Abstract
Introduction and hypothesis
Two-dimensional magnetic resonance imaging (MRI) demonstrates apical support and vaginal length contribute to anterior wall prolapse (AWP). This paper describes a novel three-dimensional technique to examine the vagina and its relationship to pelvic sidewalls at rest and Valsalva.
Methods
Twenty women (10 with AWP and 10 with normal support) underwent pelvic magnetic resonance imaging at rest and Valsalva. Three-dimensional reconstructions of the pelvic bones and anterior vaginal wall were created to assess morphologic changes occurring in prolapse.
Results
In women with AWP, Valsalva caused downward translation of the vagina along its length. A transition point separated a proximal region supported by levator muscles and a distal, unsupported region no longer in contact with the perineal body. In this latter region, sagittal and frontal plane “cupping” occurs. The distal vagina rotated inferiorly along an arc centered on the inferior pubis.
Conclusion
Downward translation, cupping, and distal rotation are three novel characteristics of AWP demonstrated by this three-dimensional technique.
Keywords: Anterior vaginal wall, Prolapse, Pelvic organ prolapse, Cystocele, Paravaginal defect
Introduction
Pelvic floor dysfunction, severe enough to result in surgical repair, affects 11% of women and is anticipated to increase in prevalence with our aging population [1-3]. The anterior vaginal wall is both the most common site of pelvic organ prolapse, with 81% of surgical repairs involving the anterior wall, [1] and the most frequent site of operative failure, with rates in the literature as high as 41% [4-9].
The contributions of apical support and vaginal length to cystocele size have been reported previously [10-12]; however, there has long been a debate concerning the occurrence and importance of midline versus paravaginal defects. Intraoperative evaluation of patients undergoing surgery for anterior vaginal wall prolapse indicated that there may indeed be a separation of the vagina from the lateral sidewall [13]. So far data have been limited to anatomical studies and two-dimensional analysis of the mechanics of cystocele formation. Objective information is therefore lacking on the role played by paravaginal defects between the vagina and arcus tendineus fascia pelvis (ATFP) and in the degree of transverse vaginal stretching.
In this paper, we develop a novel technique to allow three-dimensional (3-D) modeling of vaginal morphology at maximal Valsalva, specifically extending to the lateral fornices. The goals were to determine the 3-D relationship of the vagina to the bony pelvis both at rest and maximal strain in women with prolapse and to report on preliminary findings.
Materials and methods
Magnetic resonance imaging (MRI) scans from 20 women (10 cases and 10 controls) were selected from an ongoing University of Michigan institutional review board-approved (IRB #1999-0395) case-control study of pelvic organ prolapse. All women in the control group were asymptomatic based on Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaires, had negative full-bladder stress tests, had not had previous surgery for pelvic floor disorders, and did not have prolapse beyond the hymen. All cases were symptomatic, had not undergone previous surgery for pelvic floor disorders, and had a Ba pelvic organ prolapse quantification (POP-Q) value at least 1 cm beyond the hymenal ring on clinical examination. Selected subjects had cystocele-predominant prolapse; women in whom the cervix was the leading point of prolapse were excluded. None of the subjects had previously undergone hysterectomy. These 20 subjects all had magnetic resonance (MR) images of the pelvis that adequately demonstrated the full extent of their prolapse on dynamic MRI, allowing visualization of the changes in the anterior vaginal wall at Valsalva.
As described in our previous studies [14], each woman underwent supine MRI both at rest and during maximal Valsalva using a 3-Telsa Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands) with a six-channel, phased-array coil. Ultrasound gel was placed in the vagina to outline its contour. For standard anatomical scans made at rest, turbo spin echo (TSE) techniques were used to image the sagittal, coronal, and axial planes. At rest, 30 images were obtained in each plane (repetition time [TR] range, 2,300–3,000 ms, echo time [TE] 30 ms, 4-mm slice thickness, 1-mm gap, number of signal averages—NSA 2, 256×255 voxels). Subjects then performed a Valsalva maneuver which they held for approximately 20 s to obtain images of the pelvis with the prolapse protruding maximally. With the prolapse protruding, 14 images were serially obtained from one ischial spine to the other in sagittal plane (TR range 1,249–1253 ms, TE 80 ms; 6-mm slice thickness, 1-mm gap, SENSE factor 4, NSA 2, 320×178 voxels). Similar sequences were obtained in the axial and coronal planes, also with the prolapse at maximal extent. A research associate with the POP-Q data from each subject’s clinical examination was present during MRI to assure that the prolapse reached the same size that had been previously identified in the clinic. If the prolapse did not reach the same magnitude as had been observed on clinical examination, the MR study was repeated with additional coaching to extrude the prolapse to its fullest extent.
Computer models of the midsagittal bony pelvis, ischial spines, anterior vaginal wall, and uterovaginal junction were made using the 3-D Slicer software program (version 2.1b1; Brigham and Women’s Hospital, Boston, MA). The original axial, sagittal, and coronal Digital Imaging and Communications in Medicine (DICOM) static images were aligned using bony structures, ensuring that the bony structures colocalized in all three axes by simultaneous review of 3-D scan planes in the viewer. Satisfactory alignment was possible in all 20 scans for pelvic bones. Because of the variable deformation of soft tissue with each Valsalva, alignment of viscera in different planes is not possible. The following 3-D models were made using manual tracings of structures on MR images performed by a urogynecology fellow with supervision by the senior author: the midsagittal pelvic bones (sagittal images), ischial spines (axial images), anterior vaginal wall (sagittal images), and uterovaginal junction (sagittal images) by tracing the structure outlines and creating 3-D models from these outlines (Fig. 1a–c) Again, because of the inability to align viscera from different planes, all viscera were modeled on sagittal images to allow for best characterization of the vaginal wall. To accurately visualize the borders of the anterior vaginal wall and to decrease “lofting” errors introduced by the computer when rendering a solid object out of the originally traced outlines, minimal smoothing was used when generating the models. The efforts to decrease lofting result in the “strip-like” appearance of the anterior vaginal wall model (Fig. 1c).
Fig. 1.
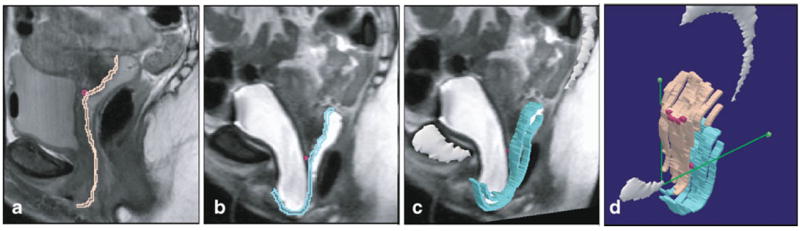
Anterior vaginal wall models at rest and with Valsalva. a Midsagittal MR slice with subsequent outline of vaginal wall at rest (pink) and with Valsalva (turquoise, panel b). Uterovaginal junction shown with dark pink square. c Addition of midsagittal pelvic bones (white) and anterior vaginal wall model. d Powerpoint image of both resting and straining anterior vaginal wall models and their relationship to the normalized ATFP, shown here as green line extending from the pubic symphysis to the ischial spines (green square), or the P-IS line. © DeLancey 2009
To analyze the deformation of the anterior vaginal wall under load and compare it with the resting vagina, 3-D models of the midsagittal pelvic bones (the pubic symphysis and sacrum) were constructed from the sagittal maximal Valsalva images and then aligned with the pelvic bones of the resting model. This identified the translational coordinates for the sagittal maximal Valsalva images such that subsequently constructed 3-D anterior vaginal wall, and uterovaginal junction models could be aligned with previously created resting models.
The resting and maximal Valsalva 3-D reconstructions were then imported into Microsoft Powerpoint. A line from the insertion of the arcus tendineus fascia pelvis (ATFP) on the pubis to the ipsilateral ischial spine (P-IS) was constructed to represent the approximate location of the ATFP and assess morphologic changes occurring in prolapse (Fig. 1d) . The reconstructions were then subjectively compared among the case and control groups.
Results
The mean age of the 20 study participants was 63±13 (SD) years for the control population and 56±7 years for those with prolapse (p=0.17). The mean body mass index (BMI) was 25.2±4.5 and 27.2±4.4 kg/m2 for controls and cases, respectively (p=0.32), while median parity was 2.5 and 2. All the control subjects and 80% of the women with prolapse were white. No subjects in either group had undergone a hysterectomy. Pelvic organ prolapse quantification (POP-Q) points for both controls and cases were statistically different between groups as expected: “Aa” -1.7±0.9 versus 1.5±1.0, “Ba” -1.6±1.0 versus 2.2±1.6, “C” -6.0±1.1 versus -3.2± 1.6, and “D” -8.9±1.1 versus -6.5±1.1, in controls versus cases, respectively.
With Valsalva, the vaginal apex descended in women both with and without prolapse, although was more pronounced in women with prolapse (Fig. 2). In addition to the more dramatic apical descent, in women with prolapse, the vagina undergoes several other morphologic changes. Their models demonstrate downward movement of the vagina throughout the length of the anterior vaginal wall, increasing the vertical distance between the lateral sulcus and P-IS line. Throughout the remainder of this manuscript, this movement is referred to as downward translation. This descent allows the lower vagina to slide below the introitus where it is no longer in contact with the perineal body, thereby revealing two distinct regions of the anterior wall: a supported region and an unsupported region separated by a transition point located at the distal end of the perineal body (Fig. 3). The supported region of the anterior wall remains flat, while the distal unsupported region shows evidence of increased transverse stretching, or vaginal “cupping” (Figs. 4 and 5). In addition, the most distal portion of the vagina does not remain fixed during Valsalva, but instead “pivots” or rotates downward along an arc in the sagittal plane centered on the inferior pubis (Fig. 2). Table 1 compares the frequency of these morphologic findings among our cases and controls.
Fig. 2.
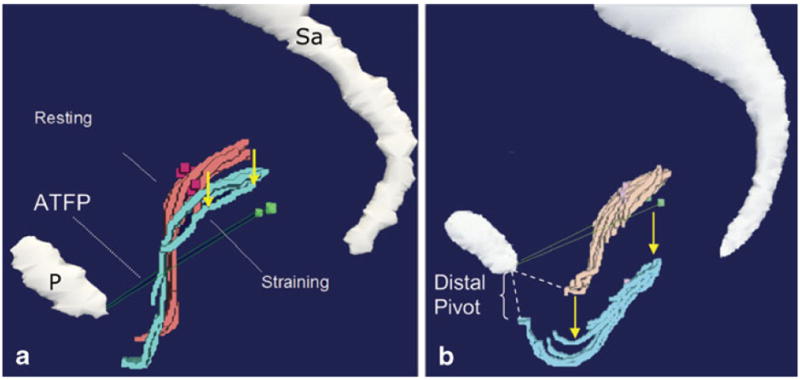
3-D model of control (a) versus case (b). Resting (pink) and straining (turquoise) cross-sections of the vagina and ATFP (green). This shows descent along the length of the vagina with Valsalva as well as the distal pivot seen in cases (b) in comparison with a representative control (a). P pubic symphysis , Sa sacrum. © DeLancey 2009
Fig. 3.
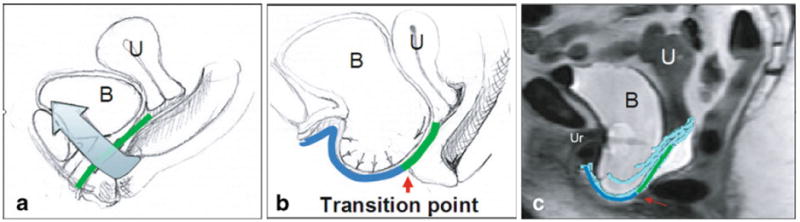
Midsagittal view of pelvis with normal support (a) and with prolapse (b). Green represents area where levators (blue arrow) provide cranial reaction forces to counteract the action of intra-abdominal pressure and caudal movement of the anterior wall. The red arrow delineates the transition to unsupported region lacking this opposing set of reaction forces (blue line), thereby creating a pressure differential acting caudally on that region. c Red arrow illustrates this point on midsagittal MRI with modeled anterior vaginal wall. U uterus, B bladder, Ur urethra. © DeLancey 2009
Fig. 4.
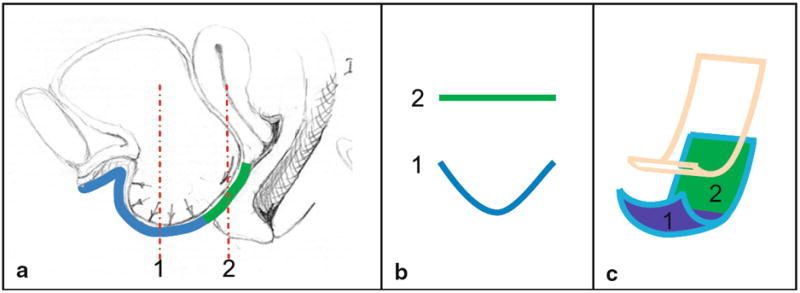
Vaginal “cupping.” a Midsagittal section with prolapse illustrating two slices through the vaginal wall (red). b Frontal plane of slices in a, illustrating the flat supported and cupped, unsupported vagina. c Oblique view of resting (pink) and straining (turquoise) vagina showing the two regions. © DeLancey 2009
Fig. 5.
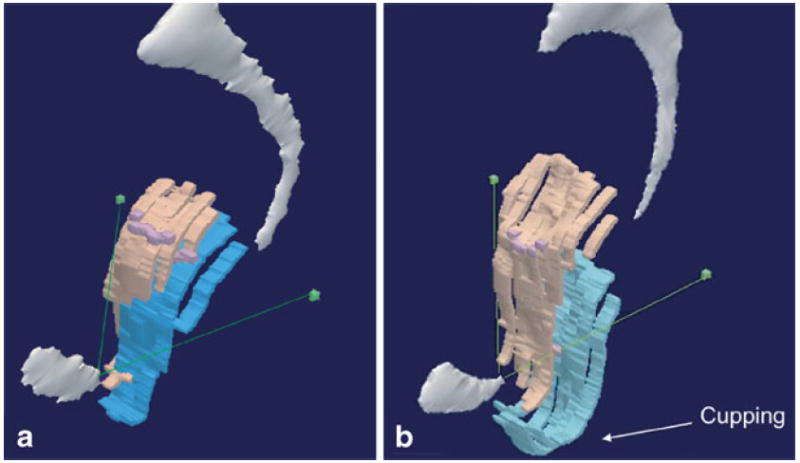
Vaginal “cupping” in case versus normal support. a Oblique 3-D model in women with normal support contrasted with b oblique 3-D model illustrating cupping of the (turquoise) vagina during straining. The cupping occurs in both the sagittal and frontal planes. © DeLancey 2009
Table 1.
Frequency of morphologic observations within cases and controls
| Cases | Controls | P | |
|---|---|---|---|
| Characteristics | (n=10) | (n=10) | (Fisher’s exact) |
| Downward translationa | 8 (80) | 2 (20) | 0.02 |
| Cuppinga | 9 (90) | 1 (10) | 0.001 |
| Distal pivota | 7 (70) | 2 (20) | 0.07 |
Data are n (%)
Discussion
This study describes the development and early observations of a 3-D modeling technique that compares the vagina’s morphologic changes when intra-abdominal pressure increases from rest to maximal Valsalva in women both with and without pelvic organ prolapse. The technique allows one to visualize changes along the full width of the anterior vagina, extending to the lateral sulci, and enables descriptions of its altered morphology in women with prolapse. Preliminary results from our pilot subjects suggest that in addition to greater apical descent when compared with controls, in women with prolapse, the anterior vaginal wall undergoes a characteristic dynamic behavior when intra-abdominal pressure is increased from resting to maximal Valsalva: downward translation increasing the distance relative to the P-IS line, vaginal cupping in the unsupported region of the anterior vaginal wall, and pivoting of the distal vagina around the inferior pubis.
The data presented in this study support published data regarding the role of apical support and cystocele formation by illustrating more significant descent of the apex in women with anterior vaginal wall prolapse [10-12, 15]. Again, in our study, some apical descent was seen in both study populations, but was more dramatic in the women with prolapse. Rooney et al. [11] reported a strong correlation between the apex and most prolapsed portion of the anterior vaginal wall, with a Spearman’s correlation coefficient of 0.835. Summers et al. [12] found that apical support is associated with half of the variation in cystocele severity. When Hsu et al. [10] factored both apical descent and midsagittal vaginal length into a linear regression model to explain the variance in cystocele size, 77% of the variation was associated with these factors. This suggests that other factors could be contributing to the variance, inviting further discovery into the mechanism(s) behind the development of cystocele.
Findings from the present study extend what literature tells us about the cystocele by providing a more detailed picture of vaginal displacement under a volitional increase in intra-abdominal pressure. Specifically, it allows the relationship between the lateral margin of the anterior vaginal wall and the normal position of the ATFP to be seen. In addition to confirming earlier observations concerning the role of apical descent in anterior vaginal wall prolapse, there were some unexpected findings. Our findings do not support our earlier concept of a distally hinged trapezoidal support of pubocervical fascia, fixed at the pubis, such that loss of apical support leads to it “hinging” downward like a trap door to form a cystocele [13]. Instead, our observations suggest that there is caudal translation along the length of the vagina with a characteristic sagittal plane rotational movement of the most distal region of the vagina. This movement causes the anterior vaginal wall to project beyond the support of the levator ani muscles and perineal body where it is unsupported by the posterior vaginal wall. In this position, it is exposed to a pressure differential between intra-abdominal pressure on its cranial surface and (lower) atmospheric pressure on its caudal surface, producing a new imbalance in forces. This imbalance results in increased load on the anterior vaginal wall, likely manifested as the “cupping” seen in the 3-D MRI-based models. Simulated vaginal deformation with Chen’s biomechanical 2-D and 3-D models with loss of apical support and impairment of the pubovisceral muscle suggests similar deformation of the distal vaginal wall [15, 16]. These models, however, do not yet incorporate the movement of the distal vaginal point that was observed in the present study.
A reasonable question is whether a paravaginal defect is a contributing factor to, or the result of, the downward movement of the vagina and subsequent misalignment? These pilot models illustrate downward translation along the length of the vagina, increasing the vertical distance to the P-IS line. This alters the direction of forces acting along the lateral vagina, suggesting changes in paravaginal support, but the determination of causality is not possible from the present data. In an earlier study, 95% to 97% of subjects had separation of the ATFP from the ischial spine, while only 80% had lower and 62% had upper anterior vaginal wall prolapse to or beyond the hymen [13]. We do not have intraoperative data from normal individuals to know the position of their ATFP; however, this result suggests that separation of the ATFP is not causal, at least in some individuals. By using the noninvasive technique described in the present study, women with normal support can be directly compared with women with cystocele, something not feasible in the operating room. In addition, any operative observations involve significant distortion of the normal structural relationships. For example, to visualize the space of Retzius, the bladder must be pulled away from the pubic bone and held in place in an abnormal position, providing a distorted view of this anatomy. Thus, the current technique allows us to make observations that are closer to reality than has previously been possible.
The clinical implications of this research concern the ability of this type of imaging to objectively capture both the full length and width of the vagina in relationship to landmarks on the pelvic sidewalls. Current surgical approaches are based upon hypotheses regarding what defect has occurred (midline, paravaginal, apical support loss), yet reliable and reproducible assessments of these defects have not been possible. In the future, based on this technique for displaying the anterior vaginal wall at maximal Valsalva, we believe it will be possible to develop measurements of vaginal widening expected from midline defects and the degree of paravaginal and apical displacements present. Such a test would allow the status of each of these defects to be quantified and surgical correction to be based on the findings. We are not suggesting that the MRI become a routine part of preoperative evaluation at present. However, we do expect that this direction of research will produce data concerning surgical selection and existing preoperative anatomy to better understand what occurs in prolapse surgery failure. This should allow the treatment approach to be better tailored to address the needs of each patient.
Several factors must be considered when interpreting the results of this study. Supine MR images were obtained, which may limit the descent of pelvic floor, although earlier studies do not document differences when compared with images obtained in the seated position in open scanners [17, 18]. Also, clinicians do make the majority of their clinical decisions based on examinations performed in women who are supine. Because the sulci are difficult to differentiate on MR images, vaginal gel was used to illuminate the lateral extent of the vagina. Although it significantly improved visualization, this gel does result in some degree distortion of the vaginal shape by filling the vagina. We suspect that this effect is minimal, as the gel’s viscosity was such that Valsalva efforts seemed to result in expulsion of gel, versus a redistribution, which could distort the vaginal wall.
Additionally, there are limitations with our study population. Our sample size included only 20 women. This study seeks to address the fundamental and common features that are found in typical cystocele formation. There is great variability in anterior vaginal wall prolapse, and subsequent research with larger samples will be needed to further define all of the variation. This current research, by pioneering a technique that allows 3-D visualization in living women with cystocele at maximal Valsalva, provides a start on this process even with the smaller sample size. Also, in selecting our cases, we chose women whose anterior prolapse was the largest element. Women in whom the uterine cervix was the leading edge were not included. Cystoceles seen with severe uterine prolapse may behave differently and likely require further investigation.
Lastly, there is an active debate and competing definitions concerning what is “normal” support. In this study, we have chosen a definition consistent with suggestions of Barber et al. [19]. Controls were required to meet two criteria: (1) all POP-Q points had to be above the hymen on examination and (2) were asymptomatic based on responses on validated questionnaires to assess pelvic floor symptoms. In addition, our own survey across southeastern Michigan as well as a six-center survey identified that large proportions of the female population fall into this “stage II” category (67% and 40%, respectively) [20, 21]. The latter of these two studies found that only a percentage of these women were symptomatic [21].
The present technique to create 3-D models of the vagina and pelvis both at rest and maximal Valsalva from MR images greatly enhances our ability to study the mechanisms of pelvic floor prolapse. Preliminary findings suggest additional factors that could contribute to cystocele size and also illuminate a method to study the role of the paravaginal defect. Further efforts with a larger number of subjects and quantification of the differences in movements between women with and without prolapse seem warranted and should yield insights into the mechanisms of anterior wall pelvic organ prolapse, expanding on the initial findings of this pilot study and potentially providing the ability to better tailor our surgical procedures.
Acknowledgments
We gratefully acknowledge support from the National Institute of Child Health and Human Development Grants R01 HD 38665 with additional investigator support from the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health 1 P50 HD044406.
Footnotes
Presented at the 35th Annual Society for Gynecologic Surgeons, New Orleans, LA, March 2009.
Conflicts of interest Dr. John OL DeLancey and Dr. James Ashton-Miller are consultants to American Medical Systems and Johnson and Johnson Personal Products. The other authors have no disclosures to report.
Contributor Information
Kindra A. Larson, Pelvic Floor Research Group, University of Michigan, Ann Arbor, MI, USA Department of Obstetrics and Gynecology, Division of Gynecology, University of Michigan, Ann Arbor, MI, USA; Women’s Hospital L4100, 1500 East Medical Center Dr., Ann Arbor, MI 48109-0276, USA, kindral@med.umich.edu.
Yvonne Hsu, Pelvic Floor Research Group, University of Michigan, Ann Arbor, MI, USA; Department of Obstetrics and Gynecology, Division of Gynecology, University of Michigan, Ann Arbor, MI, USA.
Luyun Chen, Pelvic Floor Research Group, University of Michigan, Ann Arbor, MI, USA; Departments of Mechanical and Biomechanical Engineering, University of Michigan, Ann Arbor, MI, USA.
James A. Ashton-Miller, Pelvic Floor Research Group, University of Michigan, Ann Arbor, MI, USA Departments of Mechanical and Biomechanical Engineering, University of Michigan, Ann Arbor, MI, USA.
John O. L. DeLancey, Pelvic Floor Research Group, University of Michigan, Ann Arbor, MI, USA Department of Obstetrics and Gynecology, Division of Gynecology, University of Michigan, Ann Arbor, MI, USA.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 3.He W, Sengupta M, Velkoff V, DeBarros K. 65± in the US: 2005, current population report, special studies. United States Government Printing Office; Washington DC: 2005. Dec, Available from: www.census.gov. [Google Scholar]
- 4.Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175(6):1418–1421. doi: 10.1016/s0002-9378(96)70084-4. discussion 1421–1422. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891–898. doi: 10.1097/AOG.0b013e31816a2489. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen JK. Current concepts in the diagnosis and surgical repair of anterior vaginal prolapse due to paravaginal defects. Obstet Gynecol Surv. 2001;56(4):239–246. doi: 10.1097/00006254-200104000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Maher C, Baessler K. Surgical management of anterior vaginal wall prolapse: an evidencebased literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(2):195–201. doi: 10.1007/s00192-005-1296-3. [DOI] [PubMed] [Google Scholar]
- 8.Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol. 2001;98(1):40–44. doi: 10.1016/s0029-7844(01)01378-3. [DOI] [PubMed] [Google Scholar]
- 9.Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–1373. doi: 10.1067/mob.2000.110910. discussion 1373–1374. [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–142. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195(6):1837–1840. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 12.Summers A, Winkel LA, Hussain HK, DeLancey JO. The relationship between anterior and apical compartment support. Am J Obstet Gynecol. 2006;194(5):1438–1443. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187(1):93–98. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 14.Larson KA, Hsu Y, DeLancey JO. The relationship between superior attachment points for anterior wall mesh operations and the upper vagina using a 3-dimensional magnetic resonance model in women with normal support. Am J Obstet Gynecol. 2009;200(5):554.e1–554.e6. doi: 10.1016/j.ajog.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Ashton-Miller JA, Hsu Y, DeLancey JO. Interaction among apical support, levator ani impairment, and anterior vaginal wall prolapse. Obstet Gynecol. 2006;108(2):324–332. doi: 10.1097/01.AOG.0000227786.69257.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Ashton-Miller JA, Delancey JO. A 3D finite element model of anterior vaginal wall support to evaluate mechanisms underlying cystocele formation. J Biomech. 2009;42:1371–1277. doi: 10.1016/j.jbiomech.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223(2):501–508. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
- 18.Fielding JR, Griffiths DJ, Versi E, Mulkern RV, Lee ML, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. AJR Am J Roentgenol. 1998;171(6):1607–1610. doi: 10.2214/ajr.171.6.9843296. [DOI] [PubMed] [Google Scholar]
- 19.Barber MD, Brubaker L, Nygaard I, Wheeler TL, 2nd, Schaffer J, Chen Z, Spino C. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol. 2009;114(3):600–609. doi: 10.1097/AOG.0b013e3181b2b1ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trowbridge ER, Fultz NH, Patel DA, DeLancey JO, Fenner DE. Distribution of pelvic organ support measures in a population-based sample of middle-aged, community-dwelling African American and white women in southeastern Michigan. Am J Obstet Gynecol. 2008;198(5):548.e1–6. doi: 10.1016/j.ajog.2008.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swift S, Woodman P, O’Boyle A, Kahn M, Valley M, Bland D, Wang W, Schaffer J. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192(3):795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]


