Abstract
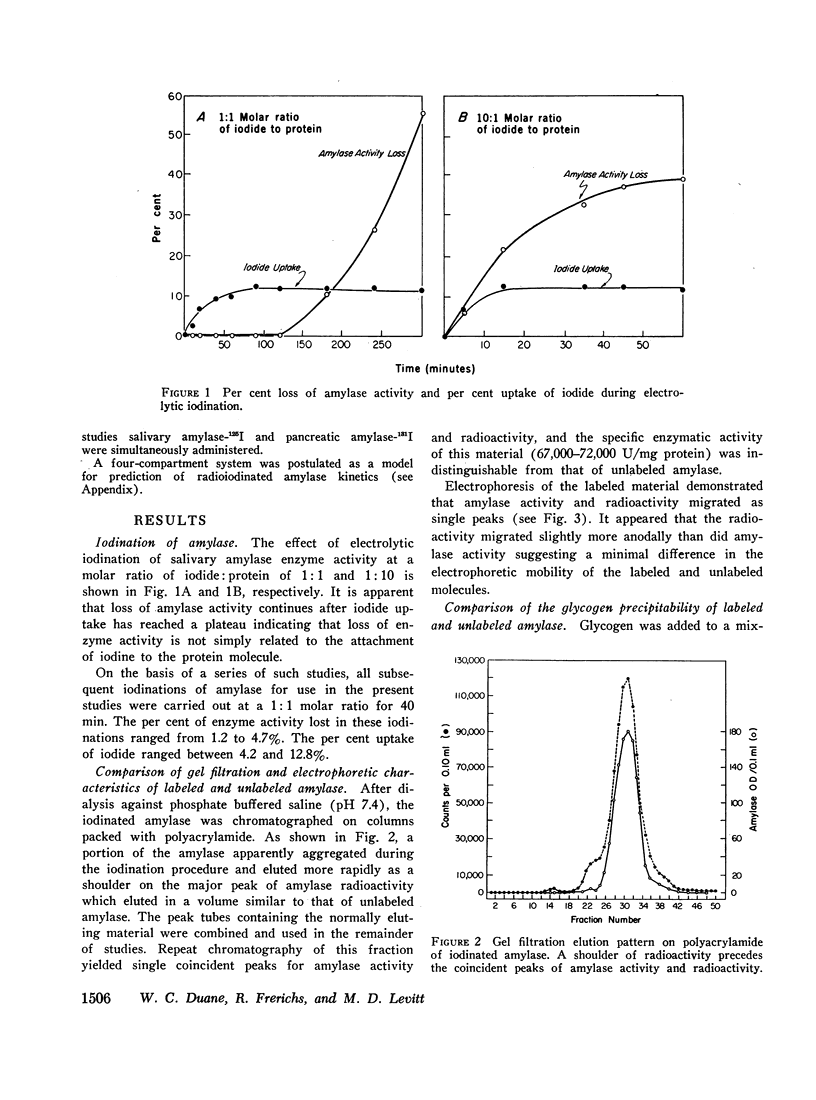
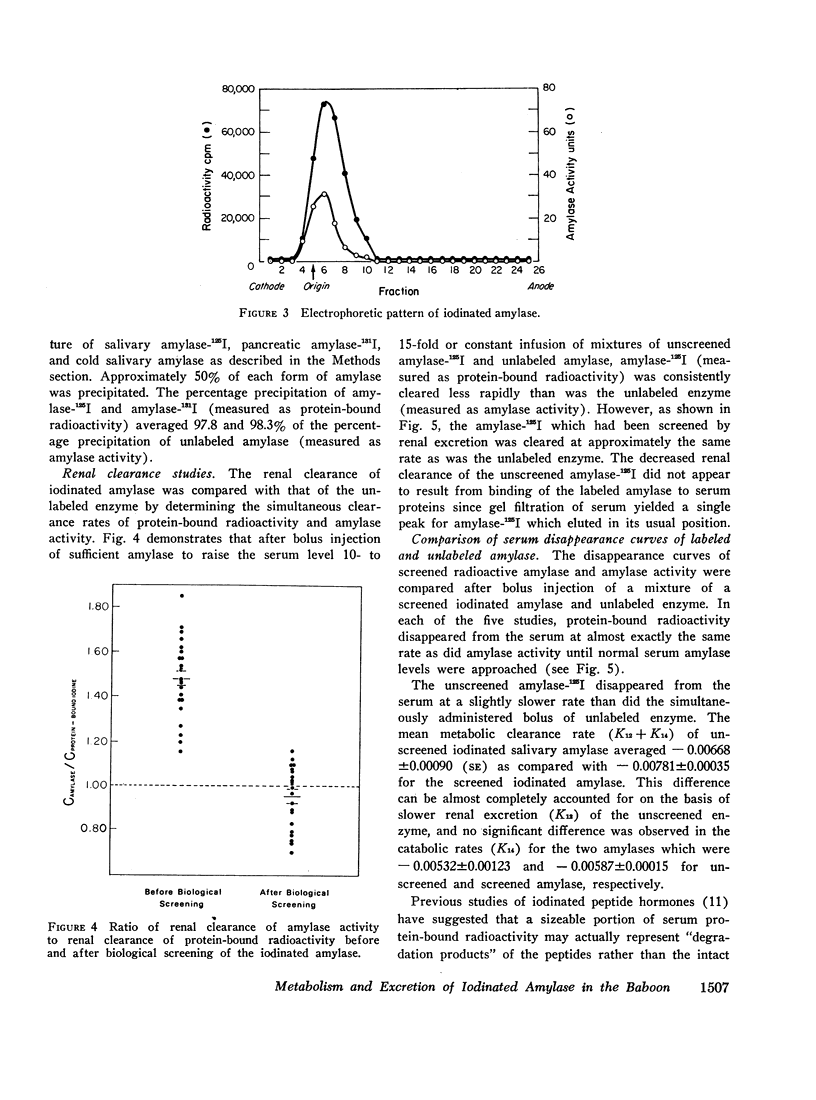
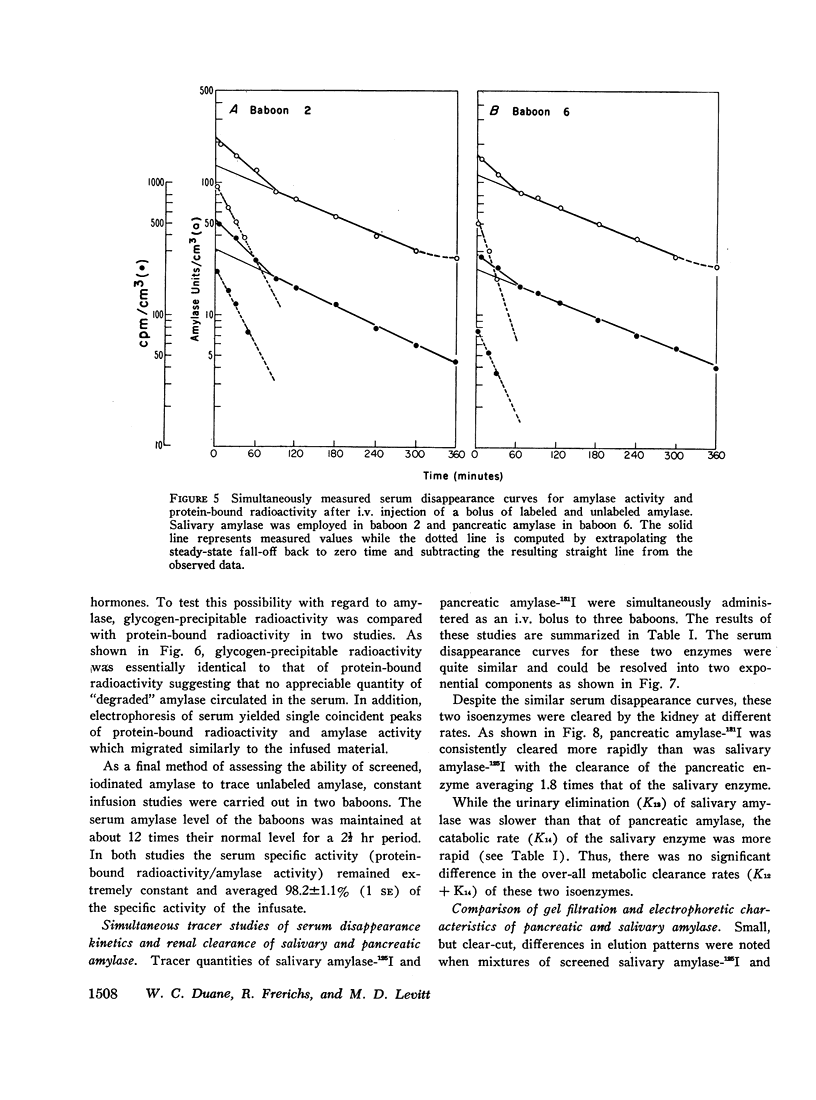
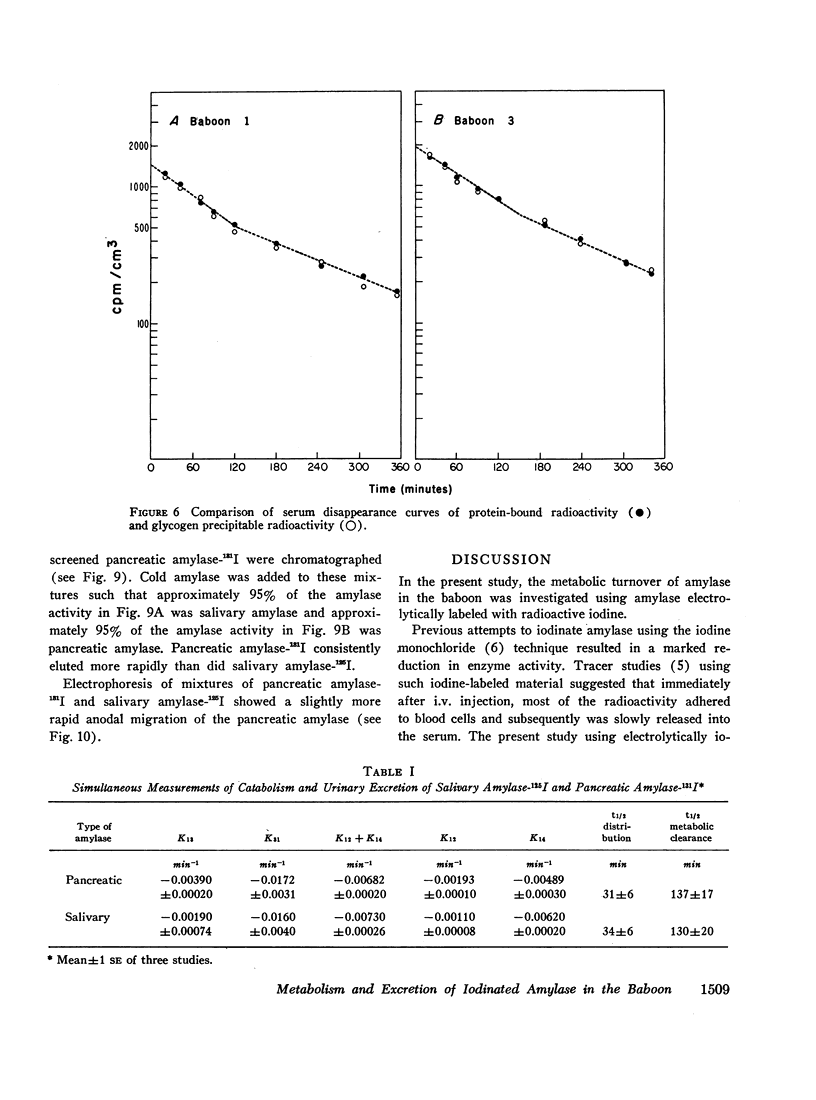
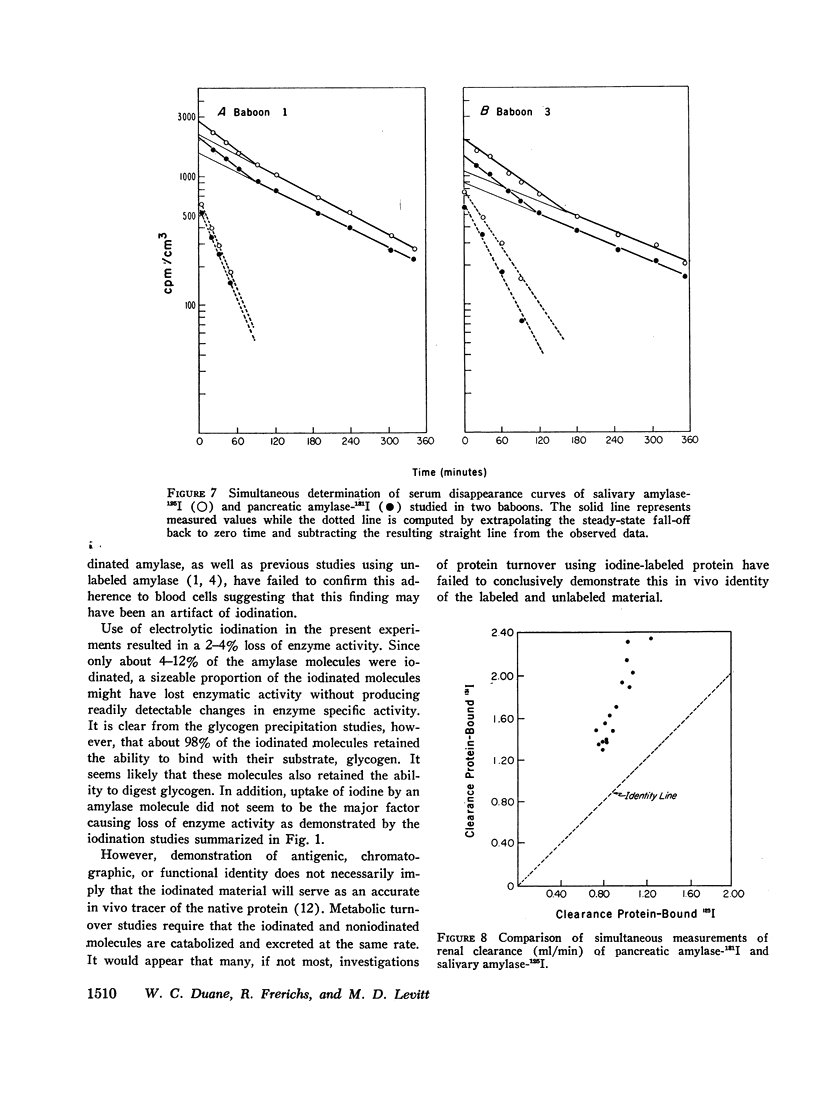
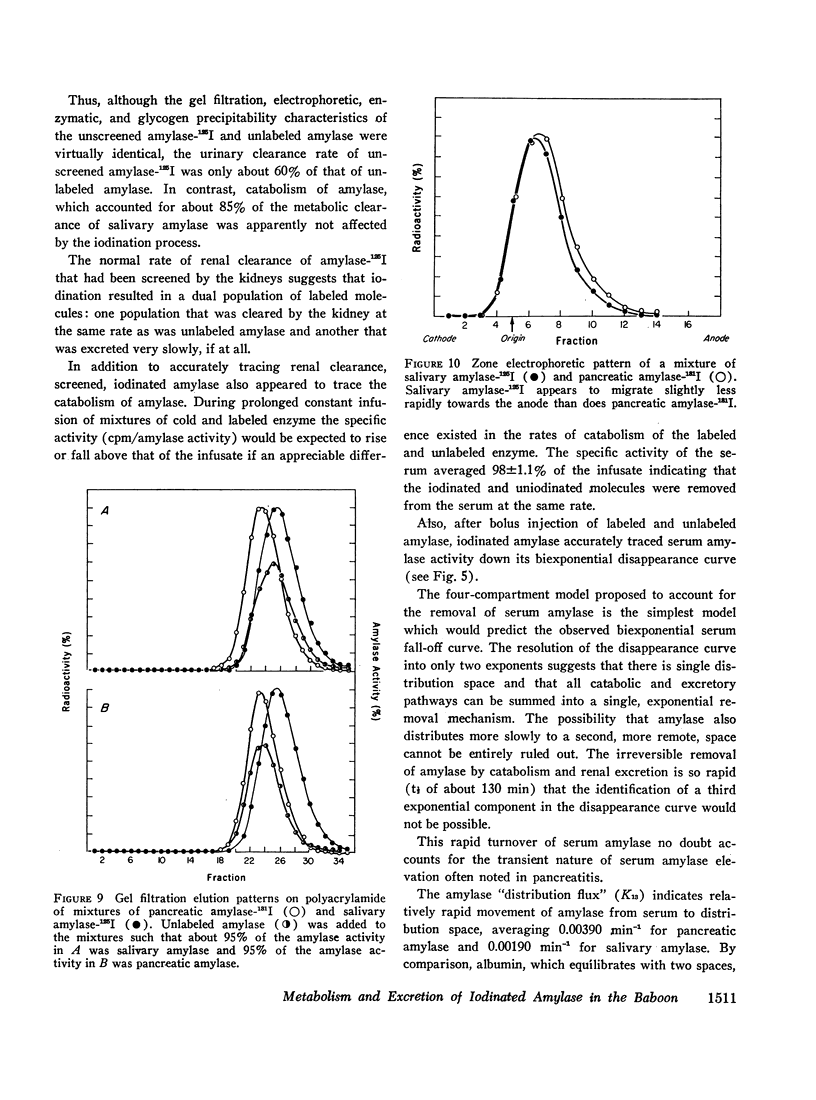
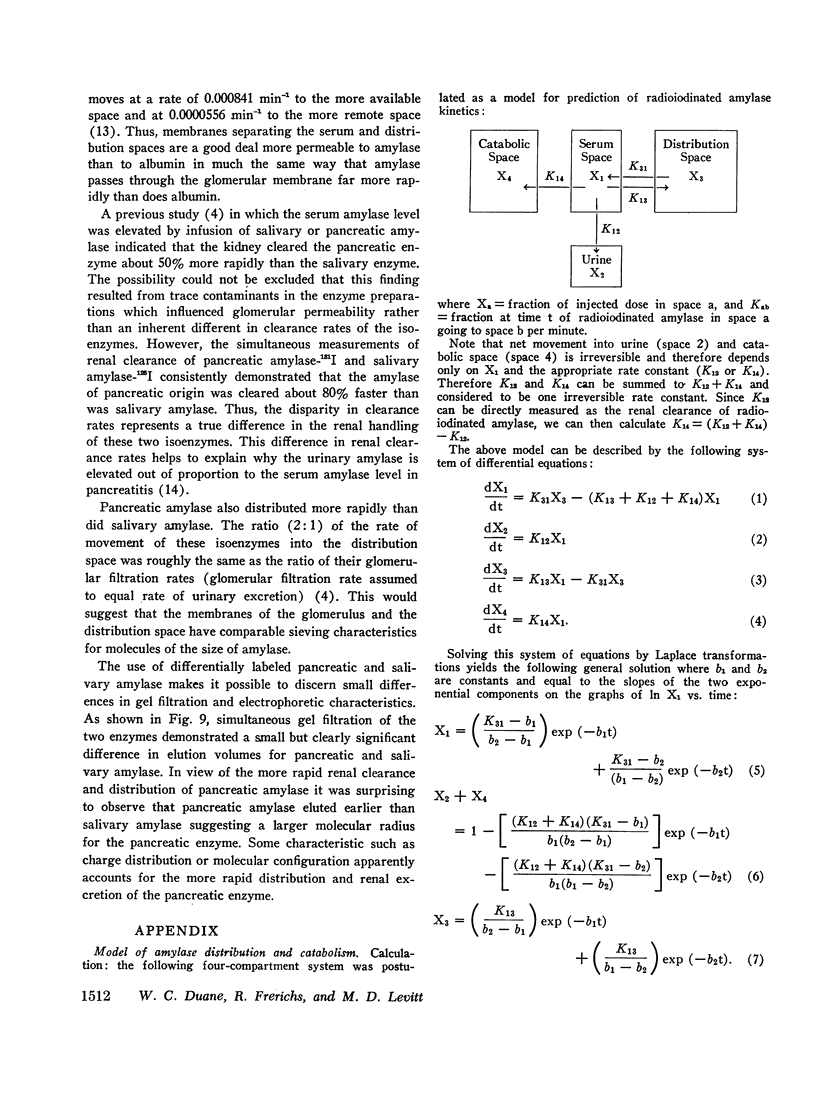
The metabolic turnover of salivary and pancreatic amylase was studied in the baboon, an animal with a serum amylase level and renal clearance of amylase similar to man. Purified amylase was electrolytically iodinated. Although iodinated and uniodinated amylase had similar gel filtration, electrophoretic, enzymatic, glycogen precipitation characteristics, the labeled enzyme was cleared less rapidly by the kidney than was the unlabeled material. However, urinary iodinated amylase which had been biologically screened by the kidney had a renal clearance and serum disappearance rate indistinguishable from unlabeled amylase and thus can serve as a tracer in metabolic turnover studies. Administration of a mixture of salivary amylase-125I and pancreatic amylase-131I made it possible to simultaneously measure the serum disappearance and renal clearance of these two isoenzymes. The metabolic clearance of both isoenzymes was extremely rapid with half-times of about 130 min. This rapid turnover of serum amylase probably accounts for the transient nature of serum amylase elevation which frequently occurs in pancreatitis. Pancreatic amylase-131I was consistently cleared more rapidly (mean clearance ratio: 1.8) by the kidney than was salivary amylase-125I. This more rapid renal clearance of pancreatic amylase may help to explain the disproportionate elevation of urinary amylase relative to serum amylase observed in pancreatitis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron D. P., Burger H. G., Catt K. J., Doig A. Metabolic clearance rate of radioiodinated human growth hormone in man. J Clin Invest. 1969 Sep;48(9):1600–1608. doi: 10.1172/JCI106125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane W. C., Frerichs R., Levitt M. D. Distribution, turnover, and mechanism of renal excretion of amylase in the baboon. J Clin Invest. 1971 Jan;50(1):156–165. doi: 10.1172/JCI106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt N., Bonorris G., Coverdale G. M., Lanchantin G. F. Hepatic deposition of 131-I labeled amylase in dogs: comparison of enzymatic and isotope measurements. Proc Soc Exp Biol Med. 1968 May;128(1):125–130. doi: 10.3181/00379727-128-32959. [DOI] [PubMed] [Google Scholar]
- Hiatt N., Bonorris G., Lanchantin G. F. Influence of reticuloendothelial blockade on the binding of amylase to blood cells. Am J Physiol. 1967 Sep;213(3):744–748. doi: 10.1152/ajplegacy.1967.213.3.744. [DOI] [PubMed] [Google Scholar]
- Katz J., Bonorris G. Electrolytic iodination of proteins with I-125 and I-131. J Lab Clin Med. 1968 Dec;72(6):966–970. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- Levitt M. D., Rapoport M., Cooperband S. R. The renal clearance of amylase in renal insufficiency, acute pancreatitis, and macroamylasemia. Ann Intern Med. 1969 Nov;71(5):919–925. doi: 10.7326/0003-4819-71-5-919. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Rosa U., Pennisi F., Bianchi R., Federighi G., Donato L. Chemical and biological effects of iodination on human albumin. Biochim Biophys Acta. 1967 Apr 11;133(3):486–498. doi: 10.1016/0005-2795(67)90553-3. [DOI] [PubMed] [Google Scholar]
- UJIHIRA I., SEARCY R. L., BERK J. E., HAYASHI S. A SACCHAROGENIC METHOD FOR ESTIMATING ELECTROPHORETIC AND CHROMATOGRAPHIC DISTRIBUTION OF HUMAN SERUM AMYLASE. Clin Chem. 1965 Feb;11:97–112. [PubMed] [Google Scholar]
- Yacoub R. S., Appert H. E., Howard J. M. Metabolism of pancreatic amylase and lipase infused intravenously into dogs. Arch Surg. 1969 Jul;99(1):54–58. doi: 10.1001/archsurg.1969.01340130056010. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Labeling of proteins--problems and practices. Trans N Y Acad Sci. 1966 Jun;28(8):1033–1044. doi: 10.1111/j.2164-0947.1966.tb02406.x. [DOI] [PubMed] [Google Scholar]


