Abstract
The complex neurodegeneration underlying Alzheimer disease (AD), although incompletely understood, is characterized by an aberrant reentry into the cell cycle in neurons. Pathological evidence, in the form of cell cycle markers and regulatory proteins, suggests that cell cycle reentry is an early event in AD, which precedes the formation of amyloid-β plaques and neurofibrillary tangles (NFTs). Although the exact mechanisms that induce and mediate these cell cycle events in AD are not clear, significant advances have been made in further understanding the pathological role of cell cycle re-entry in AD. Importantly, recent studies indicate that cell cycle re-entry is not a consequence, but rather a cause, of neurodegeneration, suggesting that targeting of cell cycle reentry may provide an opportunity for therapeutic intervention. Moreover, multiple inducers of cell cycle re-entry and their interactions in AD have been proposed. Here, we review the most recent advances in understanding the pathological implications of cell cycle re-entry in AD.
Alzheimer disease (AD) is the leading cause of senile dementia in the US where it affects 15% of people over 65 and almost 50% of those over 85 (Ref. 1). AD is characterized by severe neurodegeneration and cognitive impairment, but the precise pathogenesis has not been fully elucidated. The hallmark features of AD, neurofibrillary tangles (NFTs) and amyloid-β (Aβ) plaques, although not the sole players in neurodegeneration, are crucial to disease development and progression.
Aβ is the major component of senile plaques characteristic of AD and is derived from the amyloid-β protein precursor (APP) encoded on chromosome 21 (Ref. 2). Mutations in the APP gene are directly linked to the onset of familial AD (Ref. 3). Interestingly, recent evidence indicates that Aβ can provide oxidative protection (Ref. 4). In vitro studies of Aβ in its soluble, non-aggregated form (Refs 5, 6), demonstrate its antioxidant capacity, probably through its function as a high-valence metal chelator (Refs 5, 7). The eventual accumulation of Aβ in neuronal tissue, however, is likely to be detrimental to the cell. Specifically, Aβ-containing senile plaques elicit inflammatory responses from surrounding microglial and astrocytic cells (Refs 8, 9) and ultimately increase oxidative damage and further selfaggregation (Ref. 10). Of note here, APP is upregulated by mitogenic stimulation and APP metabolism is controlled by cell-cycle-dependant changes (Refs 11, 12, 13). Moreover, Aβ itself has been identified to be mitogenic in vitro (Refs 11, 12). Similarly, the hyperphosphorylated form of the microtubule-associated protein tau (the primary component of NFTs) (Refs 14, 15), produces neuronal dysfunction in AD through microtubule destabilization. Although tau phosphorylation is known to be effected through the action of various kinases including cdk5 and GSK-3β, similarly hyperphosphorylated tau present in mitotically active cells is thought to be partly driven by the activity of cell cycle proteins (Refs 16, 17).
Oxidative stress has become increasingly significant in the pathogenesis of AD over the past few years, and has also been identified as concordant with markers of cell cycle re-entry (Ref. 18). Although the exact origins of oxidative stress remain uncertain, it has been demonstrated to be one of the primary role-players in the onset of AD and in its development. Hence, markers of oxidative stress, such as 8-hydroxyguanosine (8OHG), precede the general signs of AD in immunohistochemically stained neurons by decades (Refs 18, 19), and it is not until gross accumulations of oxidative stress elicit overbearing amounts of Aβ that the Aβ-induced pathogenesis of AD occurs.
The pathological significance of cell cycle alteration in Alzheimer disease
The four phases of the cell cycle consist of elaborate feedback mechanisms and regulatory checkpoints that ensure its competency. The phases are: S-phase, where DNA replication takes place; M-phase, where mitosis, or cell division, occurs; and the gap phases G1 and G2, which separate the two. Quiescent cells, such as neurons in the adult hippocampus, exist in the nondividing, silent G0 phase. Such cells are terminally differentiated and generally thought to be incapable of re-entering the cell cycle (Ref. 20).
Importantly, the transition through these phases is controlled by an array of special cell cycle proteins, the cyclins and the cyclin-dependant kinases (CDKs), which fluctuate in their expression and activity as the cell cycle progresses. For instance, the expression and activation of the cyclin D1 (encoded by CCND1)–CDK4/6 complex, triggered by the presence of mitotic growth factors, controls the re-entry of resting (G0) cells into the G1 phase of cell cycle (Ref. 21). The G1-S transition is then controlled by the activation of the cyclin E1 (CCNE1)–cdk2 complex, among others (Ref. 21), such that the absence of cyclin E1 and/or the inhibition of the cyclin-E1–CDK2 complex by p21 (CDNK1A), p27 (CDKN1B) and p53 (TP53) will cause the cell cycle to be arrested at the G1 checkpoint. The subsequent fate of the G1-arrested cells depends on the presence or absence of cyclin A1 (CCNA1) (Ref. 22) such that, in the absence of cyclin A1, the cells return to G0 and redifferentiate. However, if allowed to proceed in the presence of cyclin A1, the cells are committed to division, lack the ability to redifferentiate and, if unable to complete the cell cycle, die via an apoptotic pathway (Ref. 23). Therefore, once beyond late G1, when cyclin A1 is expressed, cell cycle arrest will lead to cell death.
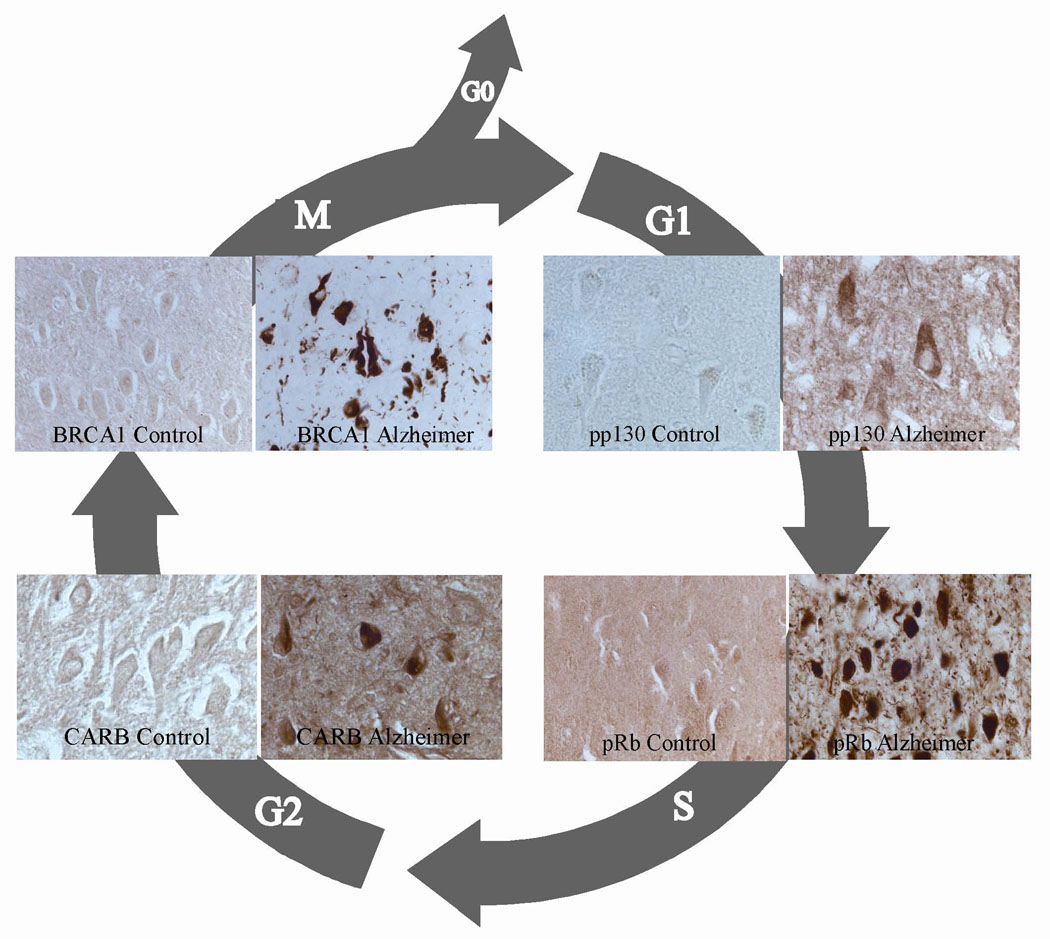
Notably, AD involves the erroneous departure of quiescent G0 neurons into G1 phase and beyond (Refs 24, 25, 26). Although the exact reasons for this re-entry are unknown, there is much pathological evidence to support it (Fig. 1). In addition to the proteins described in Figure 1, AD neurons exhibit significantly elevated levels of the cell cycle markers cyclin D1, CDK4 and Ki67 (MKI67), as well as those of the cyclin-E1–CDK4 complex, when compared with age-matched controls (Refs 18, 24, 25, 27, 28, 29), indicating a departure from quiescence. Moreover, markers of DNA replication that demonstrate a transition through S-phase, such as the chromosome-maintenance protein MCM2, are also elevated in AD neurons (Refs 30, 31, 32, 33, 34), and the mitotic signalling G-protein Ras has also been found to have altered levels in AD (Refs 35, 36). This latter protein is especially involved in the cellular transition from G0 to G1 phase through its interactions with cyclin D1 (Ref. 37), and its downstream mediators, MAPK, Raf, and MEK1/2, have all been found to be activated in AD neurons (Refs 38, 39).
Figure 1. Cell cycle markers representing all stages of the cell cycle are ectopically expressed in neurons during Alzheimer disease.
Phosphorylated p130 (pp130), a marker of G1 phase, is selectively increased in neurons in Alzheimer disease (AD) and therefore may not be able to prevent cell cycle reentry (Ref. 68). Phosphorylated retinoblastoma protein Rb (pRb), which is normally expressed during the G1-S transition, is present in nuclei, neurofibrillary tangles (NFTs) and other pathological structures (Ref. 69). CARB (CIP-1-associated regulator of cyclin B), a regulator of cyclin B in G2 phase, is also increased in NFTs (Ref. 70), as is BRCA1, an M-phase marker (Ref. 71). Reprinted from Ref. 72, with permission from Nova Science Publishers, Inc. © 2010
Interestingly, a genetic link between AD and mitotic malfunction also exists: the genes associated with a predisposition to AD, specifically APP and presenilin-1 and presenilin-2 (PS1 and PS2) (Refs 40, 41, 42), are also role players in the cell cycle and its control, and, as such, these proteins are both mitogenic in vitro (Ref. 11, 12). Moreover, the adaptor protein involved in the cleavage of APP, APP-BP1 (NAE1), is a cell cycle protein that regulates mitotic transition from S- to M-phase (Ref. 43). Overexpression of APP-BP1 would therefore push neurons into the S-phase and cause DNA replication and expression of corresponding cell cycle markers CDC2 and cyclin B1 (CCNB1) (Ref. 31, 37, 44). Similarly, PS1 and PS2 are responsible for the proteolytic cleavage of APP (Ref. 37) and are thus implicated in cell cycle control. Specifically, PS1 and PS2 have been associated with the centromere and centrosome of dividing cells (Ref. 45): overexpression of these genes in transfected HeLa cells elicited G1 arrest in the cell cycle (Refs 37, 46, 47), whereas their deficiency produces accelerated transgression from G1 through S-phase (Ref. 48).
Such evidence for mitotic alteration suggests a pivotal role of cell cycle re-entry in AD. As these markers preclude the appearance of gross indicators of the disease, such as NFTs and Aβ plaques (Ref. 18), that role may in fact be primary. Therefore, aberrant cell cycle regulation is probably a causal factor in disease progression rather than an epiphenomenon, actually eliciting neurodegeneration rather than being a result of it (Ref. 20).
The consequence of neuronal cell cycle re-entry in neurodegeneration: in vitro and in vivo studies
The early appearance of aberrant cell cycle markers in AD neurons suggests that cell cycle reentry is a causal phenomenon in neurodegeneration. Notably, although various mitotic markers are upregulated in vulnerable neurons in AD, no evidence of actual mitosis has ever been found (Ref. 33). Many AD neurons do exhibit DNA replication, expression of chromosome maintenance protein (Ref. 30) and show binucleation events (Ref. 33), indicating a completion of, or at least entrance into S-phase (Refs 30, 32). In addition, some cells have been observed in G2-phase (Refs 28, 29); however, no studies demonstrate an entrance into or completion of M-phase (Ref. 49). It seems as though these cells encounter a ‘mitotic catastrophe’ during which they are unable to complete the cell cycle as a result of inadequate control and halted protein expression (Ref. 49). Importantly, once cyclin A1 is expressed (i.e. after G1 progression), the cells become committed to division and lack the ability to return to G0. They must therefore either complete the cycle or die. Given the lack of evidence for successful completion of the cell cycle, it is likely that the reactivation of cell cycle machinery in postmitotic neurons leads, albeit slowly, to their death (Refs 50, 51).
Accordingly, when a powerful oncogene, the SV40 T antigen, is expressed specifically in maturing Purkinje cells in transgenic mice, the cells replicate their DNA (i.e. initiated cell cycle) but then degenerate and die (Ref. 52). A recent study using transgenic mice that inducibly expressed SV40T in neurons confirmed the degenerative effect of cell cycle re-entry and noted the concurrent appearance of AD-type pathologies – NFT-like profiles and Aβ deposits (Ref. 53). Similarly, the expression of SV40T by the rhodopsin gene promoter causes photoreceptor degeneration, again associated with cell cycle reactivation and DNA synthesis (Ref. 54).
It is well known that the activation of cell cycle processes is part of the mechanism by which developmental failure of trophic support leads to neuronal cell death. Additional evidence supports a role for cell cycle regulators in neuronal death evoked by various stressors (Refs 55, 56). However, the aforementioned studies in transgenic mouse and cell culture fail to accurately mimic age-related neurodegenerative diseases. In particular, the embryo-derived primary neuronal cultures used in cell culture studies do not accurately model adult neurons, and the expression of transgenes during embryogenesis (combined with neurodegeneration) at an early age in the transgenic mice might be more representative of developmental error than age-related degeneration. In addition, SV40T is derived from a virus and is not a pathophysiologically relevant entity and thus, its relevance to AD is unclear.
Recently, we circumvented these shortcomings with the generation of bitransgenic mice (CaMKII-MYC) that could be induced to overexpress human c-Myc (MYC) under the control of the CaMKII gene (CAMK2B) promoter that drives high transgene expression selectively in forebrain neurons (Ref. 57). MYC is known for its oncogenic activity and its overexpression is frequently linked to tumorigenesis (Ref. 58). Importantly, levels of MYC protein are increased in the vulnerable neurons in patients with AD (Ref. 59). In fact, MYC induction in CaMKII-MYC mice induced neuronal-specific cell cycle re-entry, neurodegeneration and cognitive decline. Therefore, these findings strongly support the idea that aberrant neuronal cell cycle re-entry is an important pathogenic mechanism responsible for the neurodegeneration in AD (Ref. 24).
The candidate inducers of cell cycle re-entry in neurons
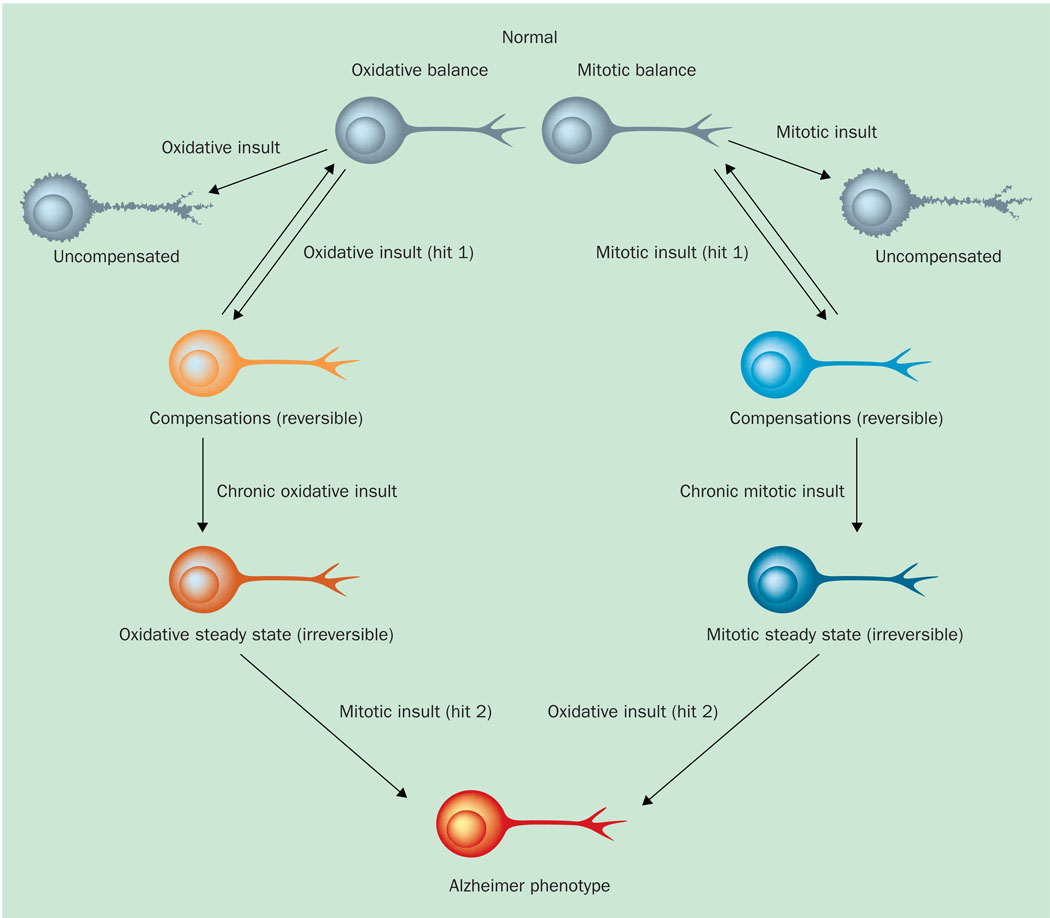
The molecular mechanisms behind the cell death associated with cell cycle re-entry seem to be closely involved with oxidative stress. As both phenomena are manifest early in the pathogenesis of AD, and as mature cases of AD exhibit markers of both (Refs 18, 60), it seems clear that there is a relationship between the two. The ‘two-hit’ hypothesis, in particular, suggests that either of the two cellular detriments (oxidative stress or mitotic dysfunction) initiates a cycle in which the first factor produces a compensatory steady state that enables its proliferation under the stressed conditions. Consequently, the weakened state of a cell makes it vulnerable to the effects of the additional insult (Ref. 60). In other words, one ‘hit’ instigates a cellular adaptation that can last for decades with coherent neuronal functioning, and adaptations of this new steady state produce vulnerabilities in affected neurons that lead to insults of the second ‘hit’. Consequently, the combination produces cellular dysfunction and death (Ref. 18). It is our hypothesis that these two hits are oxidative stress and cell cycle aberration, and the sequence of events can begin with either one, leading to the other. Figure 2 presents a pathway of the mitotic steady state as the primary factor that ultimately leads to neurodegeneration.
Figure 2. The two-hit hypothesis.
An initial insult, whether oxidative or mitotic, that is chronic and above threshold limits leads to a new steady state (either oxidative steady state or mitotic steady state). It is in this new steady state when neurons are vulnerable to the subsequent second hit, which causes the Alzheimer disease phenotype. Reprinted from Ref. 60, with permission from Elsevier © 2004.
Notably, in regard to mitotic abnormalities, it seems as though the APP mutant phenotype (of familial AD) essentially produces a ‘mitotic steady state’ that exposes affected cells to future insult through oxidative damage. Indeed, mutations in APP as well as in PS1 or PS2 elicit alterations in cell cycle control and functioning mechanisms. Interestingly, as studies with several transgenic mouse models indicate, neuronal cell cycle re-entry (as a result of mutations in APP, PS1 or PS2) precedes amyloid deposition, and thus full AD pathogenesis, by several months and occurs in an anatomical pattern that reproduces the neuronal vulnerability seen in AD (Refs 18, 61). It is thus clear that, although these mice demonstrated significant cell cycle re-entry as early as 6 months of age, they maintained cognition and cellular functioning at a near-normal level for long periods of time (Ref. 18). In other words, those mice exhibited a ‘mitotic steady state’ that eventually made the cells vulnerable to further insult, as the neurons became increasingly susceptible to molecular attack, for example, by reactive oxidative species (ROS).
However, in the much more prevalent, sporadic form of AD (or late-onset AD), an ‘oxidative steady state’ has been demonstrated, which exposes affected neurons to other insults that are due to mitotic abnormalities (Ref. 18). Although neurons are generally capable of sequestering acute levels of oxidative stress in the brain, widespread accumulation of reactive oxidative species eventually requires cellular adaptations that describe the oxidative steady state (Refs 62, 63). Specifically, affected neurons combat repetitive oxidative insults through permanent instalments of sequestration proteins and antioxidants, such as Aβ. Although these proteins do enable neurons to survive for decades under the repetitive oxidative stress (Ref. 64), their eventual accumulation might subject surrounding neurons to certain vulnerabilities, including mitotic abnormalities. Consequently, once the cells begin altered mitotic functioning in their already weakened states, they inevitably succumb to more severe dysfunction and eventual cell death. Inflammation has also been postulated as a progenitor of cell cycle re-entry in AD. A recent study using APP transgenic mice (R1.40 Tg) demonstrated that alterations in brain microglia are coincident with the first evidence of cell cycle re-entry events at 4–6 months of age, approximately 6 months before Aβ deposition. The microglial activation was found to be dependent on Aβ generation, because microglial activation was limited in mice lacking β-secretase 1 (BACE1) (Ref. 65). Additionally, treatment of young R1.40 animals with nonsteroidal anti-inflammatory drugs (NSAIDs) effectively blocked the activation of microglia in the rat brains, and thus blocked neuronal re-entry into the cell cycle.
Therapeutic potential of targeting cell cycle re-entry in Alzheimer disease
The definitive role of the cell cycle in AD affords many access points for therapeutic intervention (Ref. 66). Importantly, because the aberrant re-entry into the cell cycle appears so early in the disease progression, such treatment strategies might eventually enable a prevention or reversal of disease.
In conventional models of neurodegeneration, compounds sharing the ability to abrogate cell cycle progression from G0 to G1 have been shown to provide neuroprotection in rodent models of acute ischemic stroke and traumatic brain injury (Refs 53, 57, 65, 66). However, formal tests of the cell cycle treatment have not yet been undertaken in clinical studies. The results of retrospective and prospective analyses will have a bearing on interpretation of the usefulness of the hypothesis for development of novel therapeutics. The retrospective study reported by Breuer and Anderson (Ref. 67) provides one such example. They found that the incidence of AD was one-third lower in elderly female nursing-home residents who were treated with Tamoxifen, a well-tolerated antiproliferative cancer medication that crosses the blood–brain barrier, than in untreated residents. Importantly, the cognitive skills for daily decision-making were also significantly better in residents taking Tamoxifen. An array of other compounds which provide neuroprotective properties through the action of cell cycle inhibition includes retinoic acid, flavopiridol, simvastatin, rosiglitazone, amongst others (Ref. 66).
A number of prospective studies with possible use as incidental tests of the cell cycle hypothesis are currently ongoing or planned. A Phase II trial of epigallocatechin-gallate (ClinicalTrials.gov identifier: NCT00951834) is due to start in the near future. Epigallocatechin-gallate is a natural product that has been shown to abrogate the cell cycle at the G0–G1 phase in various tissues and is listed as a cell cycle inhibitor. This study is due to complete in the first half of 2011. Additionally, the VALID study sponsored by NIA (ClinicalTrials.gov identifier: NCT00071721) is one of number of studies investigating the effect of valproate in Alzheimer disease. Although valproate has a number of pharmacological actions, like epigallocatechin-gallate, it can abrogate the cell cycle. The VALID study is reported to be completing in the near future.
Conclusions
Although the precise origins of mitotic dysfunction in AD are not fully understood, genetic, inflammatory and oxidative defects can induce the pathways from which the altered mitotic signaling could arise, and, as is the case in AD, the complex interaction between oxidative stress and cell cycle re-entry and dysfunction seems pertinent, with mitotic abnormalities being of particular importance in familial AD. In any case, however, the cell cycle is a clear progenitor of neuronal dysfunction and as such, provides a new and possibly beneficial therapeutic avenue for disease control. Future investigation on the matter is thus of great importance.
Acknowledgments
Work in the authors’ laboratories is partly supported by the National Institutes of Health (AG031364, AG028679 and AG030096) and by the Alzheimer’s Association (NIRG-07-60164). M.S. is a paid consultant for or owns equity or stock options in Medivation, Neurotez, Neuropharm, Panacea Pharmaceuticals, and Voyager Pharmaceuticals. X.Z. is a paid consultant for and received grant funding from Medivation. We are extremely grateful and thank the peer reviewers for their helpful comments, which greatly improved the impact and focus of our paper.
References
- 1.Smith MA. Alzheimer disease. International Review of Neurobiology. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 2.Korenberg JR, et al. The Alzheimer amyloid precursor protein maps to human chromosome 21 bands q21.105-q21.05. Genomics. 1989;5:124–127. doi: 10.1016/0888-7543(89)90095-5. [DOI] [PubMed] [Google Scholar]
- 3.Hellstrom-Lindahl E, Viitanen M, Marutle A. Comparison of Abeta levels in the brain of familial and sporadic Alzheimer's disease. Neurochemistry International. 2009;55:243–252. doi: 10.1016/j.neuint.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira PI, et al. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7:3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- 5.Nunomura A, et al. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–423. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- 6.Kontush A, et al. Amyloid-beta is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radical Biology and Medicine. 2001;30:119–128. doi: 10.1016/s0891-5849(00)00458-5. [DOI] [PubMed] [Google Scholar]
- 7.Zou K, et al. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. Journal of Neuroscience. 2002;22:4833–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan D. The role of microglia in antibody-mediated clearance of amyloid-beta from the brain. CNS Neurol Disord Drug Targets. 2009;8:7–15. doi: 10.2174/187152709787601821. [DOI] [PubMed] [Google Scholar]
- 9.Piazza A, Lynch MA. Neuroinflammatory changes increase the impact of stressors on neuronal function. Biochemical Society Transactions. 2009;37:303–307. doi: 10.1042/BST0370303. [DOI] [PubMed] [Google Scholar]
- 10.Ong WY, Farooqui AA. Iron, neuroinflammation, and Alzheimer's disease. Journal of Alzheimer's Disease. 2005;8:183–200. doi: 10.3233/jad-2005-8211. discussion 209-115. [DOI] [PubMed] [Google Scholar]
- 11.Schubert D, et al. Amyloid beta protein precursor is a mitogen. Biochemical and Biophysical Research Communications. 1989;162:83–88. doi: 10.1016/0006-291x(89)91965-7. [DOI] [PubMed] [Google Scholar]
- 12.Milward EA, et al. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 13.Copani A, et al. Mitotic signaling by beta-amyloid causes neuronal death. FASEB Journal. 1999;13:2225–2234. [PubMed] [Google Scholar]
- 14.Iqbal K, et al. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathologica. 1984;62:167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- 15.Grundke-Iqbal I, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brion JP. Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer's disease. Journal of Alzheimer's Disease. 2006;9:177–185. doi: 10.3233/jad-2006-9s321. [DOI] [PubMed] [Google Scholar]
- 17.Brion JP, Octave JN, Couck AM. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994;63:895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- 18. Zhu X, et al. Alzheimer disease, the two-hit hypothesis: an update. Biochimica et Biophysica Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. This article describes the two-hit hypothesis mentioned in this text in full detail. It is a pivotal paper in the field.
- 19.Nunomura A, et al. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. Journal of Neuropathology and Experimental Neurology. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 20.McShea A, Wahl AF, Smith MA. Re-entry into the cell cycle: a mechanism for neurodegeneration in Alzheimer disease. Medical Hypotheses. 1999;52:525–527. doi: 10.1054/mehy.1997.0680. [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 22.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 23.Meikrantz W, Schlegel R. Apoptosis and the cell cycle. Journal of Cellular Biochemistry. 1995;58:160–174. doi: 10.1002/jcb.240580205. [DOI] [PubMed] [Google Scholar]
- 24.McShea A, et al. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. American Journal of Pathology. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 25. Nagy Z, Esiri MM, Smith AD. Expression of cell division markers in the hippocampus in Alzheimer's disease and other neurodegenerative conditions. Acta Neuropathologica. 1997;93:294–300. doi: 10.1007/s004010050617. This paper provides an excellent glimpse at the abundance of evidence for the role of the cell cycle in Alzheimer disease.
- 26.Smith TW, Lippa CF. Ki-67 immunoreactivity in Alzheimer's disease and other neurodegenerative disorders. Journal of Neuropathology and Experimental Neurology. 1995;54:297–303. doi: 10.1097/00005072-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer's disease? Journal of Cell Biology. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent I, et al. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. Journal of Neuroscience. 1997;17:3588–3598. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy Z, et al. Cell cycle markers in the hippocampus in Alzheimer's disease. Acta Neuropathologica. 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- 30.Bonda DJ, et al. Evidence for the progression through S-phase in the ectopic cell cycle reentry of neurons in Alzheimer disease. Aging. 2009;1:382–388. doi: 10.18632/aging.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. Journal of Neuroscience. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosch B, et al. Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. Journal of Neuroscience. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, et al. Neuronal binucleation in Alzheimer disease hippocampus. Neuropathology and Applied Neurobiology. 2008;34:457–465. doi: 10.1111/j.1365-2990.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- 34.Spremo-Potparevic B, et al. Premature centromere division of the X chromosome in neurons in Alzheimer's disease. Journal of Neurochemistry. 2008;106:2218–2223. doi: 10.1111/j.1471-4159.2008.05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartner U, Holzer M, Arendt T. Elevated expression of p21ras is an early event in Alzheimer's disease and precedes neurofibrillary degeneration. Neuroscience. 1999;91:1–5. doi: 10.1016/s0306-4522(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 36.McShea A, et al. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochimica et Biophysica Acta. 2007;1772:467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, et al. Neuronal cell cycle re-entry: a doomed journey in Alzheimer disease? In: Özben T, Chevion M, editors. Frontiers in Neurodegenerative Disorders and Aging: Fundamental Aspects, Clinical Perspectives and New Insights. Amsterdam: IOS Press; 2004. pp. 200–206. [Google Scholar]
- 38.Zhu X, et al. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the 'two hit' hypothesis. Mechanisms of Ageing and Development. 2001;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, et al. Distribution, levels, and activation of MEK1 in Alzheimer's disease. Journal of Neurochemistry. 2003;86:136–142. doi: 10.1046/j.1471-4159.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- 40.Manzano S, et al. [Genetics and Alzheimer's disease.] Neurologia. 2009;24:83–89. [PubMed] [Google Scholar]
- 41.Prat MI, et al. Presenilin 1 overexpressions in Chinese hamster ovary (CHO) cells decreases the phosphorylation of retinoblastoma protein: relevance for neurodegeneration. Neuroscience Letters. 2002;326:9–12. doi: 10.1016/s0304-3940(02)00298-7. [DOI] [PubMed] [Google Scholar]
- 42.Varvel NH, et al. Abeta oligomers induce neuronal cell cycle events in Alzheimer's disease. Journal of Neuroscience. 2008;28:10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neve RL, McPhie DL. Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis. Biochimica et Biophysica Acta. 2007;1772:430–437. doi: 10.1016/j.bbadis.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X, Raina AK, Smith MA. Cell cycle events in neurons. Proliferation or death? American Journal of Pathology. 1999;155:327–329. doi: 10.1016/S0002-9440(10)65127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, et al. Alzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell. 1997;90:917–927. doi: 10.1016/s0092-8674(00)80356-6. [DOI] [PubMed] [Google Scholar]
- 46.Janicki SM, Stabler SM, Monteiro MJ. Familial Alzheimer's disease presenilin-1 mutants potentiate cell cycle arrest. Neurobiology of Aging. 2000;21:829–836. doi: 10.1016/s0197-4580(00)00222-0. [DOI] [PubMed] [Google Scholar]
- 47.Janicki SM, Monteiro MJ. Presenilin overexpression arrests cells in the G1 phase of the cell cycle. Arrest potentiated by the Alzheimer's disease PS2(N141I)mutant. American Journal of Pathology. 1999;155:135–144. doi: 10.1016/S0002-9440(10)65108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soriano S, et al. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. Journal of Cell Biology. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa O, et al. Ectopic localization of phosphorylated histone H3 in Alzheimer's disease: a mitotic catastrophe? Acta Neuropathologica. 2003;105:524–528. doi: 10.1007/s00401-003-0684-3. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, et al. Apoptosis in Alzheimer disease: a mathematical improbability. Current Alzheimer Research. 2006;3:393–396. doi: 10.2174/156720506778249470. [DOI] [PubMed] [Google Scholar]
- 51.Perry G, et al. Apoptosis and Alzheimer's disease. Science. 1998;282:1268–1269. doi: 10.1126/science.282.5392.1265h. [DOI] [PubMed] [Google Scholar]
- 52.Feddersen RM, et al. Disrupted cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T antigen transgenic mice. Neuron. 1992;9:955–966. doi: 10.1016/0896-6273(92)90247-b. [DOI] [PubMed] [Google Scholar]
- 53.Park KH, et al. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. Journal of Neuroscience. 2007;27:2969–2978. doi: 10.1523/JNEUROSCI.0186-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.al-Ubaidi MR, et al. Photoreceptor degeneration induced by the expression of simian virus 40 large tumor antigen in the retina of transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1194–1198. doi: 10.1073/pnas.89.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giovanni A, et al. Involvement of cell cycle elements, cyclin-dependent kinases, pRb, and E2F x DP, in B-amyloid-induced neuronal death. Journal of Biological Chemistry. 1999;274:19011–19016. doi: 10.1074/jbc.274.27.19011. [DOI] [PubMed] [Google Scholar]
- 56.Park DS, et al. Cell cycle regulators in neuronal death evoked by excitotoxic stress: implications for neurodegeneration and its treatment. Neurobiology of Aging. 2000;21:771–781. doi: 10.1016/s0197-4580(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 57.Lee HG, et al. The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. American Journal of Pathology. 2009;174:891–897. doi: 10.2353/ajpath.2009.080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clinical Cancer Research. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrer I, et al. Phosphorylated c-MYC expression in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Neuropathology and Applied Neurobiology. 2001;27:343–351. doi: 10.1046/j.1365-2990.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhu X, et al. Alzheimer's disease: the two-hit hypothesis. Lancet Neurology. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, et al. Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse models. Journal of Neuroscience. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu X, et al. Oxidative imbalance in Alzheimer's disease. Molecular Neurobiology. 2005;31:205–217. doi: 10.1385/MN:31:1-3:205. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa O, et al. Mitochondrial abnormalities and oxidative imbalance in neurodegenerative disease. Sci Aging Knowledge Environ 2002. 2002:pe16. doi: 10.1126/sageke.2002.41.pe16. [DOI] [PubMed] [Google Scholar]
- 64.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. Journal of Neuropathology and Experimental Neurology. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Varvel NH, et al. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. Journal of Clinical Investigation. 2009;119:3692–3702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Woods J, Snape M, Smith MA. The cell cycle hypothesis of Alzheimer's disease: Suggestions for drug development. Biochimica et Biophysica Acta. 2007;1772:503–508. doi: 10.1016/j.bbadis.2006.12.004. This article discusses and elaborates on the therapeutic relevance of targeting the cell cycle in AD.
- 67.Breuer B, Anderson R. The relationship of tamoxifen with dementia, depression, and dependence in activities of daily living in elderly nursing home residents. Women and Health. 2000;31:71–85. doi: 10.1300/J013v31n01_05. [DOI] [PubMed] [Google Scholar]
- 68.Previll LA, et al. Increased expression of p130 in Alzheimer disease. Neurochemical Research. 2007;32:639–644. doi: 10.1007/s11064-006-9146-3. [DOI] [PubMed] [Google Scholar]
- 69.Thakur A, et al. Retinoblastoma protein phosphorylation at multiple sites is associated with neurofibrillary pathology in Alzheimer disease. International Journal of Clinical and Experimental Pathology. 2008;1:134–146. [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X, et al. Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer's disease. Journal of Neuroscience Research. 2004;75:698–703. doi: 10.1002/jnr.20028. [DOI] [PubMed] [Google Scholar]
- 71.Evans TA, et al. BRCA1 may modulate neuronal cell cycle re-entry in Alzheimer disease. International Journal of Medical Sciences. 2007;4:140–145. doi: 10.7150/ijms.4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubiak JZ, Smith MA. Ubiquitin/proteasome system in mitotic and mitotic-like regulation during brain development and pathology. In: Di Napoli M, Wojcik C, editors. The Ubiquitin Proteasome System in the Central Nervous System: From Physiology to Pathology (2008 update) NY, USA: Nova Science Publishers; 2010. pp. 113–130. [Google Scholar]