Abstract
The erythrocyte, a cell responsible for carrying and delivering oxygen in the body, has often been regarded as simply a vehicle for the circulation of hemoglobin. However, it has become evident that this cell also participates in the regulation of vascular caliber in the microcirculation via release of the potent vasodilator, adenosine triphosphate (ATP). The regulated release of ATP from erythrocytes occurs via a defined signaling pathway and requires increases in cyclic 3’ 5’ adenosine monophosphate (cAMP). It is well recognized that cAMP is a critical second messenger in diverse signaling pathways. In all cells increases in cAMP are localized and regulated by the activity of phosphodiesterases (PDEs). In erythrocytes activation of either β adrenergic receptors (β 2AR) or the prostacyclin receptor (IPR) results in increases in cAMP and ATP release. Receptor-mediated increases in cAMP are tightly regulated by distinct PDEs associated with each signaling pathway as shown by the finding that selective inhibitors of the PDEs localized to each pathway potentiate both increases in cAMP and ATP release. Here we review the profile of PDEs identified in erythrocytes, their association with specific signaling pathways and their role in the regulation of ATP release from these cells. Understanding the contribution of PDEs to the control of ATP release from erythrocytes identifies this cell as a potential target for the development of drugs for the treatment of vascular disease.
Keywords: erythrocyte, isoproterenol, iloprost, phosphodiesterases, cyclic nucleotides, adenosine triphosphate
Introduction
Within all mammalian cells, activation of specific G protein-coupled receptors can stimulate the activity of adenylyl cyclase (AC) resulting in the production of cyclic adenosine monophosphate (cAMP). With a diffusion constant of 270–780 μm2/s [4, 12, 79], it could be anticipated that cAMP would diffuse throughout the cell, activating any of several effector proteins expressed in that cell. However, indiscriminate effector protein activation does not occur since the activation of individual receptors produces highly selective cellular responses. This selectivity suggests that there is compartmentalization of cAMP signaling within cells. Multiple proteins, including ligand receptors, their G proteins, ACs as well as regulatory and scaffold proteins aid in the compartmentalization of cAMP signaling. However, this compartmentalization is suggested to be most heavily dependent on phosphodiesterase (PDE) activity [5, 79]. PDEs control the diffusion of cAMP by rapidly degrading this cyclic nucleotide within cells and, thereby, regulate its biological actions.
Eleven PDE families have currently been identified, some of which contain multiple isoforms [9]. PDE families and their individual isoforms are differentiated by their ability to hydrolyze either cAMP, cyclic guanosine monophosphate (cGMP) or both cyclic nucleotides as well as by their cellular and subcellular location, mode of regulation and sensitivity to inhibitors [9]. Although all cells contain PDE activity, the isoforms present vary according to cell type [9]. This diversity in PDE expression allows these enzymes to regulate discrete signal transduction pathways and ultimately, specific physiological processes.
Several studies provide support of a role for PDEs in regulation of discrete cell processes. For example, the hypothesis that compartmentalization of cAMP generates specificity of Gs-receptor actions, with PDEs playing a major role, was tested in adult rat ventricular myocytes by characterizing the PDEs involved in regulation of cAMP signals and L-type Ca2+ current upon stimulation of different Gs-coupled receptors [50]. These studies demonstrated that receptor-PDE coupling has functional implications downstream of cAMP and identified functional coupling of specific PDE families to specific ligand-activated Gs-coupled receptors as a major mechanism which enables cardiac cells to generate heterogeneous cAMP signals to different hormones [50]. In addition, these studies also suggest that alteration of PDE activity abolishes the compartmentalization of the cAMP signal allowing effector proteins to be activated throughout the cell eliciting aberrant responses [50]. In support of this hypothesis, using Fluorescence Resonance Energy Transfer (FRET) analysis in both HEK293 cells and cardiac myocytes, it was possible to visualize the compartmentalization of cAMP and demonstrate that this compartmentalization was dependent on PDE activity [39, 71]. In mouse embryonic fibroblasts, specific isoforms of the PDE4 gene family were shown to be functionally coupled to the β 2 adrenergic receptor. Ablation of PDE4 in mice altered the response to β 2 adrenergic receptor activation in these cells [11]. Similarly, in cardiac myocytes, disruption of cAMP pools via ablation of distinct PDE4 isoforms disrupts the tight regulation of β adrenergic signaling [77]. In the above mentioned studies, stimulation of β 1, β 2 or prostacyclin receptors (IPR), all Gs coupled receptors, increase cAMP, but produce distinct patterns of protein phosphorylation resulting in different downstream effects. In each of these studies, it was concluded that the results were partially due to either the functional coupling or localization of individual PDE families to each receptor resulting in the heterogeneity of ligand specific cAMP-mediated effects.
Subcellular localization of PDEs
The subcellular localization of PDEs is recognized to be a key mechanism for compartmentalization of cyclic nucleotide signaling. The amino terminal sequence of the PDE determines the localization of the protein within the cell. PDEs may be found either in the cytosol or associated with the plasma membrane or organelles of the cell [36, 43]. Another determinant of localization is whether or not the PDE is bound to or interacts with scaffold proteins which localize PDEs to microdomains within a cell [17, 29, 78]. Clearly, the subcellular location of PDEs is critical for coupling these enzymes to specific signal transduction pathways to permit specific PDEs to regulate local increases in cAMP produced by activation of a ligand specific receptor. This mechanism can target locally produced cAMP to selective effector proteins to stimulate a specific response. Since cAMP is synthesized in response to receptor-mediated activation of AC in erythrocytes [7, 42, 59, 61], it is logical to assume that PDEs within these cells regulate the compartmentalization of cAMP signaling allowing for specific cell responses.
PDE activity in erythrocytes
Although cAMP is considered to be a ubiquitous second messenger in cells, the presence of AC activity and cAMP production in the erythrocyte was once controversial. It was suggested that mature erythrocytes possess little or no AC activity and therefore can not synthesize cAMP [8, 21, 32, 57]. However, Nakagawa et al. demonstrated that stabilization of AC during isolation of erythrocyte membranes is critical in order to maintain enzyme activity [41]. Subsequently, it has been demonstrated that AC type II is present in both rabbit and human erythrocytes [59, 66] and that sodium fluoride, β adrenergic agonists, or prostacyclin and its analogs stimulate cAMP synthesis in these cells [1, 7, 48, 51, 60].
It has also been reported that PDE activity is present in erythrocytes, but this activity has not been well characterized. In some studies, PDE activity was determined by measurement of cAMP levels in the absence of a selective PDE inhibitor or in the presence of a nonselective PDE inhibitor, such as IBMX or theophylline, preventing the identification of the individual PDE families present in erythrocytes [3, 14, 45, 58, 70]. Using non-selective PDE inhibitors, PDE activity associated with the hydrolysis of cAMP was described in rat, rabbit, embryonic avian and human erythrocytes. However, this approach does not permit identification of the individual PDEs that hydrolyze cAMP in these cells or their association with specific signaling pathways [3, 7, 45, 70]. Importantly, in non-erythroid cells it has been shown that increases in cAMP associated with activation of β adrenergic receptors were augmented by different selective PDE inhibitors than cAMP increases associated with IP receptor activation [50, 71, 72, 74]. Such observations raise the question; are specific PDE families responsible for the regulation of receptor-mediated increases in cAMP stimulated by activation of β adrenergic and IP receptors in erythrocytes?
PDE1 in Erythrocytes
The first individual PDE isoform reported to be present in a mammalian erythrocyte was PDE1 [46]. PDE1 was reported to hydrolyze cGMP in human erythrocytes while no effect on the regulation of cAMP was reported. Identification of PDE1 in human erythrocytes was based entirely on pharmacological studies, that is, PDE1 protein was not identified [46]. There are three subfamilies of PDE1 (A–C) each with differing affinities for cAMP and cGMP [43]. Human PDE1A shows high affinity for cGMP [35], whereas PDE1B hydrolyzes cGMP with a lower Km and higher Vmax than cAMP [10] and PDE1C has a high affinity for both cyclic nucleotides [35]. In an effort to understand the role of PDE1 in mammalian erythrocytes and determine if this PDE hydrolyzes cAMP in these cells, we incubated both rabbit and human erythrocytes with the selective PDE1 inhibitor, vinpocetine, prior to stimulation of the IP and β adrenergic receptors with iloprost or isoproterenol, respectively [1, 24]. In both species, this selective inhibitor of PDE1 had no effect on increases in cAMP in either pathway suggesting that the isoform of PDE1 present in these erythrocytes is primarily involved in hydrolysis of cGMP.
PDE2 in Erythrocytes
PDE2 is a cGMP-stimulated PDE with a single gene encoding three splice variants (PDE2A1-3) [9, 38]. PDE2A has both soluble (PDE2A1) and particulate (PDE2A2 and 3) variants and binding of cGMP to GAF domains within PDE2 results in the activation of all PDE2 variants [76, 80]. PDE2 can hydrolyze both cAMP and cGMP with similar maximal rates [9, 36, 43]. In cardiac myocytes, the three classes of β adrenergic receptors, AC, and PDE2A are co-localized in lipid rafts, suggesting coupling between β adrenergic receptors and PDE2A in mediating the cAMP signal [40]. In addition, epinephrine infusion has been reported to stimulate skeletal muscle PDE2A expression in humans [73]. Recently, using Western analysis, we demonstrated that PDE2A protein is a component of human and rabbit erythrocyte membranes [1]. Moreover, the selective PDE2 inhibitor, EHNA, potentiated cAMP increases in response to isoproterenol-induced activation of the erythrocyte β adrenergic receptor, but not when cAMP levels were increased by incubation with the IP receptor agonist, iloprost [1]. These findings support the hypothesis that, in human and rabbit erythrocytes, there is coupling between the β adrenergic receptor and PDE2.
PDE4 in Erythrocytes
PDE4 is a cAMP-specific PDE which has four subfamilies, A thru D, that include over 50 isoforms [6, 27]. This family includes a number of splice variants categorized into N terminal variant groups (long, short and super short) based on the absence or presence of upstream conserved regions (UCR) that are involved in the regulation of PDE4 activity [27, 37]. These PDE4 isoforms display unique intracellular distribution patterns. PDE4 isoforms are expressed in most cell types and this PDE is considered to be an important homeostatic regulator of many cell processes [6, 13, 26]. PDE4 activation is regulated via phosphorylation by PKA in the UCR or phosphorylation by ERK in the C-terminal end of the protein, depending on the individual isoform [13, 26].
With the exception of an isoform of PDE4A, PDE4 is primarily a cytosolic enzyme [9, 26]. This restricts its interactions to intracellular scaffolding proteins such as β-arrestins and A kinase anchoring proteins (AKAPs) [6, 49, 75]. In cardiac myocytes coupling of PDE4 to the β 2 adrenergic receptor has been shown to regulate several aspects of β 2 adrenergic signaling [49, 77]. In these cells, inhibition of PDE4 increases cAMP in response to activation of β 2 adrenergic receptors, but has no effect on β 1 adrenergic signaling demonstrating the selectivity of the PDE association [49, 77]. The recruitment of PDE4 to the β 2 adrenergic receptor allows PDE4 to act locally and hydrolyze cAMP produced in response to activation of this receptor, modulating its downstream effects. Since PDE4 is a cytosolic protein, attempts to identify its presence in erythrocytes have been complicated by the large amount of hemoglobin present in this cell. However, in human and rabbit erythrocytes, activation of the β adrenergic receptor with isoproterenol results in cAMP increases that are potentiated by rolipram, a selective inhibitor of PDE4 [1]. These results suggest that PDE4 is somehow localized to the β adrenergic receptor or its signaling pathway and regulates the increases in cAMP in these cells.
PDE3 in Erythrocytes
The PDE3 family consists of 2 isoforms, 3A and 3B. Both isoforms of PDE3 contain N-terminal hydrophobic domains allowing this PDE family to be membrane-associated or targeted [55, 56]. The PDE3 family can hydrolyze both cAMP and cGMP [15, 55]. Interestingly, the hydrolysis of cGMP by PDE3 inhibits the hydrolysis of cAMP [9, 15], therefore this PDE family is also termed cGMP-inhibited PDE. Activation of PDE3 has been demonstrated to occur via phosphorylation by PKA or PKB [30, 33, 44, 55]. In adipocytes, when PDE3B is phosphorylated by PKB, it is recruited to macromolecular complexes. This recruitment is suggested to be critical for the regulation of cAMP to modulate insulin signaling pathways [2].
An inhibitor of PDE3 was first demonstrated to potentiate cAMP increases stimulated by β adrenergic agonists in embryonic avian erythrocytes [7]. Recently, we identified the membrane bound isoform of PDE3 (PDE3B) in rabbit and human erythrocytes [24]. However, inhibitors of this PDE had no effect on the cAMP increases stimulated by the β adrenergic agonist, isoproterenol, in these mammalian cells [1].
In non-erythroid cells, inhibitors of PDE3 were reported to potentiate cAMP increases stimulated by prostacyclin analogs [22, 47]. Inhibitors of PDE3 have also been shown to potentiate the vasodilation produced by prostacyclin analogs in pulmonary vessels [52, 54], suggesting that the IP receptor and PDE3 are functionally coupled. To establish a role for PDE3 in the regulation of cAMP increases produced by activation of the IP receptor in human and rabbit erythrocytes, we investigated the effect of the prostacyclin analog, iloprost, on cAMP levels in the absence and presence of PDE3 inhibitors. In cells from both species, two chemically dissimilar inhibitors of PDE3, milrinone and cilostazol, potentiated iloprost-induced cAMP increases [24]. These results suggest that in rabbit and human erythrocytes PDE3 selectively regulates cAMP synthesis stimulated by activation of the IP receptor.
Significance and Conclusions
Recently it has been demonstrated that erythrocytes participate in the regulation of vascular caliber through their ability to release adenosine triphosphate (ATP) in response to pharmacological stimuli [16, 19, 20, 64, 67]. In this construct, erythrocyte-derived ATP binds to endothelial purinergic receptors resulting in the production of endothelium-derived vasodilators such as nitric oxide, prostacyclin and endothelial hyperpolarizing factor [62, 65]. Our laboratory has proposed a signal transduction pathway that associates receptor-mediated increases in intra-erythrocyte cAMP synthesis with ATP release from erythrocytes. This pathway includes the heterotrimeric G protein Gs, AC, cAMP, protein kinase A and the cystic fibrosis transmembrane conductance regulator [42, 59, 63]. We have reported that this signaling pathway can be activated by two different Gs-coupled receptors present on the erythrocyte membrane, the β adrenergic receptor and the IP receptor [42].
Within the proposed pathways, increases in cAMP within the erythrocyte are required for receptor-mediated release of ATP [63]. Recently, we have shown that increases in cAMP within erythrocytes stimulated by activation of the β adrenergic receptor are regulated by the activity of PDEs 2 and 4 while cAMP derived from IP receptor activation is regulated by the activity of PDE3 [1, 24]. These findings are consistent with the hypothesis that the activity of specific PDEs regulates the ability of human and rabbit erythrocytes to release ATP via the compartmentalization of cAMP signaling.
Erythrocyte-derived ATP has been reported to stimulate localized increases in vessel diameter and blood flow in the microcirculation [19, 64]. The finding that both β adrenergic agonists and prostacyclin analogs stimulate ATP release from erythrocytes suggests that at least a portion of the vasodilation produced by these agents, when administered in vivo, could be mediated by erythrocyte released ATP.
Interestingly, ATP release from erythrocytes is attenuated in humans with pulmonary hypertension and type II diabetes [66, 68] suggesting that decreased ATP release from erythrocytes could contribute to the vascular disease associated with these humans conditions. Indeed, PDE inhibitors are currently used in the treatment of peripheral vascular disease (PVD) and pulmonary hypertension [23, 25, 69]. Thus, the PDE3 inhibitor cilostazol is effective in the treatment of intermittent claudication, a symptom of PVD [34] and inhibitors of PDE5 are currently used in the treatment of pulmonary hypertension. In addition PDE4 inhibitors are currently under investigation as a new therapeutic approach for inflammatory diseases as well as pulmonary hypertension [18, 23, 28, 31, 53]. In pulmonary vessels, both PDE3 and PDE4 inhibitors potentiate the vasodilation produced by prostacyclin analogs, the latter being a highly effective treatment for pulmonary hypertension [52, 54]. In none of these cases has a role for the effects of PDE inhibitors on cAMP levels and ATP release from erythrocytes been considered.
Identification of the entire compliment of PDEs that are expressed in the erythrocyte, along with an improved understanding of the role of these PDEs in various signaling pathways in this cell demands further investigation. The finding that specific PDEs are associated with individual receptor-mediated signaling pathways for ATP release from erythrocytes, and that selective inhibitors of these PDEs potentiate ATP release from these cells, suggests that the erythrocyte could be a new therapeutic target in the treatment of vascular disease.
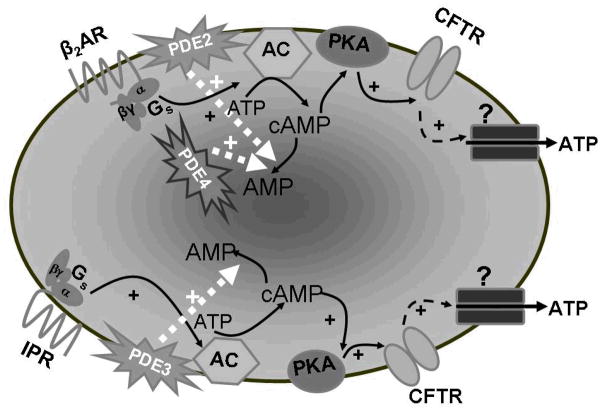
Figure 1.
Activation of the β 2AR with isoproterenol increases cAMP which then activates PKA. PKA then phosphorylates and activates CFTR which controls the activity of an ATP conduit in the erythrocyte membrane. Increases in cAMP associated with activation of the β 2AR are regulated by the activities of both PDE2 and PDE4. Similarly, activation of the IPR by iloprost results in increases in cAMP which activate ATP release from the erythrocyte. However, in the case of the IPR signaling pathway, increases in cAMP are regulated by the activity of PDE3.
Abbreviations: β 2AR = β 2 adrenergic receptor; Gs = the heterotrimeric G protein, Gs; α, β and γ = G protein subunits, PDE = phosphodiesterase; ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate, AMP = adenosine monophosphate; AC = adenylyl cyclase; PKA = protein kinase A; CFTR = cystic fibrosis transmembrane conductance regulator; ? = unknown ATP conduit; + = activation; IPR = prostacyclin receptor.
Figure 2.
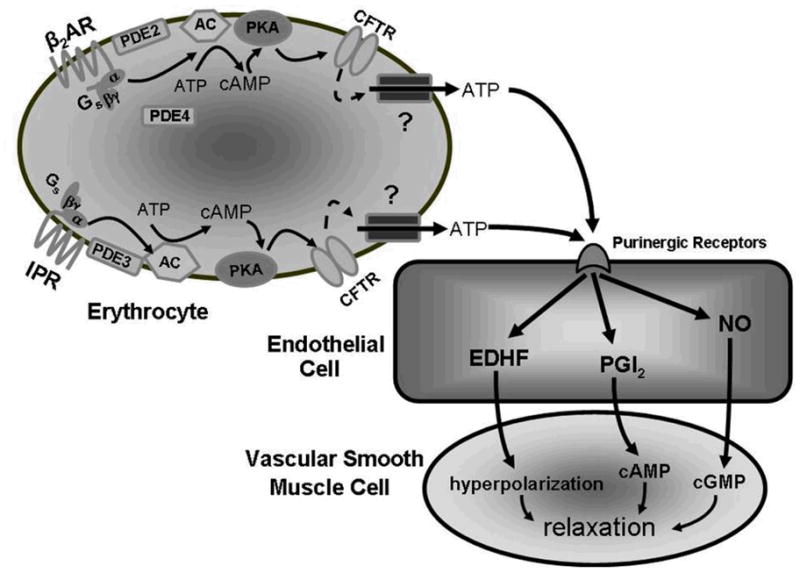
Stimulation of either the β 2AR or the IPR leads to ATP release from the erythrocyte. Once released the ATP can activate purinergic receptors on endothelial cells leading to the formation and release of EDHF, PGI2 or NO all of which can induce relaxation in the smooth muscle cell.
Abbreviations: β 2AR = β 2 adrenergic receptor; Gs = the heterotrimeric G protein, Gs; α, β and γ = G protein subunits, PDE = phosphodiesterase; ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate, AMP = adenosine monophosphate; AC = adenylyl cyclase; PKA = protein kinase A; CFTR = cystic fibrosis transmembrane conductance regulator; ? = unknown ATP conduit; IPR = prostacyclin receptor; EDHF = endothelium-derived hyperpolarizing factor; PGI2 = prostacyclin; NO = nitric oxide; cGMP = cyclic guanosine monophosphate.
Acknowledgments
The authors thank J.L. Sprague for inspiration. This work is supported by NIH grants HL-64180, HL-89094 and ADA grant RA-133.
Abbreviations
- ATP
adenosine triphosphate
- AKAP
A kinase anchoring protein
- AC
Adenylyl cyclase
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- FRET
Fluorescence Resonance Energy Transfer
- PVD
peripheral vascular disease
- PDE
phosphodiesterase
- IPR
prostacyclin receptor
- UCR
upstream conserved regions
References
- 1.Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans. Am J Physiol Heart Circ Physiol. 2009;296:H1617–1624. doi: 10.1152/ajpheart.01226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad F, Lindh R, Tang Y, Weston M, Degerman E, Manganiello VC. Insulin-induced formation of macromolecular complexes involved in activation of cyclic nucleotide phosphodiesterase 3B (PDE3B) and its interaction with PKB. Biochem J. 2007;404:257–268. doi: 10.1042/BJ20060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu CR, Azhar S, Krishna Murti CR. Loss of epinephrine stimulated synthesis of cyclic adenosine 3':5' monophosphate during maturation of rabbit and human reticulocytes. Med Biol. 1975;53:148–155. [PubMed] [Google Scholar]
- 4.Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- 5.Baillie GS. Compartmentalized signaling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. The FEBS journal. 2009;276:1790–1799. doi: 10.1111/j.1742-4658.2009.06926.x. [DOI] [PubMed] [Google Scholar]
- 6.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Baumann R, Blass C, Gotz R, Dragon S. Ontogeny of catecholamine and adenosine receptor-mediated cAMP signaling of embryonic red blood cells: role of cGMP-inhibited phosphodiesterase 3 and hemoglobin. Blood. 1999;94:4314–4320. [PubMed] [Google Scholar]
- 8.Beaumont C, Piau JP, Fischer S, Delaunay J, Schapira G. Stimulation of erythroid cells adenylate cyclase by soluble factors. Biochem Biophys Res Commun. 1979;91:1250–1257. doi: 10.1016/0006-291x(79)91201-4. [DOI] [PubMed] [Google Scholar]
- 9.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 10.Bender AT, Ostenson CL, Wang EH, Beavo JA. Selective up-regulation of PDE1B2 upon monocyte-to-macrophage differentiation. Proc Natl Acad Sci U S A. 2005;102:497–502. doi: 10.1073/pnas.0408535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruss MD, Richter W, Horner K, Jin SL, Conti M. Critical role of PDE4D in beta2-adrenoceptor-dependent cAMP signaling in mouse embryonic fibroblasts. J Biol Chem. 2008;283:22430–22442. doi: 10.1074/jbc.M803306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Nakamura T, Koutalos Y. Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophysical journal. 1999;76:2861–2867. doi: 10.1016/S0006-3495(99)77440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- 14.DeBari VA, Novak NA, Bennun A. Cyclic nucleotide metabolism in the human erythrocyte. Clinical physiology and biochemistry. 1984;2:227–238. [PubMed] [Google Scholar]
- 15.Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3) J Biol Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 17.Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ Res. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- 18.Dony E, Lai YJ, Dumitrascu R, Pullamsetti SS, Savai R, Ghofrani HA, Weissmann N, Schudt C, Flockerzi D, Seeger W, Grimminger F, Schermuly RT. Partial reversal of experimental pulmonary hypertension by phosphodiesterase-3/4 inhibition. Eur Respir J. 2008;31:599–610. doi: 10.1183/09031936.00002007. [DOI] [PubMed] [Google Scholar]
- 19.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 20.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 21.Farfel Z, Cohen Z. Adenylate cyclase in the maturing human reticulocyte: selective loss of the catalytic unit, but not of the receptor-cyclase coupling protein. Eur J Clin Invest. 1984;14:79–82. doi: 10.1111/j.1365-2362.1984.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujitani K, Kambayashi J, Murata K, Yano Y, Shinozaki K, Yukawa M, Sakon M, Murata T, Kawasaki T, Shiba E, et al. Clinical evaluation on combined administration of oral prostacyclin analogue beraprost and phosphodiesterase inhibitor cilostazol. Pharmacol Res. 1995;31:121–125. doi: 10.1016/1043-6618(95)80057-3. [DOI] [PubMed] [Google Scholar]
- 23.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 24.Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost–induced increases in cAMP. Am J Physiol Heart Circ Physiol. 2008;295:H786–793. doi: 10.1152/ajpheart.00349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemnes AR, Champion HC. Sildenafil, a PDE5 inhibitor, in the treatment of pulmonary hypertension. Expert review of cardiovascular therapy. 2006;4:293–300. doi: 10.1586/14779072.4.3.293. [DOI] [PubMed] [Google Scholar]
- 26.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signaling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 28.Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug discovery today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 29.Jarnaess E, Tasken K. Spatiotemporal control of cAMP signaling processes by anchored signaling complexes. Biochem Soc Trans. 2007;35:931–937. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T, Kitamura Y, Kuroda S, Hino Y, Ando M, Kotani K, Konishi H, Matsuzaki H, Kikkawa U, Ogawa W, Kasuga M. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol. 1999;19:6286–6296. doi: 10.1128/mcb.19.9.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee AJ, Chiao TB, Tsang MP. Sildenafil for pulmonary hypertension. The Annals of pharmacotherapy. 2005;39:869–884. doi: 10.1345/aph.1E426. [DOI] [PubMed] [Google Scholar]
- 32.Limbird LE, Gill DM, Stadel JM, Hickey AR, Lefkowitz RJ. Loss of beta-adrenergic receptor-guanine nucleotide regulatory protein interactions accompanies decline in catecholamine responsiveness of adenylate cyclase in maturing rat erythrocytes. J Biol Chem. 1980;255:1854–1861. [PubMed] [Google Scholar]
- 33.Lindh R, Ahmad F, Resjo S, James P, Yang JS, Fales HM, Manganiello V, Degerman E. Multisite phosphorylation of adipocyte and hepatocyte phosphodiesterase 3B. Biochim Biophys Acta. 2007;1773:584–592. doi: 10.1016/j.bbamcr.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Shakur Y, Yoshitake M, Kambayashi Ji J. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19:369–386. doi: 10.1111/j.1527-3466.2001.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 35.Loughney K, Martins TJ, Harris EA, Sadhu K, Hicks JB, Sonnenburg WK, Beavo JA, Ferguson K. Isolation and characterization of cDNAs corresponding to two human calcium, calmodulin-regulated, 3',5'-cyclic nucleotide phosphodiesterases. J Biol Chem. 1996;271:796–806. doi: 10.1074/jbc.271.2.796. [DOI] [PubMed] [Google Scholar]
- 36.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurice DH. Dynamic regulation of cAMP signaling by cGMP in the cardiovascular system: roles of phosphodiesterase 2 and phosphodiesterase 3 enzymes. Proceedings of the Western Pharmacology Society. 2003;46:32–36. [PubMed] [Google Scholar]
- 39.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 40.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa M, Willner J, Cerri C, Reydel P. The effect of membrane preparation and cellular maturation on human erythrocyte adenylate cyclase. Biochim Biophys Acta. 1984;770:122–126. doi: 10.1016/0005-2736(84)90120-2. [DOI] [PubMed] [Google Scholar]
- 42.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit. 2001;7:669–674. [PubMed] [Google Scholar]
- 43.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 44.Palmer D, Jimmo SL, Raymond DR, Wilson LS, Carter RL, Maurice DH. Protein kinase A phosphorylation of human phosphodiesterase 3B promotes 14-3-3 protein binding and inhibits phosphatase-catalyzed inactivation. J Biol Chem. 2007;282:9411–9419. doi: 10.1074/jbc.M606936200. [DOI] [PubMed] [Google Scholar]
- 45.Patterson WD, Hardman JG, Sutherland EW. Apparent multiple forms of cyclic AMP phosphodiesterase from rat erythrocytes. Mol Cell Endocrinol. 1976;5:51–66. doi: 10.1016/0303-7207(76)90070-8. [DOI] [PubMed] [Google Scholar]
- 46.Petrov V, Fagard R, Lijnen P. Human erythrocytes contain Ca2+, calmodulin-dependent cyclic nucleotide phosphodiesterase which is involved in the hydrolysis of cGMP. Methods Find Exp Clin Pharmacol. 1998;20:387–393. doi: 10.1358/mf.1998.20.5.485699. [DOI] [PubMed] [Google Scholar]
- 47.Phillips PG, Long L, Wilkins MR, Morrell NW. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2005;288:L103–115. doi: 10.1152/ajplung.00095.2004. [DOI] [PubMed] [Google Scholar]
- 48.Piau JP, Delaunay J, Fischer S, Tortolero M, Schapira G. Human red cell membrane adenylate cyclase in normal subjects and patients with hereditary spherocytosis, sickle cell disease and unidentified hemolytic anemias. Blood. 1980;56:963–968. [PubMed] [Google Scholar]
- 49.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. Embo J. 2008;27:384–393. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rochais F, Abi-Gerges A, Horner K, Lefebvre F, Cooper DM, Conti M, Fischmeister R, Vandecasteele G. A specific pattern of phosphodiesterases controls the cAMP signals generated by different Gs-coupled receptors in adult rat ventricular myocytes. Circ Res. 2006;98:1081–1088. doi: 10.1161/01.RES.0000218493.09370.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodan SB, Rodan GA, Sha'afi RI. Demonstration of adenylate cyclase activity in human red blood cell ghosts. Biochim Biophys Acta. 1976;428:509–515. doi: 10.1016/0304-4165(76)90059-3. [DOI] [PubMed] [Google Scholar]
- 52.Schermuly RT, Inholte C, Ghofrani HA, Gall H, Weissmann N, Weidenbach A, Seeger W, Grimminger F. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: amplification by different type phosphodiesterase inhibitors. Respir Res. 2005;6:76. doi: 10.1186/1465-9921-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schermuly RT, Kreisselmeier KP, Ghofrani HA, Samidurai A, Pullamsetti S, Weissmann N, Schudt C, Ermert L, Seeger W, Grimminger F. Antiremodeling effects of iloprost and the dual-selective phosphodiesterase 3/4 inhibitor tolafentrine in chronic experimental pulmonary hypertension. Circ Res. 2004;94:1101–1108. doi: 10.1161/01.RES.0000126050.41296.8E. [DOI] [PubMed] [Google Scholar]
- 54.Schermuly RT, Roehl A, Weissmann N, Ghofrani HA, Schudt C, Tenor H, Grimminger F, Seeger W, Walmrath D. Subthreshold doses of specific phosphodiesterase type 3 and 4 inhibitors enhance the pulmonary vasodilatory response to nebulized prostacyclin with improvement in gas exchange. J Pharmacol Exp Ther. 2000;292:512–520. [PubMed] [Google Scholar]
- 55.Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol. 2001;66:241–277. doi: 10.1016/s0079-6603(00)66031-2. [DOI] [PubMed] [Google Scholar]
- 56.Shakur Y, Takeda K, Kenan Y, Yu ZX, Rena G, Brandt D, Houslay MD, Degerman E, Ferrans VJ, Manganiello VC. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J Biol Chem. 2000;275:38749–38761. doi: 10.1074/jbc.M001734200. [DOI] [PubMed] [Google Scholar]
- 57.Sheppard H, Burghardt C. Adenyl cyclase in non-nucleated erythrocytes of several mammalian species. Biochem Pharmacol. 1969;18:2576–2578. doi: 10.1016/0006-2952(69)90374-8. [DOI] [PubMed] [Google Scholar]
- 58.Sheppard H, Burghardt CR. Age-dependent changes in the adenylate cyclase and phosphodiesterase activity of rat erythrocytes. Biochemical Pharmacology. 1973;22:427–429. doi: 10.1016/0006-2952(73)90423-1. [DOI] [PubMed] [Google Scholar]
- 59.Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep. 2005;57 (Suppl):222–228. [PubMed] [Google Scholar]
- 60.Sprague RS, Bowles EA, Hanson MS, Dufaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin Analogs Stimulate Receptor-Mediated cAMP Synthesis and ATP Release from Rabbit and Human Erythrocytes. Microcirculation. 2008;99999:1–11. doi: 10.1080/10739680701833804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation. 2008;15:461–471. doi: 10.1080/10739680701833804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 63.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 64.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep. 2009;61:183–190. doi: 10.1016/s1734-1140(09)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol. 2003;285:H693–700. doi: 10.1152/ajpheart.01026.2002. [DOI] [PubMed] [Google Scholar]
- 66.Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ. Reduced expression of G(i) in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes. 2006;55:3588–3593. doi: 10.2337/db06-0555. [DOI] [PubMed] [Google Scholar]
- 67.Sprague RS, Stephenson AH, Ellsworth ML. Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab. 2007;18:350–355. doi: 10.1016/j.tem.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Sprague RS, Stephenson AH, Ellsworth ML, Keller C, Lonigro AJ. Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med (Maywood) 2001;226:434–439. doi: 10.1177/153537020122600507. [DOI] [PubMed] [Google Scholar]
- 69.Stone WM, Demaerschalk BM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. J Stroke Cerebrovasc Dis. 2008;17:129–133. doi: 10.1016/j.jstrokecerebrovasdis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki K, Terao T, Osawa T. Studies on adenosine 3',5'-monophosphate phosphodiesterase of human erythrocyte membranes. Biochim Biophys Acta. 1980;602:78–86. doi: 10.1016/0005-2736(80)90291-6. [DOI] [PubMed] [Google Scholar]
- 71.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. The Journal of cell biology. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandecasteele G, Rochais F, Abi-Gerges A, Fischmeister R. Functional localization of cAMP signalling in cardiac myocytes. Biochem Soc Trans. 2006;34:484–488. doi: 10.1042/BST0340484. [DOI] [PubMed] [Google Scholar]
- 73.Viguerie N, Clement K, Barbe P, Courtine M, Benis A, Larrouy D, Hanczar B, Pelloux V, Poitou C, Khalfallah Y, Barsh GS, Thalamas C, Zucker JD, Langin D. In vivo epinephrine-mediated regulation of gene expression in human skeletal muscle. The Journal of clinical endocrinology and metabolism. 2004;89:2000–2014. doi: 10.1210/jc.2003-031733. [DOI] [PubMed] [Google Scholar]
- 74.Willoughby D, Baillie GS, Lynch MJ, Ciruela A, Houslay MD, Cooper DM. Dynamic regulation, desensitization, and cross-talk in discrete subcellular microdomains during beta2-adrenoceptor and prostanoid receptor cAMP signaling. J Biol Chem. 2007;282:34235–34249. doi: 10.1074/jbc.M706765200. [DOI] [PubMed] [Google Scholar]
- 75.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. Embo J. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu AY, Tang XB, Martinez SE, Ikeda K, Beavo JA. Molecular determinants for cyclic nucleotide binding to the regulatory domains of phosphodiesterase 2A. J Biol Chem. 2004;279:37928–37938. doi: 10.1074/jbc.M404287200. [DOI] [PubMed] [Google Scholar]
- 77.Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. Phosphodiesterase 4D is required for beta2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc Natl Acad Sci U S A. 2005;102:909–914. doi: 10.1073/pnas.0405263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yarwood SJ, Steele MR, Scotland G, Houslay MD, Bolger GB. The RACK1 signaling scaffold protein selectively interacts with the cAMP-specific phosphodiesterase PDE4D5 isoform. J Biol Chem. 1999;274:14909–14917. doi: 10.1074/jbc.274.21.14909. [DOI] [PubMed] [Google Scholar]
- 79.Zaccolo M, Di Benedetto G, Lissandron V, Mancuso L, Terrin A, Zamparo I. Restricted diffusion of a freely diffusible second messenger: mechanisms underlying compartmentalized cAMP signalling. Biochem Soc Trans. 2006;34:495–497. doi: 10.1042/BST0340495. [DOI] [PubMed] [Google Scholar]
- 80.Zoraghi R, Corbin JD, Francis SH. Properties and functions of GAF domains in cyclic nucleotide phosphodiesterases and other proteins. Mol Pharmacol. 2004;65:267–278. doi: 10.1124/mol.65.2.267. [DOI] [PubMed] [Google Scholar]