Abstract
Understanding visual signal processing can benefit from simultaneous measurement of different types of retinal neurons working together. In this paper we demonstrate that intrinsic optical signal (IOS) imaging of frog retina slices allows simultaneous observation of stimulus-evoked responses propagating from the photoreceptors to inner neurons. High resolution imaging revealed robust IOSs at the photoreceptor, inner plexiform and ganglion cell layers. While IOSs of the photoreceptor layer were mainly confined to the area directly stimulated by the visible light; IOSs of inner retinal layers spread from the stimulus site into relatively large areas with a characteristic near to far time course.
As the first part of the visual system, the retina is responsible for effective capture of photons, conversion of light energy to biochemical and electrical activity, and early visual information processing via interactions among different types of neural cells. Advanced investigations of retinal signal processing mechanisms not only leads to a better understanding of the operation of the normal retina, but also can bring insight to improving retinal disease diagnosis and treatment outcome monitoring. In-depth understanding of retinal signal processing has been hindered by technical limitations of the traditional methods of retinal neural studies. It is well established that the retina is a complex neural network which contains multiple cell types that are stratified in different functional layers. Single and multiple-channel electrophysiological recording provides valuable information about retinal information processing at the cellular level [1]. However, the spatial resolution of conventional electrophysiological measurement and direct information about the interactions between retinal neurons are limited. A better understanding of visual signal processing would benefit from simultaneous measurement of stimulus-evoked responses of multiple retinal layers/neuron types working together.
Intrinsic optical signals (IOSs) have been detected in retinal photoreceptors [2] and inner neurons [3]. Fast IOSs, recorded using near infrared (NIR) light imaging, have time courses that are comparable to electrophysiological kinetics [4, 5], and thus may provide a new method to evaluate the functional connectivity of photoreceptors and inner neurons. By focusing the NIR light at different depths of isolated frog (Rana Pipines) retina, rapid IOS imaging has revealed complex spatiotemporal patterns at the photoreceptor and inner retinal layers [6]. However, simultaneous monitoring of stimulus-evoked signal transmission over multiple retinal layers/cells is still technically challenging. In principle, functional optical coherence tomography (OCT) can provide the spatial and temporal resolutions required for in-depth revolved IOS imaging of intact retina. Both time-domain [7, 8] and frequency-domain [9] OCT systems have recently demonstrated functionality for in vitro [7, 8] and in vivo [9] IOS imaging of the retina. However, because of the limited signal-to-noise ratio (SNR) due to unavoidable speckle noise of the coherent light source of the OCTs, reliable single-pass IOS imaging of retinal signal propagation is still difficult to achieve. Although averaging techniques can improve the SNR for OCT measurement, averaging of multiple experimental passes is a time-consuming procedure. In addition, given the requirement for multiple measurements with repeated stimuli, at least some components of dynamic optical responses may be reduced, distorted, or lost because of the imperfection of synchronization for averaged stimulations, degradation or movement of the retina over time, and the possibility that some fast IOSs may vary in phase relative to the stimulus in individual trials. In this paper we demonstrate the feasibility of single-pass IOS imaging of a stimulus activated frog retina slice preparation that allows simultaneous observation of stimulus-evoked responses from the photoreceptors to inner neurons.
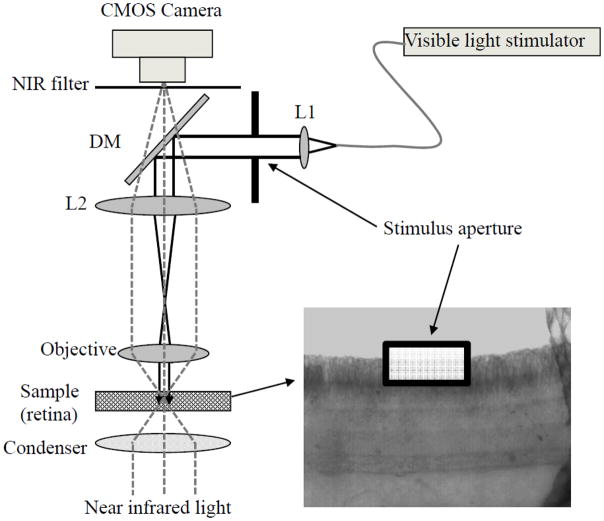
Figure 1 illustrates the schematic diagram of the experimental setup. Equipped with a high-speed CMOS camera (PCO1200, PCO AG, Kelheim, Germany), the imaging system can support ~ms temporal resolution and ~μm spatial resolution. The imaging system consisted of two light sources: a NIR light for IOS recording, and a visible light for retinal stimulation. In order to reduce the effect of stray light on retinal stimulation, the visible light was collimated, to ensure minimized beam blur of the stimulus.
Fig. 1.
Schematic diagram of experimental setup. During the measurement, the retinal slice was continuously illuminated by the NIR light for recording of stimulus-evoked IOSs; while the collimated visible light stimulator with a square aperture was used for retinal stimulation. At the dichroic mirror (DM), visible stimulus light was reflected and NIR recording light was passed through. The NIR filter was used to block visible stimulus light, and allow the NIR probe light to reach the CMOS camera. A 10X system magnification, from the retina to the camera, was used for this study.
Isolated frog (Rana Pipiens) retinal slices were used for demonstration of the feasibility of simultaneous optical monitoring of visual signal propagation from the photoreceptor outer segments to inner retina. The preparation of retinal slices was conducted in a dark environment with dim red light illumination. Frogs were first dark-adapted for 20–30 minutes before rapid euthanasia and retina isolating surgery were conducted [6]. After isolating the intact retina from the retinal pigment epithelium, a retinal patch (~ 4 mm × 4 mm) was laid flat on a round acrylic plate with retinal anterior (i.e. ganglion cell layer) facing upwards. A sharp surgical scalpel was used to slice the middle part of the isolated retina. Finally, the acrylic plate holding the sliced retina (~200 μm thickness) was transferred into a recording chamber for IOS imaging. The retina was stabilized in the recording chamber with a slight pressure using a micromesh sheet [7]. Slices within a flat mounted retina opened a cross-section of the retina, and thus allowed parallel IOS observation of retinal signal propagation across retinal layers. During the recording, the retina was immersed in Ringer’s solution, and was continuously illuminated by a ~1 mW NIR light. A 500 ms visible light (~105 550-nm photons·μm−2· ms−1) flash was used to stimulate the retina slice. As shown in Fig. 1, a square aperture which was conjugated to the sample (retina) was used to limit the stimulus light at the central part of the photoreceptor layer.
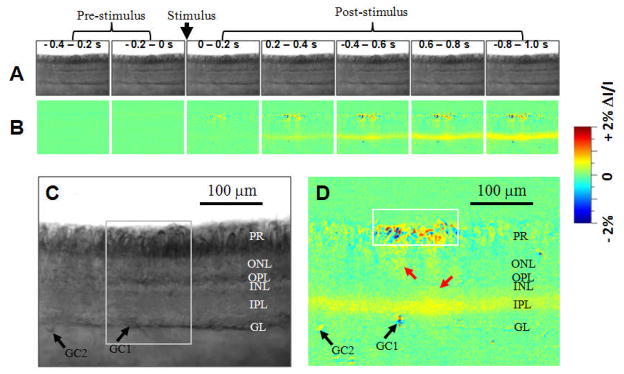
Figure 2 illustrates the results of single-pass IOS imaging of light-evoked frog retinal slices. Rapid IOS images revealed a complex but consistent spatiotemporal pattern of retinal activation over multiple functional layers. Robust IOSs were consistently detected from stimulus activated photoreceptor outer segments, IPL, and ganglion cells (black arrowheads in Fig. 2D); and weak IOSs (red arrowheads in Fig. 2D) could be occasionally identified in the outer nuclear layer (ONL), outer plexiform layer (OPL), and inner nuclear layer (INL) between the photoreceptor and IPL. At the photoreceptor layer, high magnitude IOSs were mainly confined to the area (~120 μm) covered by the visible stimulus light (white square shown in Fig. 2D). In comparison, IOSs of the IPL and ganglion layer could spread beyond the whole image area (~ 400 μm). GC1 and GC2 in Fig. 2D show ganglion cell layer activities directly beneath the visible light stimulus and at the edge of the recording area, respectively.
Fig. 2.
(A) Representative raw image sequence of the retinal slice before differential processing. (B) IOS image sequence. The raw CMOS images were recorded with a speed of 500 frame/s. Each illustrated frame is an average over 200 ms interval (100 frames). The IOS images were reconstructed by processing the raw images as follows: 1) The pre-stimulus baseline images were averaged, pixel by pixel, and was taken as the background light intensity, I, of each pixel; 2) The background light intensity was subtracted from each recorded frame sequentially, pixel by pixel, to get the dynamic optical response, ΔI, of each pixel of the images; 3) The image sequence of ΔI/I was constructed to show the dynamic intrinsic optical changes of the retina activated by a visible light stimulus. (C) Enlarged image of the fifth frame in A. (D) Enlarged image of the fifth frame in B. The white square indicates the visible light stimulus aperture. Black arrowheads point to the somas of two ganglions cells (GC1 and GC2). Black arrowheads point to the somas of two ganglions cells (GC1 and GC2).
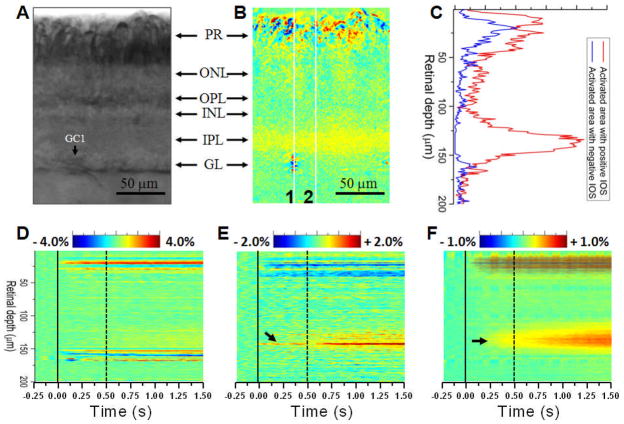
Both positive (increasing) and negative (decreasing) optical responses were observed in a retinal slice activated by a single stimulus. Fig. 3C shows the statistics of positive and negative optical signals throughout retinal depth, from the photoreceptor to ganglion layers. High resolution IOS images showed complex spatial distributions of positive and negative IOSs at different retinal layers (Fig. 3B). At the photoreceptor and ganglion layers, positive and negative optical responses were mixed at a sub-cellular scale. We speculate that the positive and negative signal complexity might result from different types of localized light scattering and transmission changes that could happen on a single activated photoreceptor or ganglion cell. Previous investigations have indicated that both cellular (i.e., swelling or shrinking change of the cell body) [10, 11] and sub-cellular (e.g., nuclear infoldings) [12] changes occur during neural activation. We consistently observed that the IPL was dominated by positive-going optical responses (Figs. 2 and 3). It is well established that the bipolar, amacrine and ganglion cells interact in the IPL through nerve terminals, and thus we hypothesize that the positive signal that dominated the IOS response in the IPL might be related to light scattering changes due to light-regulated release of synaptic vesicles at nerve terminals [13].
Fig. 3.
(A) and (B) show enlarged raw and IOS images of the area marked by the white square in Fig. 2C. (C) Statistics of positive and negative signals over the retinal slice shown in Fig. 3B. (D) and (E) are spatiotemporal images of the line areas 1 and 2 in Fig. B. (F) Averaged spatiotemporal image of the retinal slice shown in Fig. B. At each time point (i.e. each frame), the IOSs were averaged in the lateral direction, and thus a two dimensional IOS image, such as Fig. 3B, was condensed to a one dimensional vertical line in the spatiotemporal image Fig. 3F. In Fig. 3D–F, the vertical solid and dash lines indicate the onset and offset of the visible light stimulus.
Fig. 3D–F show spatiotemporal IOS images that provide simultaneous depiction of the temporal (lateral coordination) and spatial (vertical coordination) dynamics of IOSs in the retinal slice. In the photoreceptor and ganglion layers, fast optical responses occurred almost immediately, and rapidly reached their magnitude peaks within ~100 ms after the onset of the visible light stimulus (Fig. 3D). At the IPL, IOS images consistently disclosed detectable optical signal in middle part (arrowheads in Fig. 3E and F) of the IPL first, and the response gradually spread into the whole layer of the IPL. Overall optical response of the IPL reached its magnitude peak after the offset of the stimulus (Fig. 3F).
In summary, we have demonstrated the feasibility of parallel optical monitoring of visual signal propagation through the entire depth of the retina. Fast NIR imaging revealed three robust sources of fast IOS, i.e., photoreceptors, IPL and ganglion cells. These optical responses showed complex but consistent spatiotemporal dynamics during the retinal activation. We believe that IOS imaging of retinal slices provides a simple but valuable platform for advanced study of the functional connectivity of retinal neurons, particularly the interactions among IPL cell dendrites that cannot be simultaneously recorded in multiple cells by other techniques. A better understanding of the retinal neural network will advance our understanding the mechanisms of visual information processing in retina, and will also provide valuable information to improve retinal disease diagnosis and treatment outcome evaluation.
Acknowledgments
This research is supported by Dana Foundation (Brain and Immuno-Imaging Grant Program), Eyesight Foundation of Alabama, and NIH (1R21RR025788-01).
References
- 1.Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke AM, Chichilnisky EJ. Electrical stimulation of mammalian retinal ganglion cells with multielectrode arrays. J Neurophysiol. 2006;95:3311–3327. doi: 10.1152/jn.01168.2005. [DOI] [PubMed] [Google Scholar]
- 2.Pepperberg DR, Kahlert M, Krause A, Hofmann KP. Photic modulation of a highly sensitive, near-infrared light-scattering signal recorded from intact retinal photoreceptors. Proc Natl Acad Sci U S A. 1988;85:5531–5535. doi: 10.1073/pnas.85.15.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawis SM, Rossetto M. Light-evoked changes in near-infrared transmission by the ON and OFF channels of the anuran retina. Visual Neurosci. 1993;10:687–692. doi: 10.1017/s0952523800005381. [DOI] [PubMed] [Google Scholar]
- 4.Li YG, Zhang QX, Liu L, Amthor FR, Yao XC. High spatiotemporal resolution imaging of fast intrinsic optical signals activated by retinal flicker stimulation. Opt Express. 2010;18:7210–7218. doi: 10.1364/OE.18.007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao XC, George JS. Dynamic neuroimaging of retinal light responses using fast intrinsic optical signals. Neuroimage. 2006;33:898–906. doi: 10.1016/j.neuroimage.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 6.Yao XC, Zhao YB. Optical dissection of stimulus-evoked retinal activation. Opt Express. 2008;16:12446–12459. doi: 10.1364/oe.16.012446. [DOI] [PubMed] [Google Scholar]
- 7.Yao XC, Yamauchi A, Perry B, George JS. Rapid optical coherence tomography and recording functional scattering changes from activated frog retina. Appl Opt. 2005;44:2019–2023. doi: 10.1364/ao.44.002019. [DOI] [PubMed] [Google Scholar]
- 8.Bizheva K, Pflug R, Hermann B, Povazay B, Sattmann H, Qiu P, Anger E, Reitsamer H, Popov S, Taylor JR, Unterhuber A, Ahnelt P, Drexler W. Optophysiology: depth-resolved probing of retinal physiology with functional ultrahigh-resolution optical coherence tomography. Proc Natl Acad Sci U S A. 2006;103:5066–5071. doi: 10.1073/pnas.0506997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan VJ, Chen Y, Duker JS, Fujimoto JG. In vivo functional imaging of intrinsic scattering changes in the human retina with high-speed ultrahigh resolution OCT. Opt Express. 2009;17:3861–3877. doi: 10.1364/oe.17.003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao XC, Rector DM, George JS. Optical lever recording of displacements from activated lobster nerve bundles and Nitella internodes. Appl Opt. 2003;42:2972–2978. doi: 10.1364/ao.42.002972. [DOI] [PubMed] [Google Scholar]
- 11.Tasaki I, Byrne PM. Rapid structural changes in nerve fibers evoked by electric current pulses. Biochem Biophys Res Commun. 1992;188:559–564. doi: 10.1016/0006-291x(92)91092-5. [DOI] [PubMed] [Google Scholar]
- 12.Wittmann M, Queisser G, Eder A, Wiegert JS, Bengtson CP, Hellwig A, Wittum G, Bading H. Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling. J Neurosci. 2009;29:14687–14700. doi: 10.1523/JNEUROSCI.1160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salzberg BM, Obaid AL, Gainer H. Large and rapid changes in light scattering accompany secretion by nerve terminals in the mammalian neurohypophysis. J Gen Physiol. 1985;86:395–411. doi: 10.1085/jgp.86.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]