Abstract
The identification of monotypic light chains is an important adjunct to the diagnosis of B-cell lymphoma, yet is often difficult to reliably perform on formalin-fixed paraffin sections. We have evaluated a new set of monoclonal antibodies to kappa and lambda light chain that are reactive in paraffin sections. In reactive lymphoid tissues, polytypic staining was noted in greater than 95% of cases, with strong staining of plasma cells, moderate staining of the follicular dendritic cell network, and weak staining of mantle zone cells. Strong staining for the appropriate light chain was seen in each of 7 cases of multiple myeloma. In a series of 58 cases of B-cell lymphoma, correlation between the results of immunohistochemistry and flow cytometry was obtained in 36 cases (62%), including 32 cases (21 kappa and 11 lambda) in which a single light chain was expressed. Monotypic staining also seen in 6 additional cases (10%) in which the flow cytometry had been negative. Thirty of 46 cases (65%) of follicular lymphoma showed monotypic light chain expression, in contrast to 64 of 67 cases (95%) of reactive lymphoid hyperplasia, which showed polytypic light chain expression. These antibodies may provide an effective adjunct to the diagnosis of B-cell lymphoma in routine diagnostic work.
Keywords: immunoglobulin light chain, immunohistochemistry, kappa, lambda, follicular lymphoma, reactive follicular hyperplasia
The identification of monotypic light chain expression, while not diagnostic alone, is an important criterion to use when one is attempting establish a diagnosis of B-cell lymphoma, as only rare B-cell proliferations are monotypic, yet polyclonal (e.g.\, multisystemic Castleman disease being one exception) (1). The demonstration of monotypic light chains can be accomplished by either flow cytometry or frozen section immunohistochemical studies, when fresh or frozen tissue is available, however many times such tissue is not available in routine diagnostic work.
The detection of immunoglobulin light chains is one of the oldest applications of paraffin section immunohistochemistry, with the first publications appearing more than 30 years ago (2-4). It widely acknowledged to be an effective means of detecting monotypic light chain expression in plasma cells, however its efficacy in detecting the smaller amounts of immunoglobulin present on B-lymphocytes and B-cell lymphomas is still controversial. While a minority of authors report relatively reliable detection of immunoglobulin in B-cells and their neoplasms, using either enzyme digestion combined with microwave-heating antigen retrieval (5), enzyme digestion alone (6), or heat-induced epitope retrieval alone (7), the majority of investigators have not obtained similar good results (8-14). In addition, our own practical experience, both from our laboratory and reviewing diagnostic slides prepared by other laboratories, suggests that there is not widespread satisfaction with current practical light chain immunoglobulin studies. A demonstration of the dissatisfaction of light chain immunohistochemical studies is the popularity of in situ hybridization studies for the detection of light chain immunoglobulin mRNA (15,16). These studies have never been shown to be of high sensitivity, and are typically only useful in proliferations showing frank plasmacytoid features.
Recently, we evaluated a new set of monoclonal anti-kappa and anti-lambda antibodies. This report summarizes our experience with the antibodies. In addition to a survey of non-Hodgkin lymphomas, with comparison to the results of flow cytometry, we focused on the diagnostic problem of reactive follicular hyperplasia vs. follicular lymphoma, since this differential diagnosis remains a significant one in everyday practice and could potentially benefit from an effective assessment of light chains.
Materials and Methods
Monoclonal antibodies R10135 (anti-kappa) and R10141 (anti-lambda) were obtained from Silver Lake Research Corporation (Monrovia, CA).
We studied formalin-fixed, paraffin embedded specimens from 7 cases of myeloma and 58 cases of B-cell lymphoma, each of which had flow cytometry studies available for comparison. Representative strips of the blocks were prepared in checkerboards or multitumor blocks, as previously reported (17,18). We also studied additional 26 cases of follicular lymphoma (without concomitant flow cytometry studies available), organized into multitissue areas using a Beecham microarrayer. Finally we studied 67 cases of reactive follicular hyperplasia. For 60 of these cases, multitissue arrays were prepared using a Beecham microarrayer, while the other cases were studied as a full tissue section (2 cases) or multitumor blocks (5 cases).
The immunohistochemical staining was performed on 5-um thick sections prepared from formalin-fixed, paraffin-embedded tissue. Tissues sections were deparaffinized in xylene and rehydrated in graded alcohol. Samples were then quenched in 3% hydrogen peroxide and pretreated to promote antigen retrieval by steaming with EDTA buffer (pH8.0) solution to a temperature of 98° C for 20 minutes, using a Black and Decker steamer. After antigen retrieval, the slides were then placed onto an autostainer (Dako, Carpinteria, CA) using the following protocol: 1. protein block (Dako) for 5 minutes; 2. incubation with the appropriate antibody for 30 minutes at room temperature; and 3. incubation with secondary labeled polymer anti-mouse (Dako, K4001) for 30 minutes at room temperature. After washes in buffer (Dako), the slides were incubated with diaminobenzidine tetrahydrochloride, and counterstained with hematoxylin for 3 minutes.
Results
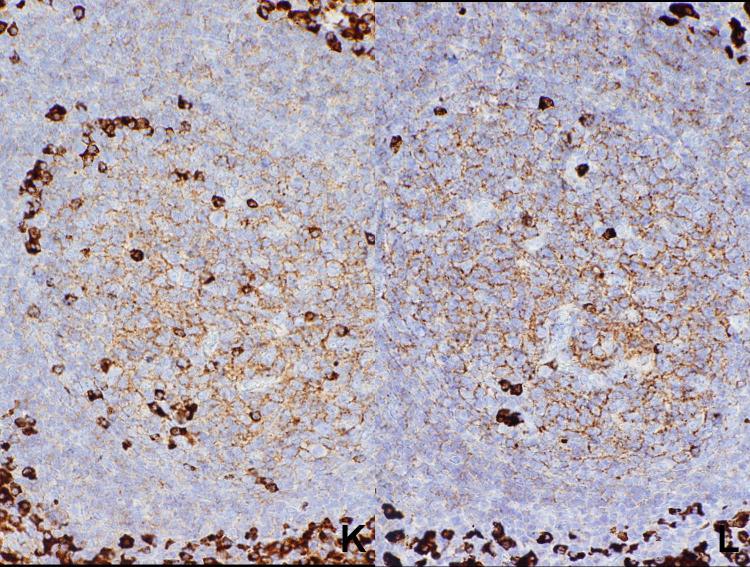
In cases of reactive lymphoid hyperplasia, variable staining for both light chains was seen in 64 of the 67 cases (Figure 1). In three cases, no staining for either kappa or lambda light chain was seen, probably related to poor fixation of the samples. In the stained cases, cases the intensity of the staining varied, probably varying with the quality of the fixation, and there was always a close correlation between intensity of the two light chain stains. In general, there were scattered cells in germinal centers strongly positive for light chain, with a moderate staining on the follicular dendritic network. Because of the staining on the network, it was difficult to assess individual staining of germinal center cells. There was weak staining of the mantle zone cells, and there were scattered plasma cells intensely stained in the interfollicular areas, along with scattered small to moderately sized lymphoid cells weakly to moderately positive in the interfollicular areas. Although difficult to discern in some cases, in general the staining was cytoplasmic rather than membranous, even in lymphoid cells with sparse amounts of cytoplasm.
Figure 1.
Reactive follicular hyperplasia. Note strong polytypic staining of scattered cells in the germinal center as well as plasma cells in the interfollicular areas, moderate staining of the processes of follicular dendritic cells, and weak staining of mantle zone cells.
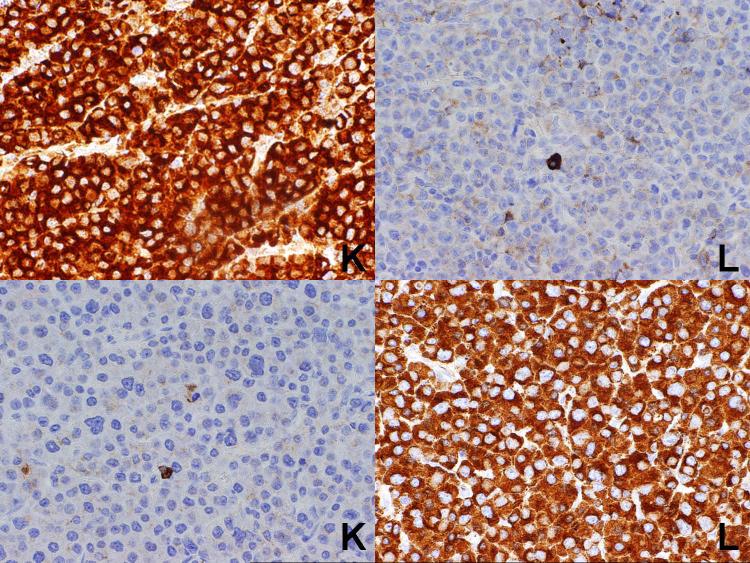
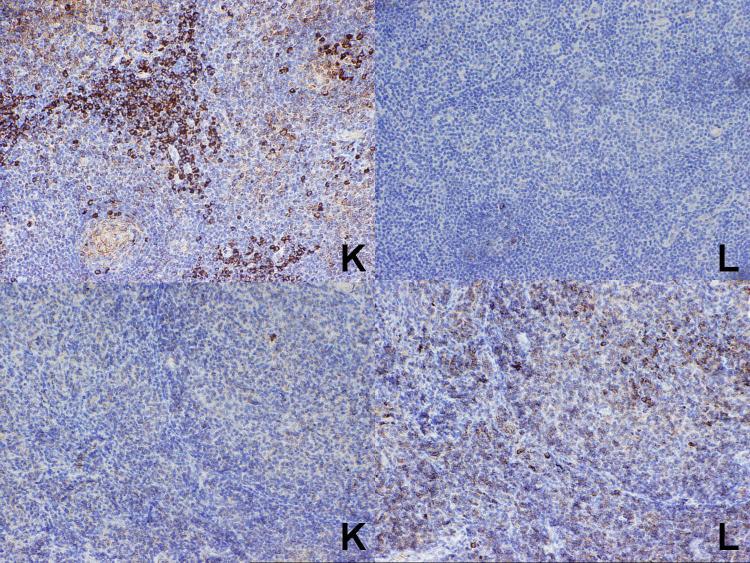
In the seven cases of plasma cell myeloma, strong staining for the appropriate light chain was seen in each case (Figure 2). The results for the 58 cases of B-cell lymphoma for which the results of flow cytometry studies were known are summarized in Table 1. Overall, correlation between the results of immunohistochemistry and flow cytometry was obtained in 36 of the 58 cases (62%), including 32 cases (21 kappa and 11 lambda) (58%) in which a single light chain was expressed. In 16 cases, flow cytometry studies demonstrated a monotypic light chain, while the immunostains were negative, while in 6 cases (10%), the immunostains demonstrated a monotypic light chain while the flow cytometry studies were polytypic or negative (Figure 3).
Figure 2.
Plasma cell myeloma. A known kappa-positive case is shown at top, while a known lambda-positive case is shown at the bottom. Strong cytoplasmic staining is seen for the appropriate light chain.
Table 1.
Results in B-Cell Lymphoma (excluding Myeloma)
| FLOW | ||||
|---|---|---|---|---|
| IM | K | L | N | |
| K | 21 | 0 | 4 | |
| L | 0 | 11 | 2 | |
| N | 5 | 11 | 4 | |
Flow= Flow cytometry studies; IM= Paraffin section immunohistochemical studies for light chain; K= Kappa light chain; L=lambda light chain; N=not detected
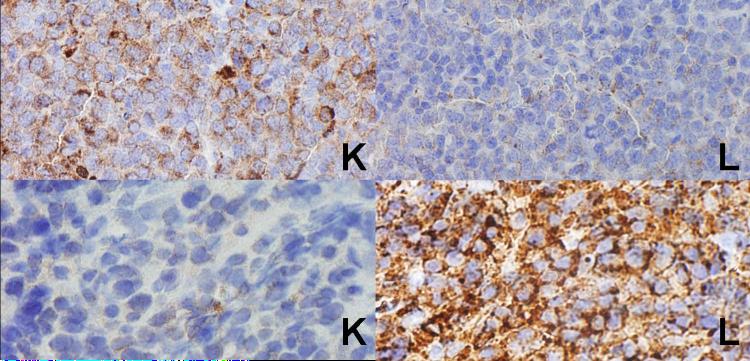
Figure 3.
At top is a case of nodal marginal zone B-cell lymphoma. Although the flow cytometry studies were negative, the paraffin section immunohistochemical studies clearly show a kappa-positive interfollicular cell population. At the bottom, as a case of follicular lymphoma, grade 3. Although the flow cytometry studies were also negative, the paraffin section immunohistochemical studies show a lambda-light restricted population of large lymphoid cells in both the follicular and interfollicular areas.
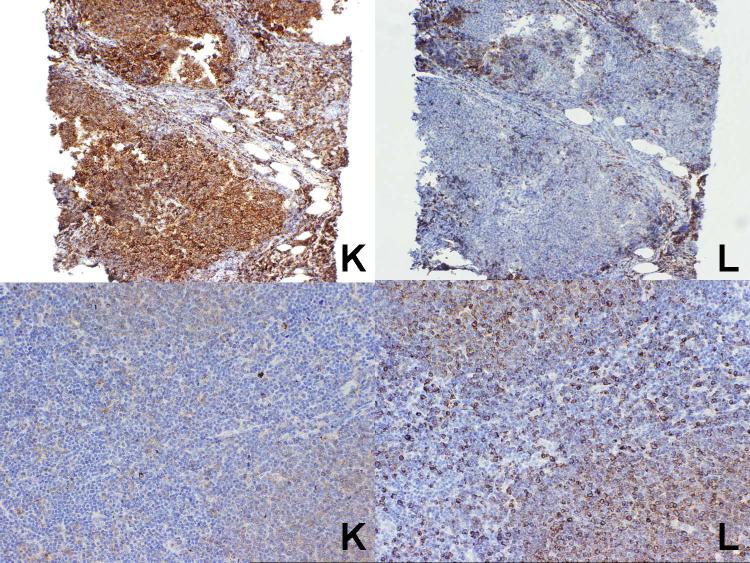
The specific results obtained for follicular lymphoma are summarized in Table 2 and illustrated in Figure 4). Overall, correlation between the results of immunohistochemistry and flow cytometry was obtained in 13 of the 20 cases (65%), including 12 cases (60%) (9 kappa and 3 lambda) in which a single light chain was expressed. Nine of the 20 cases were cytologic grade 3, and of these 5 (56%) expressed a single light chain (all of but one of which correlated with the flow cytometry studies).
Table 2.
Results in Follicular Lymphoma
| FLOW | ||||
|---|---|---|---|---|
| IM | K | L | N | |
| K | 9 | 0 | 0 | |
| L | 0 | 3 | 1 | |
| N | 1 | 5 | 1 | |
Flow= Flow cytometry studies; IM= Paraffin section immunohistochemical studies for light chain; K=Kappa light chain; L=lambda light chain; N=not detected
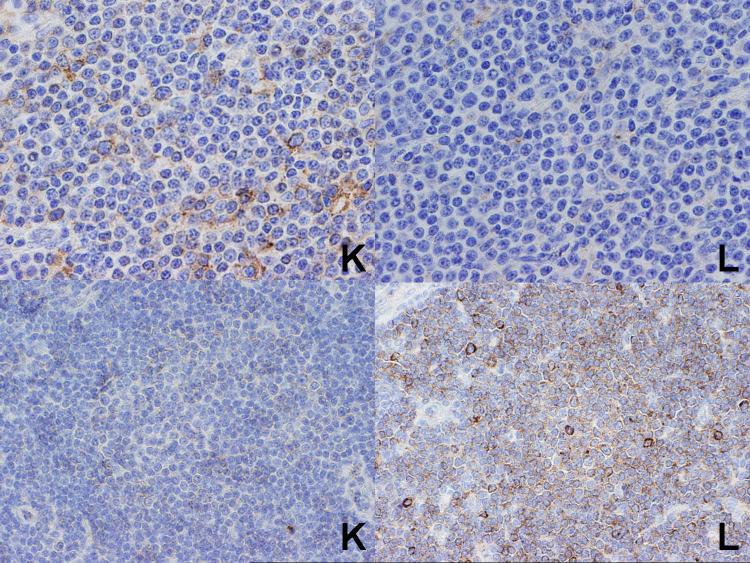
Figure 4.
Two cases of follicular lymphoma. At top is a case which was kappa light chain restricted, in agreement with the flow cytometry studies, while at the bottom is a lambda light chain restricted case, again in agreement with the flow cytometry studies. Note the weak staining of the follicles with kappa does not prevent assessment of the lower case as clearly showing lambda light chain restriction.
The specific results obtained for diffuse large B-cell lymphoma are summarized in Table 3 and illustrated in Figure 5. Overall, correlation between the results of immunohistochemistry and flow cytometry was obtained in 10 of 15 cases (67%), including 7 cases (4 kappa and 3 lambda) in which a single light chain was expressed. There were 8 cases of splenic marginal zone B-cell lymphoma, of which 3 showed correlation with the flow cytometry studies (all kappa light chain), while 5 cases showed a light chain by flow cytometry but was negative by immunohistochemistry. Thee were 5 cases of nodal or extranodal marginal zone B-cell lymphoma, of which 2 showed correlation between the results immunohistochemistry and flow cytometry (one kappa and one lambda light chain-restricted), one case showed lambda light-chain restriction by flow cytometry but was negative by immunohistochemistry, while two cases were negative by flow cytometry, yet positive by immunohistochemistry (Figure 3). There were 5 cases of chronic lymphocytic leukemia/small lymphocytic lymphoma, all of which showed correlation between flow cytometry and immunohistochemistry. In each case, the positivity was limited to larger cells, particularly within proliferation centers (Figure 6). There were 4 cases of mantle cell lymphoma, with three showing correlation between the two methodologies (1 kappa and 2 lambda), and one showing lambda light-chain restriction by flow cytometry, but negative by immunohistochemistry. Finally, there was one case of Burkitt lymphoma, which showed lambda light-chain restriction by flow cytometry, but was negative by immunohistochemistry.
Table 3.
Results in Diffuse Large B-Cell Lymphoma
| FLOW | ||||
|---|---|---|---|---|
| IM | K | L | N | |
| K | 4 | 0 | 2 | |
| L | 0 | 3 | 1 | |
| N | 1 | 1 | 3 | |
Flow= Flow cytometry studies; IM= Paraffin section immunohistochemical studies for light chain; K= Kappa light chain; L=lambda light chain; N=not detected
Figure 5.
Two cases of diffuse large B-cell lymphoma. At top is a case which was kappa light chain restricted, while at the bottom is a lambda light chain restricted case, both results in agreement with the flow cytometry studies.
Figure 6.
Two cases of chronic lymphocytic leukemia/small lymphocytic lymphoma. At top, only scattered large cells are showing kappa light chain restriction, while at bottom, a proliferation center is showing lambda light chain restriction. Both results were in agreement with the flow cytometry studies.
Including the 26 cases of follicular lymphoma for which there were no flow cytometry studies available, there were a total of 46 cases of follicular lymphoma studied. Of these, 30 (65%) showed kappa (21 cases) or lambda (9 cases) light chain restriction. For the 21 cases of cytologic grade 3, 15 (71%) showed light chain restriction.
Discussion
Paraffin immunohistochemistry for the detection of light chain protein has always been problematic. The presence of light chains in interstitial fluid, specific binding onto the processes of follicular dendritic cells, as well as non-specific binding onto proteins such as collagen all complicate interpretation the specific staining of immunoglobulin-producing cells. In addition, it seems that light chain epitopes are highly sensitive to tissue fixation. Several apparently successful methodologies have been published, most often requiring enzymatic or heat antigen retrieval, or both (2-4). Yet, the success of at least some of these protocols has been difficult to replicate (8-14). For example, our laboratory has not found the high sensitivity claimed using the procedure published by Hartford, and in fact most of the cases in the present study were negative when using that procedure in our laboratory (data not shown). The reasons for the discrepancy in the results probably relates to some degree to differences in fixation, as we occasionally note superb studies, albeit in a minority of cases.
In the current study, we evaluated novel mouse monoclonal antibodies to kappa and lambda light chain that had been generated through immunization with the EAP System, using constant region sequences of human Ig-kappa and Ig-lambda. Most prior light-chain antibodies have been polyclonal, since it has been thought that monoclonal antibodies may show false negative results in occasional cases. However, use of constant region sequences probably minimizes this possibility. We generally found robust results for our cases, generally fixed for a wide variety of fixation times, although there were differences in staining intensities, and occasional cases were completely negative. In contrast to frozen section immunoglobulin studies, in which the staining of lymphocytes is usually membranous, the paraffin light chain staining was cytoplasmic in distribution when one could discern cytoplasmic from membranous staining.
In general, there was good correlation between the results of flow cytometry and light chain immunohistochemistry. Splenic marginal zone B-cell lymphoma had a particularly high incidence of cases that were monotypic by flow cytometry, yet negative by light chain immunohistochemistry. We speculate that non-optimal fixation time may account for this discrepancy, since these cases were spleens rather than lymph nodes. Conversely, nodal and extranodal marginal zone B-cell lymphoma had a particularly high incidence of cases that were monotypic by light chain immunohistochemistry yet negative by flow cytometry. Review of these cases, as illustrated in Figure X, suggested that the staining was real and may have reflected positivity within a plasmacytoid component of the tumor. It is well known that flow cytometry may not detect cytoplasmic antigens unless special procedures such as permeabilization are performed prior to the analysis.
We focused on follicular lymphoma, as it still represents a major diagnostic problem area in surgical pathology. Bcl-2 coexpression is seen in the large majority of low grade follicular lymphomas, but is not seen in a significant proportion of high grade follicular lymphomas (19). The current study shows that detection of monotypic light chains by paraffin section immunohistochemistry may be a useful adjunct to the diagnosis of follicular lymphoma, and is just as effective (if not more effective) in grade 3 follicular lymphoma as low-grade follicular lymphoma.
Despite the good results reported in the current study, it is clear that the detection of light chain immunochemistry in paraffin sections is not a panacea. We unable to detect monotypic light chains in cases in which monotypic light chains were demonstrated by flow cytometry in 16 of the 58 (28%) cases of B-cell lymphoma in which flow cytometry results were available. This may be due to fixation issues, the intrinsic level of immunoglobulin expression in the neoplastic B-cells, the presence of interstitial immunoglobulins, or non-specific binding by proteins in the tissue (such as collagen in a cutaneous lymphoid lesion with significant fibrosis). In situ hybridization studies for light chain mRNA may be useful as a supplemental analysis in the latter two circumstances, as these factors should not influence the results of mRNA studies (15,16).
Acknowledgements
The authors thank Silver Lake Research Corporation of Monrovia, CA, USA for providing the monoclonal antibodies used in the study. Dr. Weiss is partially supported by National Cancer Institute grants CA33572 and CA107399.
Reference List
- 1.Du MQ, Liu H, Diss TC, et al. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97:2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CR, Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalin-fixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974;27:14–20. doi: 10.1136/jcp.27.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor CR, Mason DY. The immunohistological detection of intracellular immunoglobulin in formalin-paraffin sections from multiple myeloma and related conditions using the immunoperoxidase technique. Clin Exp Immunol. 1974;18:417–429. [PMC free article] [PubMed] [Google Scholar]
- 4.Pinkus GS, Said JW. Specific identification of intracellular immunoglobulin in paraffin sections of multiple myeloma and macroglobulinemia using an immunoperoxidase technique. Am J Pathol. 1977;87:47–57. [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton-Key M, Jessup E, Isaacson PG. Immunoglobulin light chain staining in paraffin-embedded tissue using a heat mediated epitope retrieval method. Histopathology. 1996;29:525–531. doi: 10.1046/j.1365-2559.1996.d01-534.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurtin PJ, Hobday KS, Ziesmer S, et al. Demonstration of distinct antigenic profiles of small B-cell lymphomas by paraffin section immunohistochemistry. Am J Clin Pathol. 1999;112:319–329. doi: 10.1093/ajcp/112.3.319. [DOI] [PubMed] [Google Scholar]
- 7.Marshall-Taylor CE, Cartun RW, Mandich D, et al. Immunohistochemical detection of immunoglobulin light chain expression in B-cell non-Hodgkin lymphomas using formalin-fixed, paraffin-embedded tissues and a heat-induced epitope retrieval technique. Appl Immunohistochem Mol Morphol. 2002;10:258–262. doi: 10.1097/00129039-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Ho J, Shintaku P, Preston M, et al. Can microwave antigen retrieval replace frozen section immunohistochemistry in the phenotyping of lymphoid nelplasms? Appl Immunohistochem. 1994;2:282–286. [Google Scholar]
- 9.Leers MP, Theunissen PH, Ramaekers FC, et al. Clonality assessment of lymphoproliferative disorders by multiparameter flow cytometry of paraffin-embedded tissue: an additional diagnostic tool in surgical pathology. Hum Pathol. 2000;31:422–427. doi: 10.1053/hp.2000.6551. [DOI] [PubMed] [Google Scholar]
- 10.Tashiro K, Ohshima K, Suzumiya J, et al. Clonality of primary pulmonary lymphoproliferative disorders; using in situ hybridization and polymerase chain reaction for immunoglobulin. Leuk Lymphoma. 1999;36:157–167. doi: 10.3109/10428199909145960. [DOI] [PubMed] [Google Scholar]
- 11.Bergman R, Kurtin PJ, Gibson LE, et al. Clinicopathologic, immunophenotypic, and molecular characterization of primary cutaneous follicular B-cell lymphoma. Arch Dermatol. 2001;137:432–439. [PubMed] [Google Scholar]
- 12.Tworek JA, Singleton TP, Schnitzer B, et al. Flow cytometric and immunohistochemical analysis of small lymphocytic lymphoma, mantle cell lymphoma, and plasmacytoid small lymphocytic lymphoma. Am J Clin Pathol. 1998;110:582–589. doi: 10.1093/ajcp/110.5.582. [DOI] [PubMed] [Google Scholar]
- 13.El-Zimaity HM, El-Zaatari FA, Dore MP, et al. The differential diagnosis of early gastric mucosa-associated lymphoma: polymerase chain reaction and paraffin section immunophenotyping. Mod Pathol. 1999;12:885–893. [PubMed] [Google Scholar]
- 14.Takahashi K, MacDonald DG, Kinane DF. Analysis of immunoglobulin-synthesizing cells in human dental periapical lesions by in situ hybridization and immunohistochemistry. J Oral Pathol Med. 1996;25:331–335. doi: 10.1111/j.1600-0714.1996.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiss LM, Movahed LA, Chen Y-Y, et al. Detection of immunoglobulin light-chain mRNA in lymphoid tissues using a practical in situ hybridization method. Am J Pathol. 1990;137:979–988. [PMC free article] [PubMed] [Google Scholar]
- 16.Beck RC, Tubbs RR, Hussein M, et al. Automated colorimetric in situ hybridization (CISH) detection of immunoglobulin (Ig) light chain mRNA expression in plasma cell (PC) dyscrasias and non-Hodgkin lymphoma. Diagn Mol Pathol. 2003;12:14–20. doi: 10.1097/00019606-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Battifora H, Mehta P. The checkerboard tissue block. An improved multitissue control block. Lab Invest. 1990;63:722–724. [PubMed] [Google Scholar]
- 18.Battifora H. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986;55:244–248. [PubMed] [Google Scholar]
- 19.Lai R, Arber DA, Chang KL, et al. Frequency of bcl-2 expression in non-Hodgkin’s lymphoma. A study of 798 cases with comparison of marginal zone lymphoma and monocytoid B cell hyperplasia. Mod Pathol. 1998;11:864–869. [PubMed] [Google Scholar]