action potential-evoked calcium transients in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:4325–36. doi: 10.1523/JNEUROSCI.19-11-04325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose CR, Konnerth A. Stores not just for storage. intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 31.Lu YF, Hawkins RD. Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol. 2002;88:1270–8. doi: 10.1152/jn.2002.88.3.1270. [DOI] [PubMed] [Google Scholar]
- 32.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–7. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco MA, Jaimovich E, Kemmerling U, Hidalgo C. Signal transduction and gene expression regulated by calcium release from internal stores in excitable cells. Biol Res. 2004;37:701–12. doi: 10.4067/s0716-97602004000400028. [DOI] [PubMed] [Google Scholar]
- 34.Brown GR, Sayers LG, Kirk CJ, Michell RH, Michelangeli F. The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem J. 1992;282( Pt 2):309–12. doi: 10.1042/bj2820309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang SS, Han KS, Ku BM, Lee YK, Hong J, Shin HY, Almonte AG, Woo DH, Brat DJ, Hwang EM, Yoo SH, Chung CK, Park SH, Paek SH, Roh EJ, Lee SJ, Park JY, Traynelis SF, Lee CJ. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010;70:1173–1183. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steer P, Flenady V, Shearman A, Charles B, Gray PH, Henderson-Smart D, Bury G, Fraser S, Hegarty J, Rogers Y, Reid S, Horton L, Charlton M, Jacklin R, Walsh A. High dose caffeine citrate for extubation of preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2004;89:F499–503. doi: 10.1136/adc.2002.023432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juárez-Méndez S, Carretero R, Martínez-Tellez R, Silva-Gómez AB, Flores G. Neonatal caffeine administration causes a permanent increase in the dendritic length of prefrontal cortical neurons of rats. Synapse. 2006;60:450–5. doi: 10.1002/syn.20318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cortical neurons transiently transfected with CRE-luciferase were treated with 50 mM KCl in the presence or absence of caffeine as indicated for 5 hours prior to harvesting for luciferase assays. Data shown are average of quadruplicate samples plotted relative to untreated neurons. All samples were normalized using TK Renilla expression. Data shown is the average of triplicates with standard error is indicated.


