Abstract
Introduction
The amount of myocardial perfusion required for successful defibrillation after prolonged cardiac arrest is not known. Coronary perfusion pressure (CPP) is a surrogate for myocardial perfusion. One limited clinical study reported that a threshold of 15 mmHg was necessary for return of spontaneous circulation (ROSC), and that CPP was predictive of ROSC. A distinction between threshold and dose of CPP has not been reported.
Objective
To test the hypothesis that swine achieving ROSC will have higher preshock mean CPP and higher preshock area under the CPP curve (AUC) than swine not attaining ROSC.
Methods
Data from four similar swine cardiac arrest studies were retrospectively pooled. Animals had undergone 8–11 minutes of untreated ventricular fibrillation, 2 minutes of mechanical cardiopulmonary resuscitation (CPR), administration of drugs, and 3 more minutes of CPR prior to the first shock. Mean CPP ± standard error of the mean (SEM) was derived from the last 20 compressions of each 30-second epoch of CPR and compared between ROSC/no-ROSC groups by repeated-measures analysis of variance (RM-ANOVA). AUC for all compressions delivered over the 5 minutes was calculated by direct summation and compared by Kruskal-Wallis test. Prediction of ROSC was assessed by logistic regression.
Results
Throughout the first 5 minutes of CPR (n = 80), mean CPP ± SEM was consistently higher in animals with ROSC (n = 63) (maximum CPP 41.2 ± 0.6 mmHg) than animals with no ROSC (maximum CPP 20.1 ± 0.3 mmHg) (p = 0.0001). Animals with ROSC received more total reperfusion (43.9 ± 17.6 mmHg × 102) than animals without ROSC (21.4 ± 13.7 mmHg × 102) (p < 0.001). Two regression models identified CPP (odds ratio [OR] 1.11; 95% confidence interval [CI] 1.05, 1.18) and AUC (OR 1.10; 95% CI 1.05, 1.16) as predictors of ROSC. Experimental study also predicted ROSC in each model (OR 1.70; 95% CI 1.15, 2.50; and OR 1.59; 95% CI 1.12, 2.25, respectively).
Conclusion
Higher CPP threshold and dose are associated with and predictive of ROSC.
Keywords: cardiopulmonary resuscitation, CPR, resuscitation, heart arrest, animal model, swine
Introduction
After more than 3–4 minutes of cardiac arrest, myocardial reperfusion is necessary prior to defibrillation to achieve successful resuscitation.1-4 Prior animal and human studies demonstrate that chest compressions and/or vasoactive medications are needed to achieve adequate myocardial reperfusion, as indicated by a coronary perfusion pressure (CPP) of at least 15–25 mmHg.5-14 However, published animal studies6-13 simulate brief untreated arrest duration (0-5 minutes) that rarely extends beyond the first phase of cardiac arrest.15 As such, these animal data do not reflect the most current model of out-of-hospital cardiac arrest with an untreated duration of 10 minutes followed by resuscitation.16-23 The foremost human data published in 1990 by Paradis et al. describe a necessary CPP of 15 mmHg for return of spontaneous circulation (ROSC) in out-of-hospital cardiac arrest (OHCA).14 By nature of the steps needed for data collection, these were gathered very late during resuscitation (8–42 minutes after loss of pulses) and do not reflect the state of the myocardium during early therapies. As the understanding and modeling of time intervals in OHCA evolve to reflect the most recent literature, it is necessary to reexamine CPP required for ROSC.
We retrospectively analyzed four experiments using our porcine model of ventricular fibrillation (VF) OHCA to reevaluate the necessary threshold CPP for ROSC. We also sought to determine whether calculating the total “dose” of myocardial perfusion pressure as measured by the area under the CPP curve would predict ROSC. We hypothesized that higher CPP than previously reported is required for ROSC in our swine model of prolonged cardiac arrest.
Materials and Methods
Each of the studies analyzed was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The care and handling of the animals were in accord with National Institutes of Health (NIH) guidelines. We conducted a retrospective analysis of four experiments performed in our laboratory using mixed-breed domestic swine of either gender.24-27 Animals that received cardiopulmonary resuscitation (CPR) and medications prior to the first rescue shock were included in the analyses.
Experimental Protocol
Swine were between 12 and 16 weeks of age, and our supplier has remained the same during the course of these experiments. Sedation and anesthesia to a surgical plane were achieved using intramuscular ketamine (10.0 mg/kg) and xylazine (4.0 mg/kg) and intravenous alpha-chloralose (50 mg/kg bolus) followed by continuous infusion (40 mg/kg/h). After induction of a surgical plane of anesthesia, paralysis was induced with a 4-mg bolus of pancuronium, with additional 2-mg boluses administered as needed to maintain paralysis. Anesthesia was maintained throughout the instrumentation and VF induction periods with a continuous infusion of alpha-chloralose.
Swine were orally intubated with a 5-0 cuffed endotracheal tube. Animals were ventilated by a volume-cycled ventilator (Harvard Apparatus, South Natick, MA) with room air (tidal volume of 15–20 mL/kg, ventilatory rate of 12 breaths/min and an inspiration- to-expiration ratio of 40%). Ventilation rate and tidal volume were adjusted to maintain an end-tidal carbon dioxide (CO2) value between 35 and 45 mmHg measured by sidestream capnometry. Three limb-lead electrodes were secured in place to correspond to a standard lead II electrocardiogram (ECG), which was monitored continuously throughout the experiments (LifePak 12 Monitor-defibrillator, Medtronic Emergency Response Systems, Redmond, WA).
Arterial and venous transducers were inserted via right femoral cutdown. The arterial transducer (Mikro-Tip transducer model SPC 3705, Millar Instruments, Houston, TX) was advanced into the ascending aorta and the venous transducer was inserted into the right atrium. Correct positioning of the catheters was confirmed by interpretation of the pressure tracings. Heart rate and arterial and venous pressure were monitored continuously throughout the remaining procedures. All data were acquired digitally at a sampling rate of 1,000 points/sec with a commercially available software package (Chart, AD Instruments, Castle Hill, Australia).
Arterial blood gases were obtained after arterial access was established, at any time ventilator settings were changed, and just prior to the induction of VF (Portable Clinical Analyze, I-Stat, Heska Corporation, Waukesha, WI). Anesthesia time was defined as the time from the initial bolus of alpha-chloralose until the time VF was induced. The interval of anesthesia was standardized by initiating VF as close to 40 minutes of anesthesia time as possible. In all studies we induced VF by delivering a 3-second, 60-Hz, 100-mA alternating current (AC) externally across the thorax. VF was left untreated for 8–11 minutes depending on the experimental study.
Chest compressions were delivered manually, mechanically, via high-impulse, or continuously. Manual compressions were delivered by one investigator (JJM), using the sound of the mechanical device as a metronome. Mechanical chest compressions were applied using an oxygen-driven resuscitation device (Thumper, Michigan Instruments, Grand Rapids, MI). High-impulse compressions were accomplished using a high-impulse Thumper (Model 100/, Michigan Instruments). Continuous compressions with intermittent ventilations were performed using a LUCAS device (Jolife, Lund, Sweden). All compressions were performed in an anterior-posterior direction at a rate of 100 per minute with a compression depth of 1.5 inches and a duty cycle of 50%. Compression-to-ventilation ratios of 5:1, 15:1, or 30:1 were dictated by experimental protocol. Ventilation was performed with 100% fractional concentration of oxygen in inspired gas (FiO2) for all experiments at a tidal volume of approximately 400 mL.
Cardiopulmonary resuscitation was performed for 5 minutes before delivery of the first rescue shock. All animals received drugs after 2 minutes of compressions. All animals received high-dose epinephrine (0.1 mg/kg), propranolol (1 mg), vasopressin (40 IU), and bicarbonate (1 mEq/kg) as an initial drug cocktail. Thereafter, the animals may have received additional doses of epinephrine (0.015 mg/kg) every 3 minutes as the resuscitation continued and pulses were not restored. If pulses were restored, sodium bicarbonate was administered for acidemia as necessary. Dosing of sodium bicarbonate was based on an arterial blood gas (NaHCO3 = 0.3 mEq × weight in kg × base deficit).
We used an impedance-compensating, truncated exponential biphasic defibrillation waveform (LifePak 12, 3-D, Medtronic Physio-Control) with a fixed energy dose of 150 J for all rescue shocks. All countershocks were delivered by one investigator (JJM) to eliminate interuser variability.
Return of spontaneous circulation was defined as an organized electrical rhythm with a systolic blood pressure of at least 80 mmHg for at least 1 minute continuously at any time during the resuscitation effort. We considered 1 minute of sustained ROSC to be an “electrophysiologic success after defibrillation,” whereas more prolonged maintenance of pulses can depend on many other variables. After a period of short-term survival (20–120 minutes), the animal was euthanized with 40 mEq of potassium chloride. Resuscitation efforts consisting of continued CPR, further rescue shocks, standard dose epinephrine (0.015 mg/kg), and sodium bicarbonate were continued for 20 minutes in those animals that did not experience ROSC. Twenty minutes of failed resuscitation indicated no ROSC.
Coronary Perfusion Pressure Calculation
Coronary perfusion pressure was defined as aortic diastolic pressure minus right atrial diastolic pressure and was determined at the time point immediately prior to the compression upsurge in pressure (ie: end-relaxation). Using our data acquisition software, we devised a simple algorithm to record CPP measurements based on time expired between compressions. Random pressure tracings were also validated manually to confirm the accuracy in detection (DDS). CPP measurements were extracted from the electronic data into a spreadsheet. Mean CPP ± standard error of the mean (SEM) was then calculated for 20 chest compressions before each 30-second time epoch. The pressures generated by compressions were summed to derive an area under the curve (AUC) as an estimate of total perfusion prior to the first rescue shock (Fig. 1).
Figure 1.
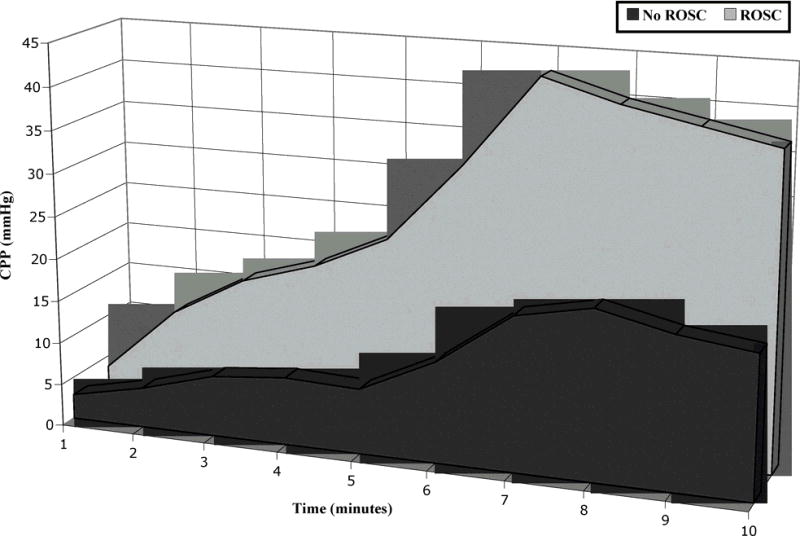
Derivation of the area under the curve. Successive coronary perfusion pressures (CPPs) generated by chest compressions were summed to derive an area under the curve as an estimate of total measured perfusion. ROSC = return of spontaneous circulation.
Data Analysis
Mean CPP ± SEM, experimental study, resuscitation protocol, gender, weight, CPR ratio (compressions to ventilations), preresuscitation anesthesia duration, VF duration, CPR duration, ROSC, and survival were abstracted from the experimental records. We used Microsoft Excel 2007 (Microsoft Corp., Redmond, WA) and STATA (StataCorp LP, College Station, TX) to record and analyze the data. CPP was plotted against time in 30-second epochs for animals with and without ROSC that received 5 minutes of CPR. Continuous variables were compared with repeated-measures analysis of variance (RM-ANOVA). A trend line was created for the CPP measurements from drug delivery to the peak CPP to quantify physiologic response to exogenous medications. We constructed multivariate logistic regression models to assess the relationship between ROSC and key predictors. Univariate logistic regression was used to screen predictors with a p < 0.1 threshold for entrance into the multivariate models. Candidate predictors included gender, weight, duration of instrumentation, duration of untreated VF, ratio of compressions to ventilations, duration of CPR prior to the first rescue shock, experimental study, the maximum CPP achieved (maxCPP), and the total dose of CPP as measured by calculating the AUC. Experimental study was used to account for variation in the method of chest compressions, drug cocktail used, survival time, and year. An alpha of 0.05 was used for RM-ANOVA and the multivariate logistic regression.
Results
A total of 80 animals were included in the analyses from four previous studies. Animals received 5 minutes of compressions before the first rescue shock and drugs were delivered after the first 2 minutes of compressions. See Table 1 for a further summary of animal and experimental characteristics.
Table 1.
Data from the Individual Studies Reviewed*
| Experimental Study (n) [Reference]† |
Gender–% Male | Weight (kg) | CPR Ratio | Anesthesia Time (min) |
VF Duration§ (min) |
CPR Duration (min) |
% ROSC |
|---|---|---|---|---|---|---|---|
| 1 (n = 35) [17] | 31.4 | 26.1 (2.2) | 15:1 | 35.2 (7.6) | 8(0) | 5(0) | 68.6 |
| 2(n = 17) [18] | 70.6 | 26.2 (1.7) | 30:1 | 39.8 (8.0) | 8(0) | 5(0) | 100.0 |
| 3 (n = 21) [19] | 57.1 | 27.1 (2.9) | 5:1 | 31.1 (4.5) | 8(0) | 5(0) | 76.2 |
| 4 (n = 7) [20] | 42.9 | 22.3 (1.3) | 5:1 | 44.6 (3.9) | 11.6 (1.3) | 5 (0) | 100.1 |
| Mean (N = 80) | 50.5 | 25.7 (1.8) | 37.7 (5.8) | 8.9 (1.8) | 5.0 (0) | 86.2 |
Data are reported as mean (standard deviation), unless otherwise specified.
For complete reference citations, see the reference list.
CPR ratio: external compressions to ventilations.
VF duration: duration of untreated VF.
CPR duration: CPR duration until the first rescue shock was delivered. CPR = cardiopulmonary resuscitation; ROSC = return of spontaneous circulation; VF = ventricular fibrillation.
Animals without ROSC began resuscitation with a CPP of 2.8 ± 0.5 mmHg, while animals with ROSC began at 3.1 ± 0.3 mmHg. Immediately prior to drug delivery, animals without ROSC achieved a CPP of 4.7 ± 0.2 mmHg with external compressions alone, while animals with ROSC achieved a CPP of 8.8 ± 0.3 mmHg. After medications and further compressions, animals without ROSC achieved a maxCPP of 20.1 ± 0.3 mmHg, while animals with ROSC achieved a maxCPP of 41.2 ± 0.6 mmHg (Fig. 2). RM-ANOVA yielded a difference between animals with and without ROSC (p < 0.001). Immediately following medication administration, animals without ROSC demonstrated a slope in CPP of 8.9, whereas animals with ROSC demonstrated a slope of 22.9 (Fig. 3). The CPP measurements summed over time in animals without ROSC yielded an AUC of 21.4 ± 13.7 mmHg × 102, whereas the CPP measurements in animals with ROSC yielded an AUC of 43.9 ± 17.6 mmHg × 102 (p < 0.001). Univariate regression identified maxCPP, AUC, and experimental study as three candidate variables for multivariate analysis. MaxCPP and AUC were extremely colinear and were placed in separate multivariate models with experimental study. MaxCPP (odds ratio [OR] 1.11; 95% confidence interval [CI] 1.05, 1.18) and AUC (OR 1.10; 95% CI 1.05, 1.16) predicted ROSC. Experimental study also predicted ROSC in each model (OR 1.70; 95% CI 1.15, 2.50; and OR 1.59; 95% CI 1.12, 2.25, respectively) (Table 2). Nonsignificant predictors were gender, weight, duration of instrumentation, duration of untreated VF, ratio of compressions to ventilations, and duration of CPR prior to the first rescue shock.
Figure 2.
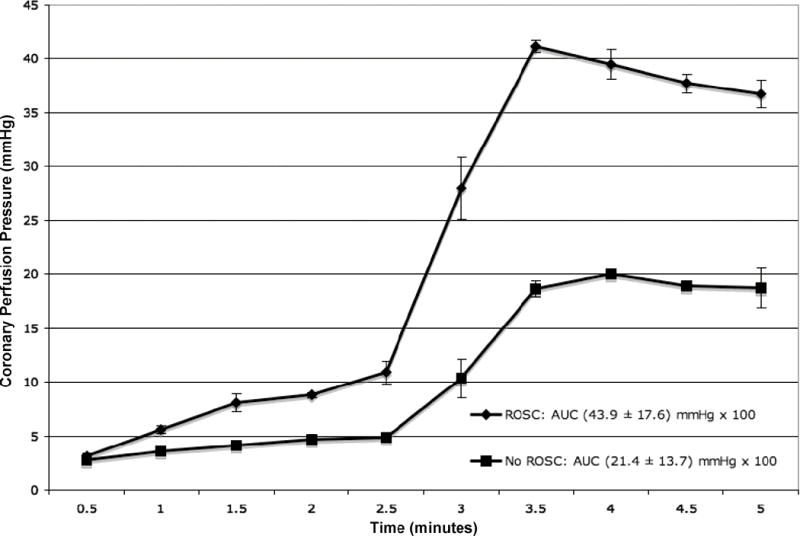
Mean coronary perfusion pressure ± standard error for animals receiving 5 minutes of cardiopulmonary resuscitation with and without return of spontaneous circulation (ROSC). AUC = area under the curve.
Figure 3.
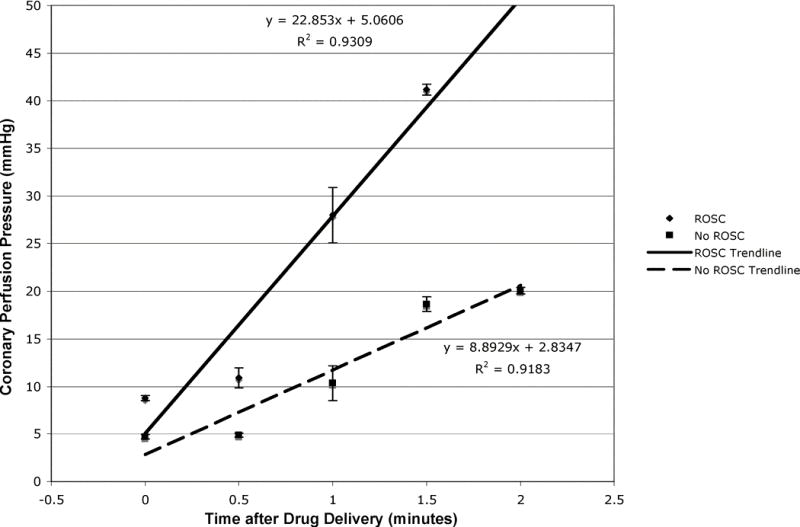
Slope analysis of coronary perfusion pressure (CPP) between drug delivery and maximum CPP for animals with and without return of spontaneous circulation (ROSC).
Table 2.
Multivariate Logistic Regression for Predictors of Return of Spontaneous Circulation
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Threshold model | |||
| MaxCPP (mmHg) | 1.11 | 1.05-1.18 | <0.001 |
| Experimental study | 1.70 | 1.15-2.50 | 0.008 |
| Dose model | |||
| AUC (mmHg × 102) | 1.10 | 1.05-1.16 | <0.001 |
| Experimental study | 1.59 | 1.12-2.25 | 0.009 |
AUC = area under the curve; CI = confidence interval; MaxCPP = maximum mean coronary perfusion pressure achieved during resuscitation.
Discussion
The CPP required for ROSC in our porcine model of OHCA is significantly higher than the often-quoted necessary threshold CPP of 15–25 mmHg for ROSC derived from prior animal and human data.5-14
One objective of CPR and medication delivery is to generate an adequate CPP to reperfuse the myocardium before administering a rescue shock.28 During the first 2 minutes of resuscitation, resuscitated animals achieved higher CPP from compressions alone compared with unresuscitated animals. These animals may have higher residual levels of endogenous catecholamines, upregulated catecholamine receptors, or increased receptor sensitivity. The resuscitated animals also demonstrated much higher maxCPP after exogenous catecholamine administration and continuing chest compressions than the unresuscitated animals. Slope analysis of the CPP curve immediately after medication administration shows a response from the resuscitated animals twice that of the unresuscitated animals. Animals without ROSC appear to be “hyporesponders” to exogenous catecholamine administration and to have lost a greater degree of vascular tone. Further investigation into residual catecholamine levels and the activity of catecholamine receptors during cardiac arrest is warranted.
A likely reason for the threshold discrepancy between prior animal models and our data is the timing of the experimental protocol. Previous studies were conducted with briefer untreated arrest intervals (0–5 minutes). These studies modeled cardiac arrest that rarely extended beyond the electrical phase.15 Our studies were conducted with an untreated VF duration of 8–11 minutes (circulatory and metabolic phases), which reflects a reasonable response time in OHCA. Prior human data were collected in persons suffering 8–12 minutes of untreated cardiac arrest, yet there is still a significant difference in the necessary threshold CPP for ROSC. A likely reason for this discrepancy is the timing of data collection. Human data collection began very late into cardiac arrest (24–25 minutes), whereas our data were collected immediately at the onset of resuscitation (8–11 minutes). A different conception of myocardial perfusion may shed light onto this inconsistency.
Coronary perfusion pressure in resuscitation is typically considered in terms of a threshold; that is, a certain level of perfusion must be achieved to predict defibrillation success. Previous animal and human studies focus on this threshold concept of myocardial perfusion reported as a maxCPP achieved during resuscitation. However, it may be useful to conceive of CPP as a “total dose” of perfusion over time, as reflected in our AUC analysis.
Previously reported animal data used too brief an arrest period to be compared with current OHCA modeling. The only published human data describe a required preshock threshold CPP of 20–25 mmHg that was measured very late in resuscitation. Our animal model reveals a required preshock threshold CPP of 35–40 mmHg measured early in resuscitation. It is possible that a lower threshold CPP over a longer resuscitation approximates a higher threshold CPP over a shorter resuscitation; that is, the “total doses” of reperfusion are similar. Essentially, human subjects maintaining CPP so late in resuscitation were likely more responsive to both endogenous and exogenous catecholamines, able to maintain CPP for a prolonged period of time, and thus able to yield a substantial AUC for the entire resuscitation. Our regression models of both threshold and dose had predictive value of ROSC, but were extremely colinear. Pauses in compressions to deliver other therapies during resuscitation as well as the total durations of compressions might favor either peak (maxCPP) or total (AUC) perfusion pressure as a better predictor of ROSC. The relationship between dose and threshold of CPP warrants further investigation.
Our higher threshold of the necessary preshock CPP for ROSC emphasizes the importance of chest compressions and timely use of resuscitative medications in OHCA to reperfuse the myocardium and render it more amenable to defibrillation. Ventricular fibrillation waveform analyses with a variety of methods are available measures that can be used to determine the amenability of VF to defibrillation and to guide titration of resuscitative therapies.29 Correlation of CPP to the VF waveform would prove useful since these electrical analyses could act as a marker of myocardial perfusion and conceptually link changes in the waveform to changes in reperfusion. Also, should a relatively noninvasive method of measuring CPP be developed, this threshold CPP could be used for titration of early resuscitative therapy.
Limitations
There were several limitations to our study. First, we analyzed young healthy swine whose physiology may not reflect that of typical cardiac arrest patients. Second, there may be a species difference between swine, canine, and human cardiopulmonary physiologies. Third, we analyzed CPP, not myocardial blood flow directly. Typical cardiac arrest patients have a high burden of cardiovascular disease with increased coronary vascular resistance, adversely affecting the translation of pressure into flow. Fourth, VF was electrically induced in all of these cases, not induced by ischemia. Finally, we examined ROSC, not long-term survival or neurologic outcomes.
Conclusions
A higher threshold CPP than previously reported is necessary to achieve ROSC in a porcine model of prolonged OHCA. It may be useful to redefine CPP as a “total dose” rather than a threshold required for successful resuscitation.
Acknowledgments
Supported by contract RO1 HL080483, from the National Heart, Lung, and Blood Institute, National Institutes of Health (to Dr. Menegazzi). Dr. Menegazzi is a coinventor of a patented quantitative method of ECG analysis (the scaling exponent), which has been licensed to Medtronic Physio-Control, from which he receives royalties. Neither of the other authors have anything to disclose. The authors alone are responsible for the content and writing of the paper.
Footnotes
Presented at the American Heart Association Resuscitation Science Symposium, New Orleans, Louisiana, November 2008.
Contributor Information
Joshua C. Reynolds, Department of Emergency Medicine, University of Maryland, Baltimore, Maryland.
David D. Salcido, Department of Emergency Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
James J. Menegazzi, Department of Emergency Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–8. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 2.Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 3.Wik L, Hansen TB, Fylling F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–95. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 4.Berg RA, Hilwig RW, Ewy GA, Kern KB. Precountershock cardiopulmonary resuscitation improves initial response to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Crit Care Med. 2004;32:1352–7. doi: 10.1097/01.ccm.0000127780.01362.e5. [DOI] [PubMed] [Google Scholar]
- 5.Babbs CF. New versus old theories of blood flow during CPR. Crit Care Med. 1980;8:191–5. doi: 10.1097/00003246-198003000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–50. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 7.Redding JS, Pearson JW. Evaluation of drugs for cardiac resuscitation. Anesthesiology. 1963;24:203–7. doi: 10.1097/00000542-196303000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ditchey RV, Winkler JV, Rhodes CA. Relative lack of coronary blood flow during closed-chest resuscitation in dogs. Circulation. 1982;66:297–302. doi: 10.1161/01.cir.66.2.297. [DOI] [PubMed] [Google Scholar]
- 9.Sanders AB, Ewy GA, Alferness CA, Taft T, Zimmerman M. Failure of one method of simultaneous chest compressions, ventilation, and abdominal binding during CPR. Crit Care Med. 1982;10:509–13. doi: 10.1097/00003246-198208000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Michael JR, Guerci AD, Koehler RC, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;9:822–35. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 11.Niemann JT, Rosborough JP, Niskanen RA, Alferness CA, Criley JM. Mechanical “cough” cardiopulmonary resuscitation during cardiac arrest in dogs. Am J Cardiol. 1985;55:199–204. doi: 10.1016/0002-9149(85)90328-5. [DOI] [PubMed] [Google Scholar]
- 12.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14:521–8. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 13.Sanders AB, Kern KB, Atlas M, Bragg S, Ewy GA. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. J Am Coll Cardiol. 1985;6:113–8. doi: 10.1016/s0735-1097(85)80261-8. [DOI] [PubMed] [Google Scholar]
- 14.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–13. [PubMed] [Google Scholar]
- 15.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3- phase time-sensitive model. JAMA. 2002;288:3035–8. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 16.Mader TJ. Prolonged cardiac arrest: a revised model of porcine ventricular fibrillation. Resuscitation. 2008;76:481–4. doi: 10.1016/j.resuscitation.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) study. JAMA. 1994;271:678–83. [PubMed] [Google Scholar]
- 18.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 19.Fan KL, Leung LP. Prognosis of patients with ventricular fibrillation in out-of-hospital cardiac arrest in Hong Kong: prospective study. Hong Kong Med J. 2002;8:318–21. [PubMed] [Google Scholar]
- 20.Herlitz J, Engdahl J, Svensson L, Angquist KA, Young M, Holmberg S. Factors associated with an increased chance of survival among patients suffering from an out-of-hospital cardiac arrest in a national perspective in Sweden. Am Heart J. 2005;149:61–6. doi: 10.1016/j.ahj.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Stiell IG, Wells GA, DeMaio VJ, et al. Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS study phase I results. Ontario Prehospital Advanced Life Support. Ann Emerg Med. 1999;33:44–50. doi: 10.1016/s0196-0644(99)70415-4. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds JC, Rittenberger JC, Menegazzi JJ. Drug administration in animal studies of cardiac arrest does not reflect human clinical experience. Resuscitation. 2007;74:13–26. doi: 10.1016/j.resuscitation.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112(suppl 24):IV–1. IV–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 24.Menegazzi JJ, Rittenberger JC, Suffoletto BP, Logue ES, Salcido DD, Sherman LD. Effects of pre-arrest and intra-arrest hypothermia on ventricular fibrillation and resuscitation. Resuscitation. 2009;80:126–132. doi: 10.1016/j.resuscitation.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suffoletto BP, Salcido DD, Logue ES, Caprio T, Menegazzi JJ. Ethyl pyruvate enhances intra- and post-resuscitation hemodynamics in prolonged ventricular fibrillation arrest. Resuscitation. 2009 doi: 10.1016/j.resuscitation 2009.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Betz AE, Menegazzi JJ, Logue ES, Callaway CW, Wang HE. A randomized comparison of manual, mechanical, and high-impulse chest compression in a porcine model of prolonged ventricular fibrillation. Resuscitation. 2006;69:495–501. doi: 10.1016/j.resuscitation.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Menegazzi JJ, Callaway CW, Sherman LD, et al. Ventricular fibrillation scaling exponent can guide timing of defibrillation and other therapies. Circulation. 2004;109:926–31. doi: 10.1161/01.CIR.0000112606.41127.D2. [DOI] [PubMed] [Google Scholar]
- 28.Frenneaux M. Cardiopulmonary resuscitation—some physiologic considerations. Resuscitation. 2003;58:259–265. doi: 10.1016/s0300-9572(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 29.Callaway CW, Menegazzi JJ. Waveform analysis of ventricular fibrillation to predict defibrillation. Curr Opin Crit Care. 2005;11:192–9. doi: 10.1097/01.ccx.0000161725.71211.42. [DOI] [PubMed] [Google Scholar]


