SYNOPSIS
Over many years, fear extinction has been conceptualized as one dominant process, new inhibitory learning, which serves to dampen previously acquired fear. Here we present an alternative view, that brain region-specific processing of representations, expectations and emotional attributes of the fear-provoking event, recruits unique mechanisms that interdependently contribute to the conditioning and extinction of fear. The co-occurrence of these mechanisms within the fear circuit can thus be tracked and differentiated at a molecular and cellular level. Among others, the transcriptional regulators cFos, cAMP-dependent response element binding protein (CREB), Zif268, and extracellular signal-regulated kinases (Erk) stand out as hippocampal nuclear markers signaling novelty, arousal, retrieval, and prediction error, respectively. Consistent with evidence from human studies, these findings indicate that, beyond inhibitory learning, fear extinction requires modification of the emotional attributes and expectations that define the threatening context. Given the likely dysregulation of one or more of these processes in anxiety disorders, a key research challenge for the future is the identification and enhancement of individual extinction mechanisms to target the specific components of fear.
Environmental stimuli lacking affective properties (conditioned stimuli, CS) rapidly become threatening if presented with stressful events (unconditioned stimuli, US). Consequently, based on a CS-US association, the presentation of the CS triggers species-specific fear responses until the US consistently stops occurring. At that point, new learning takes place and the fear response declines, a phenomenon termed extinction. The view that extinction occurs because a new, inhibitory CS-noUS association gains control over behavior 106, has remained dominant in the field (reviewed by 20,33,35,100). The implications of impaired fear regulation in the development of anxiety disorders have stimulate-d intense research in this area. Rodent studies identified the circuits involved in the conditioning and extinction of fear of salient cues 99,98,85,93,150, generating data that were confirmed in humans with brain imaging approaches 114,130. Nevertheless, research with experimental animals has not fully taken advantage of human data in order to better interpret extinction mechanisms in the framework of learning, expectation and emotion governing fear-motivated behavior.
The present article aims to summarize recent molecular evidence on fear extinction, focusing on hippocampal mechanisms and experimental models of contextual fear, and compare the results with other relevant fear paradigms and human imaging studies. Instead of conceptualizing extinction learning as one process, such as CS-noUS association or inhibitory learning 19,26,96, we propose that fear extinction reflects the behavioral output of several region-specific learning processes that modify different components of the conditioning memory. The significance of these findings is discussed in the framework of fear regulation and anxiety disorders.
Keywords: context, valence, partial reinforcement, continuous reinforcement, hippocampus, protein kinase, actin rearrangement, neurotransmitter receptors, protein synthesis, chromatin remodeling, post-traumatic stress disorder
1. CONDITIONING AND EXTINCTION OF CONTEXTUAL FEAR
Contextual stimuli play an important modulatory (“occasion setting”) role in fear to explicit cues 18 but also directly associate with stressful events 84. As one of the most robust and rapid forms of associative learning, contextual fear conditioning has been extensively used in molecular studies of memory 1,134,142. Extinction of contextual fear however, has only been recently studied. Advances in this area are important because contextual fear might best reflect the aversive expectation about potential dangers that characterizes anxiety. This view is based on observations that anxious patients are overly sensitive to threatening contexts, and that anxiety is neither triggered nor suppressed by explicit cues 7,49. Accordingly, among multiple responses elicited by fear, context-specific freezing has been proposed as one of the main risks factors for the development of anxiety 23. Whereas the relationship of contextual fear conditioning to individual disorders is yet to be systematically defined, its contribution to spatial phobias and posttraumatic stress disorder is considerable.
2. ANIMAL MODELS
In animal models, contextual fear memories are acquired after pairing a specific environment with an electric shock serving as an aversive reinforcer (US). These memories are behaviorally expressed as freezing 16 if the exposure to the context is inescapable as in contextual fear conditioning, or as avoidance if animals have the option to remain in a safe compartment as in passive avoidance paradigms 17. The duration of freezing and latency to step-through or step-down from a safe to a shock-associated compartment are used as measures of learned fear. Passive avoidance and contextual fear conditioning have much in common, because both paradigms involve conditioning to the environmental context, exposure to a brief, mild shock, and induction of fear-motivated behavior (Fig. 1).
Fig. 1.
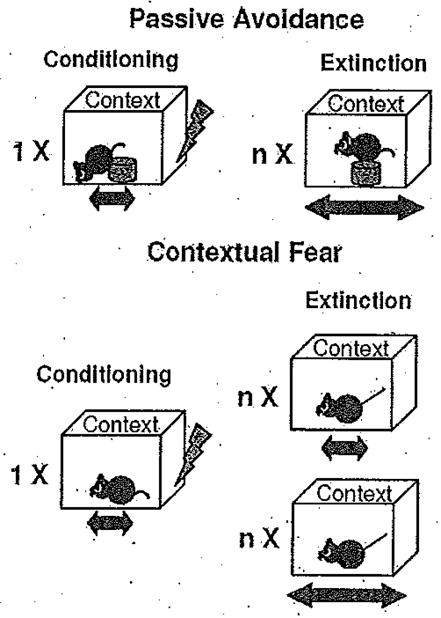
Schematic representation of the conditioning and extinction procedures employed in passive avoidance (upper panel) and fear conditioning (lower panel) paradigms.
However, the training and extinction protocols have important differences. In contextual fear conditioning, animals are exposed for a period of time to a distinctive context, sufficient to form a representation of that context, and then presented with a brief, mild footshock. Extinction trials consist of re-exposure to the shock-paired context, leading to memory retrieval, induction of a central fear state, and freezing behavior. In contrast, passive avoidance is conducted in a two-compartment box, and initial exposure is to the non-preferred compartment or elevated platform. Rodents typically run to the preferred compartment or step-down where they immediately receive a footshock and are removed from the apparatus, Extinction consists of re-exposure to the safe area of the compartment, and the latency to move into the shock-paired area is measured. Here, the very short contextual exposure during training and ability to avoid the shock-paired context are critical differences from contextual fear conditioning.
2.1. Reinforcement during conditioning
The rate of fear extinction depends on the protocol used to condition fear 60 Indeed, several different reinforcement schedules can be used to induce fear learning. A single trial of a context-shock pairing is most common, in which the length of time in the context is sufficient to form and store a contextual representation. Other raining schedules include continuous reinforcement, consisting of multiple pairings of a given context with shock, or partial reinforcement, consisting of randomly paired and unpaired shock presentations (Fig. 2). In addition, fear can be acquired through second-order conditioning by pairing novel stimuli with the previously conditioned instead of an unconditioned stimulus.
Fig. 2.
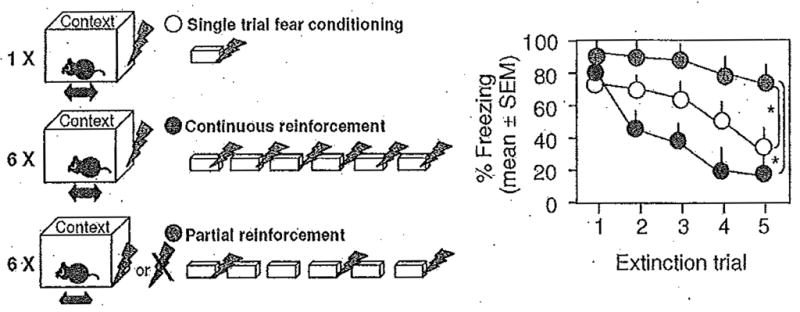
Effect of reinforcement during conditioning on the rate of fear extinction. Schematic representation of different reinforcement schedules (left) and freezing behavior over multiple extinction trials (right). Note rapid extinction after continuous and lack of extinction after partial reinforcement. Adapted from Huh et al., 2009.
Typically, one presentation of shock after adequate contextual exposure is sufficient for the generation of robust and lasting freezing and avoidance behavior. Multiple or spaced trials are rarely employed for these paradigms, but the few studies performed so far indicate that their molecular requirements for fear conditioning are different 70,124 This omission leaves a significant gap for future research, because variations in US number, intensity, and contingency with CS, despite producing similar levels of avoidance or freezing, cause marked differences in the extinction rate of these behaviors 6,60. More importantly, depending on the conditioning procedure, fear can be acquired by different processes that cause susceptibility or resistance to extinction. For example, whereas first-order conditioning requires a CS-US association, second-order conditioning does not depend on the US representation 119, but probably on a CS-response or CS-affective state associations 117,53. Furthermore, continuous reinforcement fosters the formation of specific expectations of an imminent US, whereas a partial reinforcement schedule fails to do so and instead enhances the aversive emotional attributes (negative valence, arousal) of the context 60 Finally, single reinforcement probably engages both the expectancy and affective components, although they appear to be weaker than those found after continuous or partial reinforcement, respectively (Fig. 3). Although all the described fear conditioning situations trigger similar levels of freezing, extinction is rapid when fear is based on shock expectancy while markedly impaired if controlled by an aversive contextual valence 60 These findings are consistent with human observations indicating susceptibility and resistance to extinction after expectancy and evaluative learning, respectively 54,147
Fig. 3.
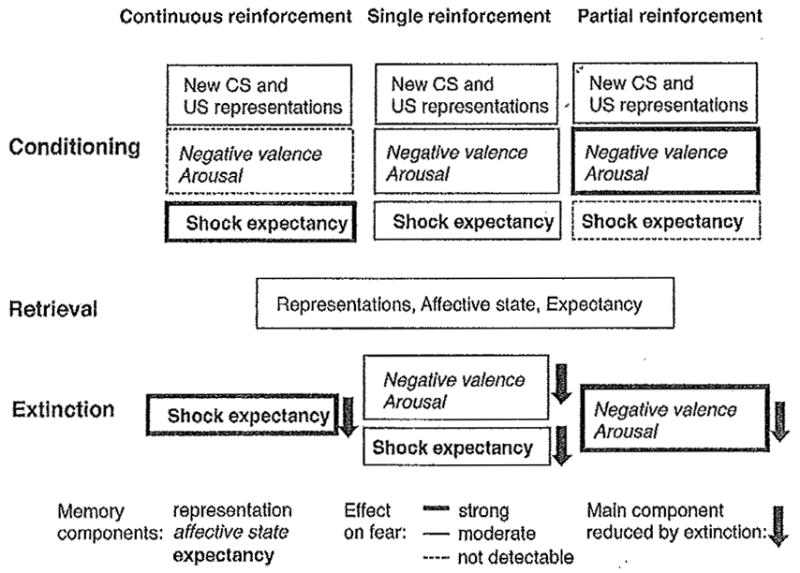
Proposed processes of conditioning and extinction of fear. Continuous, single, and partial reinforcement are established similar contextual representations but different levels of associated expectancy and affective attributes. Successful extinction therefore needs to target the main fear-provoking component.
2.2 Extinction protocols
The reduction of fear also depends on the timing of the extinction trials that can be presented as massed versus spaced 24 or short versus long (reviewed by 100). In that respect, exposures performed after fear conditioning and passive avoidance training are very different. In passive avoidance, it is unclear when contextual representations are formed because the training exposure seems much too short for their formation 152 Context exposures during extinction are always much longer than the conditioning episode as they are controlled by the animal’s avoidance behavior, and likely trigger additional processing of contextual representations. Further, it is notable that extinction largely involves exposure to the safe compartment of the apparatus, as animals are immediately removed from the shock-paired compartment after entry. Finally, it is not clear whether the option to avoid the threatening compartment attenuates the development of a central fear state.
In fear conditioning, exposure to the context is tightly controlled by the experimenter and optimized for the formation of contextual representation 152. During extinction, the context is inescapable and the animals thus experience intense fear. The trials involve exposures of similar or longer duration when compared to training. It is believed, based on the generally anticipated amnestic effects of anisomycin, that short trials trigger memory reconsolidation whereas long trials trigger extinction 107. In our view, this differentiation is not substantiated, unless additional evidence is provided for reconsolidation versus extinction, because although short nonreinforced trials are not sufficient to trigger within-session extinction they do result in a stable reduction of fear after several repetitions. Therefore, most known molecular mechanisms of contextual fear extinction are based on short repetitive exposures, as discussed below. Long trials, on the other hand, cause rapid but transient within-session effects 82. These behavioral findings alternatively suggest that the extinction seen after short and long trials recruits different learning processes and molecular mechanisms.
Based on these paradigmatic differences and related molecular findings (discussed below), we will argue that hippocampal mechanisms of extinction of passive avoidance leads to the formation of new contextual representations embedded in a competing, but not necessarily inhibitory, memory. Extinction mechanisms of conditioned fear, on the other hand, predominantly involve modifications of shock expectancy and a decrease of the affective contextual attributes.
3. HIPPOCAMPAL PROCESSES IN FEAR EXTINCTION
Whereas the amygdala is a major mediator of conditioned fear responses in general, the hippocampus is selectively involved in contextual and trace fear conditioning. Contextual fear conditioning involves hippocampal encoding of contextual representations that are subsequently associated with the US 89,156. Here, contextual stimuli can be the main CS, or a background modulator of a discrete cue. Trace conditioning, which occurs when the CS and US are separated in time, requires hippocampal activity to bridge the temporal gap between these stimuli 90. Delay paradigms, in which a salient cue co-terminates with or is immediately followed by a US, are hippocampus-independent 131,65,108
Hippocampal lesions produce severe deficits in habituation and extinction processes in a number of paradigms37,75,36,66,128,71,11. Yet, because the hippocampus is neither involved in conditioned inhibition 136 nor direct control of fear-motivated behavior, this area was overlooked in fear extinction research until recently. Currently, both animal 148,126,41 and human studies 94,5,74 show hippocampal involvement in fear extinction when the main CS is a threatening environmental context. Most theories of hippocampal function, with the exception of the cognitive mapping theory 102, take into account its significance in processing affective states. Hirsh’s contextual retrieval theory 56 posits that information transfer from storage to performance is prompted by motivational cues resulting in anticipation of stimuli. According to Cormier’s match-mismatch theory 30 and consistent with later comparator views of hippocampal function 135,127,50,48, this brain area performs continual analysis of the relationship of cues to reinforcement and thus, when reinforcement is omitted, contributes to habituation and extinction. This is supported by evidence for significant functional interactions between the hippocampus and amygdala 59,132. In addition, the hippocampus processes almost all modalities of sensory input 149, and surprising events such as novelty and prediction errors 58, 109,113,73. Finally, emotional and motivational states strongly alter hippocampal activity associated with specific contexts and goals 97,63. Together, these theoretical and experimental analyses indicate that the hippocampus contributes to fear extinction by processing the sensory/discriminative, motivational/affective, and unexpected properties of contextual stimuli.
4. MOLECULAR MECHANISMS
4.1 Extinction of passive avoidance
Much initial data on hippocampal mechanisms of extinction came from studies utilizing the passive avoidance paradigm. In this protocol, many of the mechanisms required for consolidation are replicated during extinction. Like conditioning, extinction of passive avoidance is disrupted by anisomycin 148,110 and dependent on protein kinase A, ERK, NMDA receptors, CaMKII 143 and p38 MAPK 120 These data supported the concept of extinction as new learning, resulting in a memory that competes for retrieval with the original fear-provoking memory 21. However, these studies have not revealed mechanisms for an extinction-specific, inhibitory association, consistent with the lack of earlier behavioral evidence for associative inhibition 72,116,80
4.2 Extinction of contextual freezing
In contrast to passive avoidance, hippocampal mechanisms of contextual fear extinction exhibit many differences when compared to fear conditioning, independently of whether exposures involve short or long trials. These differences are notable at multiple levels and encompass general biochemical and metabolic processes as well as signaling within individual neurotransmitter and transduction pathways.
4.2.1. General biochemical processes
The use of anisomycin, cycloheximide, or other drugs that block protein synthesis [but also exhibit many other cellular effects on signal transduction and neurotransmitter release 46,112 is known to produce strong and lasting amnesia in many learning paradigms. Consistent with the view that protein synthesis is a biochemical process required for learning, these compounds impair fear conditioning, when injected systemically or locally, before or immediately after training 140, 125 Unexpectedly, extinction of contextual fear progresses when protein synthesis is blocked throughout the brain 76 or within the hippocampus 41,77,86. These initial data suggested that molecular mechanisms of extinction would fundamentally differ from those governing contextual fear conditioning.
It should be noted that some general processes, such as dependence on cytoskeletal rearrangement 41, glutamate receptors 153, and histone acetylation 78,22 appear to be common. Given the primary role of histone acetylation in gene expression and protein synthesis, the evidence for a role of histone acetylation but not protein synthesis is puzzling. Among other possibilities, the expression of regulatory, non-protein coding RNAs 92, rather than production of new proteins, may be the predominant consequence of gene expression in fear extinction. The activation of the hypothalamo-pituitary-adrenal axis has been strongly associated with general metabolic changes and other effects of stress hormones (corticotropin-releasing factor, corticosterone) contributing to fear-motivated behavior 28. Somewhat surprisingly, elevated levels of stress hormones enhance fear conditioning 122,118, and also enhance fear extinction 155,2,47 (but see opposite effects for passive avoidance 144), Notably, low doses of stress hormones also seem to have beneficial effects in patients with PTSD, arachnophobia, and social phobia 3,137. The extinction-enhancing effect of corticosterone has been attributed to impaired memory retrieval 2, however this contradicts the view that retrieval is required for extinction (see below) and needs to be further clarified.
4.2.2. Hippocampal mechanisms exhibiting opposite activity patterns and roles after fear conditioning and extinction
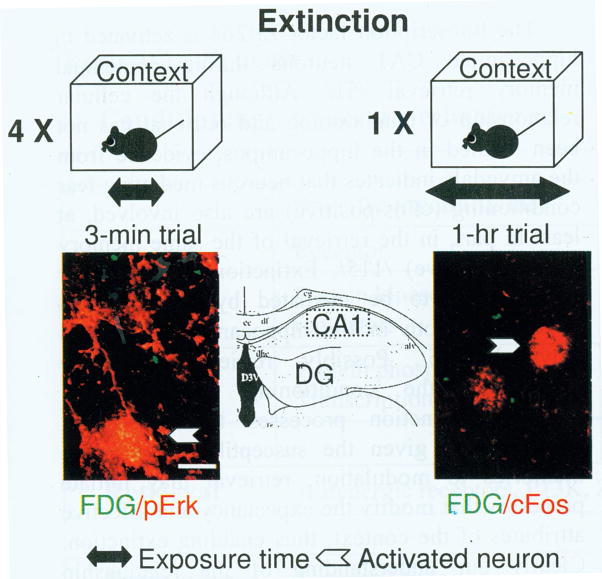
A number of molecules exhibiting a key role in fear conditioning emerge as irrelevant or inhibitory to fear extinction. cFos, an immediate early gene product and potent transcriptional regulator, is strongly activated by environmental stimuli and exhibits a highly conserved time course independently of stimulus duration. Hippocampal cFos is triggered by novel contexts 111,59,88 but rapidly habituates thereafter 105,95,151. On this basis, the role of cFos in fear conditioning is most likely to promote the formation of contextual representations that are the integral part of a fear conditioning memory. Absence of cFos after short extinction trials therefore suggests that formation of a new contextual representation does not take place during extinction. Interestingly, extending the exposure to 1 hr increases cFos levels (Fig. 4), implying that despite the same spatial configuration, duration can also provide a novelty signal. Unlike cFos, the activation time-course of the transcription factor CREB both in vitro 15 and in ivo is highly variable. In the hippocampus, and in other brain areas 68,25 CREB regulation may be more related to arousal and anxiety, processes that markedly support consolidation of emotionally-relevant memory, as discussed earlier 139. Accordingly, conditioning performed with spaced trials overcomes the requirement for CREB for contextual fear conditioning. Hippocampal pCREB levels decline after repeated short and individual long extinction trials along with the decrease of freezing 145,86 and may thus reflect a change of the emotional attributes, such as valence and arousal, linked to the conditioning context. Although mice with a disrupted CREB gene exhibit impairments of contextual fear extinction, these effects seem to be hippocampus-independent and are instead mediated by the amygdala and prefrontal cortex 86. A number of signaling pathways affecting gene expression and synaptic function exert opposite effects on conditioning and extinction. Brain-derived neurotrophic factor (BDNF) is an important mediator of contextual fear conditioning 83. The same factor, however, impairs fear extinction, as revealed by enhancing effects of post-session injections of BDNF antisense or prevention of pro-BDNF processing in the hippocampus 10.
Fig. 4.
Molecular changes triggered in the CA1 hippocampal area after short and long extinction trials. Neurons activated by fear conditioning are fluorescently labeled (green) as described in Tronson et al., 2009. Short and long extinction trials activate either ERK (left) or cFos (right), respectively. Scale bar = 5 μm. FDG, fluorescein-di-beta-D-galactopyranoside New contextual representations that are not associated with shock may thus preferentially contribute to extinction after long exposures
Protein kinase A (PKA) and protein kinase C (PKC) 4 as well as cyclin dependent kinase 5 (CDK5) 40, have been strongly implicated in the consolidation of contextual fear. These kinases, however, exhibit an inhibitory effect on fear extinction 61,123,101. Notably, most of the protein kinases that are strongly activated by fear conditioning are not triggered by extinction trials. It is rather their baseline activity or subcellular redistribution that opposes fear extinction 123,145. Contrary to these kinases, the phosphatase calcineurin that inactivates serine-threonine kinases, is required for extinction, but exerts an opposite, limiting effect on conditioning 52. The protein kinases exhibiting an inhibitory role in extinction are therefore likely substrates for calcineurin.
4.2.3. Hippocampal mechanisms specific for fear extinction
Contextual fear extinction requires several mechanisms that are not induced during conditioning. Cannabinoids acting via CB1 receptors are one of the major mediators of fear extinction. Pharmacological inhibition of hippocampal CB1 impairs 14,104, whereas CB1 stimulation exhibits an opposite, enhancing effect on fear extinction 34. Consolidation of contextual fear conditioning, on the other hand, is not affected by CB1-acting drugs9 The cytoplasmic polyadenylation element binding protein (CPEB) also emerges as an important and selective mediator of contextual fear extinction, as revealed by persistent freezing behavior to shock-paired context in the CPEB knockout mouse 13 Although this mouse shows baseline changes in the expression of several hippocampal genes, the direct hippocampal role in this phenotype remains to be demonstrated.
Another interesting question is whether the extinction effects of CPEB are due to its RNA-regulatory or prion-like actions 133. More subtle signaling differences, such as activation of ERK at different subcellular locations, occur between conditioning and extinction. After conditioning, pERK is predominantly localized to fibers, whereas during non-reinforced trials nuclear activation of ERK is required for extinction to progress 39, 146. A differential role and pattern of ERK signaling in extinction compared to conditioning has also been suggested from transgenic mice with overexpression of Rap2. In these mice, both fear and pERK levels after conditioning are normal. In contrast, extinction and pERK induced by non-reinforced sessions are impaired 121. Importantly, sustained nuclear activation of ERK linked to extinction was specifically observed after detection of prediction error, when expectations of shock were violated by lack of shock delivery, but not by novelty, retrieval, habituation or reinforcement 60 These findings demonstrate that habituation (as revealed by a downregulation of cFos and pCREB) of CA1 neurons mediating fear conditioning may contribute to, but is not sufficient for extinction of fear. Activation of a separate, pErk-positive cell subset by prediction errors plays a critical, extinction-specific key role in the reduction of fear.
4.2.4. Hippocampal mechanisms specific for memory retrieval
The most clearly defined process differentially required for extinction and consolidation is retrieval. Several extinction mechanisms initiated during nonreinforced contextual exposures have been attributed to retrieval processes. Ouyang and Thomas 103 demonstrated that adrenergic signaling mediates contextual memory retrieval, and that this is critical for extinction. Similarly, hippocampal PI3K is required for both retrieval and extinction 27. This molecular evidence has been supported by electrical stimulation studies showing that the hippocampus also modulates the recall of fear extinction38
The transcription factor Zif268 is activated in hippocampal CA1 neurons during contextual memory retrieval 51. Although the cellular relationship of conditioning and retrieval has not been studied in the hippocampus, evidence from the amygdala indicates that neurons mediating fear conditioning (cFos-positive) are also involved, at least in part, in the retrieval of the same memory (Zif268-positive) 115. Extinction, on the other hand, seems to be mediated by different cell populations both in the hippocampus 146 and amygdala 55. Possibly, retrieval transiently destabilizes the conditioning memory 79, allowing extinction processes to take place. Alternatively, given the susceptibility of active memories to modulation, retrieval may initiate processes that modify the expectancy and affective attributes of the context, thus enabling extinction. Clearly our understanding of the relationship between memory retrieval and fear extinction needs to be further advanced.
4.2.5. Summary
The extinction of contextual fear requires an entirely different pattern of molecular and cellular responses when compared to conditioning (Fig. 5). These identified molecular mechanisms likely represent multiple processes engaged by extinction including retrieval, detection of prediction error, and mechanisms to alter contextual valence and arousing attributes.
Fig. 5.
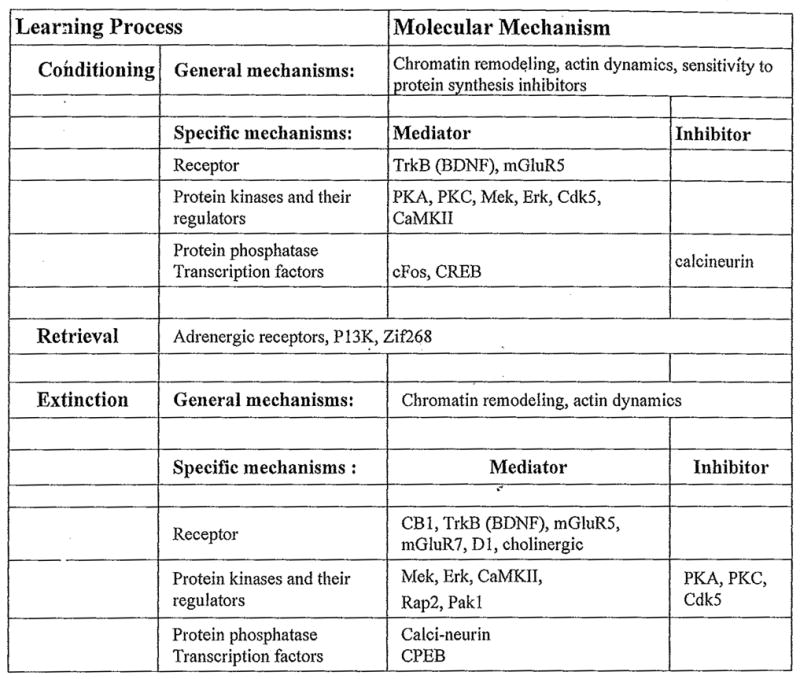
Overview of hippocampal molecular mechanisms mediating or inhibiting contextual fear extinction.
5. A MULTITUDE OF LEARNING PROCESSES CONTRIBUTING TO FEAR EXTINCTION
The presented findings indicate that the model of CS-US and CS-noUS associations referring to the learning processes underlying conditioning and extinction of fear, respectively, may neither be sufficiently specific nor accurate. In addition to the animal studies presented above, evidence from human studies also supports this view. Emotion theorists have long acknowledged that fear conditioning does not entail “a” CS-US association but instead a multitude of different associations formed between the CS and the sensory/discriminative properties of the US, the CS and the affective/motivational properties of the US, the CS and different conditioned responses 42, the US and affective/hedonic states 44, and more (reviewed in detail by 32). Depending on the conditioning protocol, the CS may be endowed with the emotional attributes of the US, such as negative valence or arousing properties, or linked to specific expectations that a CS will be followed by a US.54 Based on a large body of human imaging studies, it appears that the specific components of these learning processes are mostly regionalized whereas the associative aspects are likely to reflect a network property. Specifically, the amygdala and orbitofrontal cortex encode the arousing attributes and valence of a reinforcing stimulus 8,64,81; the prefrontal-hippocampal network is involved in formation and modification of expectancy 141,73 and responses to surprising events such as novelty 109, 154 and prediction errors; and the entire orbitofrontalamygdalar-hippocampal circuit is active during contextual conditioning and extinction 69, 94 Because of the general concordance between animal and human neurocorrelates of fear extinction 62,5, electrophysiological and molecular analyses with experimental animals have the potential to significantly expand our detailed knowledge at a mechanistic and microcircuit level. For example, the better resolution of electrophysiological approaches allows detection of different amygdalar populations representing positive and negative valence 12, whereas imaging studies only show responses to but not valence 64. Similarly, molecular analyses identify segregated hippocampal populations responding to novelty and prediction errors 146, two processes that in imaging studies activate the hippocampus indistinguishably 109
6. SIGNIFICANCE
Studies of contextual fear conditioning and extinction have begun to unravel novel hippocampal mechanisms with significant pathophysiological implications for anxiety disorders. Although many of the learning processes discussed may take place after a single training trial, it has been known for a long time that, both from animal studies and patients suffering from anxiety disorders, conditioned responses, expectancies of aversive outcomes, and fear itself, extinguish independently from one another and at different rates. For example, somatic fear responses can show reduction even when expectations of aversive outcomes persist 67 Accordingly, anxious patients also differentially extinguish fear-motivated conditioned behaviors, somatic responses (heart rate, skin conductance), threat expectancies (human) and fear affect 31. Possibly, hippocampal abnormalities observed in patients with PTSD 45 contribute to the persistence of selected aspects of fear. Identifying and activating those extinction mechanisms that specifically oppose the process governing fear in individual patients will enhance the success of developing therapeutic approaches for anxiety disorders.
7. FUTURE DIRECTIONS
Neuroanatomical and electrophysiological approaches in animals have advanced the understanding on how representations of stimuli, value, response, outcome and prediction error contribute to appetitive learning (reviewed by 129, 57). Although the main brain circuit involved in fear conditioning is known 87,138, how other memory components are regionally processed remains unclear. The detection of prediction errors occurring when expected aversive events are omitted has been found in the periaquaeductal graymatter 91 and hippocampus 60 This type of expectation violation emerges as an important and specific determinant of fear extinction when compared to conditioning. Alterations of valence and arousal are also critical factors mediated by the amygdala 12, and orbitofrontal cortex 81. These findings open broad opportunities for future research aiming to dissect the molecular substrates of individual process underlying conditioning and extinction of fear. While facing the difficulty of directly quantifying expectancies in animals, the use of continuous reinforcement schedules is very likely to foster this type of learning and associated molecular changes. The use of partial reinforcement, on the other hand, may be useful to preferentially study the role of affective states in fear regulation. The identification of molecular correlates of emotional valence and arousal will clarify whether contextual representations gain stable aversive attributes endowed in a single fear memory, or if emotional attributes are reevaluated on a trial-by trial basis. In addition to contextual fear extinction, the hippocampus serves as an important mediator of context-specific regulation of aversively 29 and appetitively motivated behaviors 43 in response to corresponding cues. It remains to be determined whether the molecular pathways described above also contribute to extinction in these situations.
Identifying a common mechanism would significantly advance treatment options for simultaneous extinction of contextual and other unwanted, context-specific, behaviors motivated by fear or reward. The relationship between memoiy retrieval and fear extinction remains to be further clarified as does the contribution of emotional factors caused by general arousal, anticipatory anxiety. Whether different memories compete for retrieval or if one contextual representation is retrieved and the decision made based on appraisal and US expectancy is still unclear. These important questions will be answered once the brain region-specific molecular correlates of the aforementioned processes have been elucidated. Ultimately, the determination of the molecular identity of hippocampal and other regional processes governing fear extinction would stimulate the development of specialized molecular approaches toward fear relief based on distinctive abnormalities in individual patients with anxiety disorders.
Acknowledgments
We would like to thank Dan Sylvester for his assistance with the preparation of the manuscript, and Yomayra Guzman, Anita Guedea, Kevin Corcoran, Can Gao, and Eva Redei for their helpful comments. This work was supported by the NIMH grant MH073669 and Dunbar Funds to J.R
References
- 1.Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev. 1998;26:360–78. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 2.Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: dependence upon training intensity. Neurobiol Learn Mem. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose Cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 4.Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: corticalhippocampal and amygdala contributions. J Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrogi Lorenzini C, Baldi E, Bucherelli C, Tassoni G. Forced extinction as a means to evaluate consolidation gradient of a passive avoidance response in the rat. Physiol Behav. 1993;53:873–877. doi: 10.1016/0031-9384(93)90263-f. [DOI] [PubMed] [Google Scholar]
- 7.Ameli R, Ip C, Grillon C. Contextual fearpotentiated startle conditioning in humans: replication and extension. Psychophysiology. 2001;38:383–390. [PubMed] [Google Scholar]
- 8.Anderson AK, Sobel N. Dissociating intensity from valence as sensory inputs to emotion. Neuron. 2003;39:581–583. doi: 10.1016/s0896-6273(03)00504-x. [DOI] [PubMed] [Google Scholar]
- 9.Arenos JD, Musty RE, Bucci DJ. Blockade of cannabinoid CB1 receptors alters contextual learning and memory. Eur J Pharmacol. 2006;539:177–183. doi: 10.1016/j.ejphar.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Barnes P, Thomas KL. Proteolysis of proBDNF is a key regulator in the formation of memory. PLoS ONE. 2008;3:e3248. doi: 10.1371/journal.pone.0003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker JT, Olton DS. Object discrimination by rats: the role of frontal and hippocampal systems in retention and reversal. Physiol Behav. 1980;24:33–38. doi: 10.1016/0031-9384(80)90010-4. [DOI] [PubMed] [Google Scholar]
- 12.Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger-Sweeney J, Zeaifoss NR, Richter JD. Reduced extinction of hippocampal-dependent memories in CPEB knockout mice. Learn Mem. 2006;13:4–7. doi: 10.1101/lm.73706. [DOI] [PubMed] [Google Scholar]
- 14.Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–14. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 17.Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48. [Google Scholar]
- 18.Bouton M, Swartzentruber D. Analysis of the associateive and occasion-setting properties of contexts participating in a Pavlovian discrimination. J Exp Psychol Anim Behav Process. 1986;12:333–350. [Google Scholar]
- 19.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 20.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 21.Bouton ME, Peck CA. Context effects on conditioning, extinction, and reinstatement in an appetitive conditioning preparation. Anim Learn Behav. 1989;17:188–198. [Google Scholar]
- 22.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context-specific freezing and associated physiological reactivity as a dysreguiated fear response. Dev Psychol. 2004;40:583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- 24.Cain CK, Blouin AM, Barad M. Temporally massed CS presentations generate more fear extinction than spaced presentations. J Exp Psychol Anim Behav Process. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- 25.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Chan KH, Morell JR, Jarrard LE, Davidson XL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- 28.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 29.Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cormier SM. A match-mismatch theory of limbic system function. Physiological Psychology. 1981;9:3–36. [Google Scholar]
- 31.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Dalgleish T. Cognitive approaches to posttraumatic stress disorder: the evolution of multirepresentational theorizing. Psychol Bull. 2004;130:228–260. doi: 10.1037/0033-2909.130.2.228. [DOI] [PubMed] [Google Scholar]
- 33.Davis M, Myers KM. The role of glutamate and gamma-am inobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- 34.de Oliveiia Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. Opposite action of hippocampal CBI receptors in memory reconsolidation and extinction. Neuroscience. 2008;154:1648–1655. doi: 10.1016/j.neuroscience.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Delamater AR. Experimental extinction in Pavlovian conditioning: behavioural and neuroscience perspectives. Q J Exp Psychol B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- 36.Douglas RJ. The Hippocampus and Behavior. Psychol Bull. 1967;67:416–442. doi: 10.1037/h0024599. [DOI] [PubMed] [Google Scholar]
- 37.Ellen P, Wilson AS. Perseveration in the rat following hippocampal lesions. Exp Neurol. 1963;8:310–317. [Google Scholar]
- 38.Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyciin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;24:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 43.Gabriele A, Packard MG. Evidence of a role for multiple memory systems in behavioral extinction. Neurobiol Learn Mem. 2006;85:289–299. doi: 10.1016/j.nlm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Garcia J. Learning without memory. J Cogn Neurosci. 1990;2:287–305. doi: 10.1162/jocn.1990.2.4.287. [DOI] [PubMed] [Google Scholar]
- 45.Gilbertson MW, Williston SK, Paulus LA, Lasko NB, Gurvits TV, Shenton ME, Pitman RK, Orr SP. Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol Psychiatry. 2007;62:513–520. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold PE. Protein synthesis inhibition and memory: formation vs amnesia. Neurobiol Learn Mem. 2008;89:201–211. doi: 10.1016/j.nlm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray J, McNaughton N. The neuropsychology of anxiety. Oxford: Oxford University Press; 2000. [Google Scholar]
- 49.Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 50.Grossberg S, Merrill JW. A neural network model of adaptively timed reinforcement learning and hippocampal dynamics. Brain Res Cogn Brain Res. 1992;1:3–38. doi: 10.1016/0926-6410(92)90003-a. [DOI] [PubMed] [Google Scholar]
- 51.Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–93. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Havekes R, Nijholt IM, Visser AK, Eisel UL, Van der Zee EA. Transgenic inhibition of neuronal calcineurin activity in the forebrain facilitates fear conditioning, but inhibits the extinction of contextual fear memories. Neurobiol Learn Mem. 2008;89:595–598. doi: 10.1016/j.nlm.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Helmstetter FJ, Fanselow MS. Differential second-order aversive conditioning using contextual stimuli. Anim Learn Behav. 1989;17:205–212. [Google Scholar]
- 54.Hermans D, Vansteenwegen D, Crombez G, Baeyens F, Eelen P. Expectancy-learning and evaluative learning in human classical conditioning: affective priming as an indirect and unobtrusive measure of conditioned stimulus valence. Behav Res Ther. 2002;40:217–234. doi: 10.1016/s0005-7967(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 55.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 56.Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- 57.Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Honey RC, Watt A, Good M. Hippocampal lesions disrupt an associative mismatch process. J Neurosci. 1998;18:2226–2230. doi: 10.1523/JNEUROSCI.18-06-02226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huh KH, Guzman YF, Tronson NC, Guedea AL, Gao C, Radulovic J. Hippocampal Erk mechanisms linking prediction error to fear extinction: roles of shock expectancy and contextual aversive valence. Learn Mem. 2009;16:273–278. doi: 10.1101/lm.1240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isiegas C, Park A, Kandel ER, Abel T, Lattal KM. Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction. J Neurosci. 2006;26:12700–12707. doi: 10.1523/JNEUROSCI.2743-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci USA. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci USA. 2004;101:3310–5. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 66.Kimble DP. Hippocampus and internal inhibition. Psychol Bull. 1968;70:285–295. doi: 10.1037/h0026470. [DOI] [PubMed] [Google Scholar]
- 67.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 68.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 69.Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kogan JH, Frankland PW, Blendy JA, Cobientz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induges normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- 71.Kohler C. Habituation after dorsal hippocampal lesions: a test dependent phenomenon. Behav Biol. 1976;18:89–110. doi: 10.1016/s0091-6773(76)91780-6. [DOI] [PubMed] [Google Scholar]
- 72.Konorski J, Szwejkowska G. Chronic extinction and restoration of conditioned reflexes. I. Extinction against the excitatory background. Acta Biol Exp (Warsz) 1950;15:155–170. [Google Scholar]
- 73.Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29:823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lash L. Response discriminability and the hippocampus. J Comp Physiol Psychol. 1964;57:251–6. doi: 10.1037/h0047851. [DOI] [PubMed] [Google Scholar]
- 76.Lattal KM, Abel T. Different requirements for protein synthesis in acquisition and extinction of spatial preferences and context-evoked fear. J Neurosci. 2001;21:5773–5780. doi: 10.1523/JNEUROSCI.21-15-05773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci U S A. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Kim H, Kaang BK. Synaptic protein degradation underlies destabilization of retrieved fearmemory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 80.Leung HT, Bailey GK, Laurent V, Westbrook RF. Rapid reacquisition of fear to a completely extinguished context is replaced by transient impairment with additional extinction training. J Exp Psychol Anim Behav Process. 2007;33:299–313. doi: 10.1037/0097-7403.33.3.299. [DOI] [PubMed] [Google Scholar]
- 81.Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cereb Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li SH, Westbrook RF. Massed extinction trials produce better short-term but worse long-term loss of context conditioned fear responses than spaced trials. J Exp Psychol Anim Behav Process. 2008;34:336–351. doi: 10.1037/0097-7403.34.3.336. [DOI] [PubMed] [Google Scholar]
- 83.Liu IY, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical roie in contextual fear conditioning. J Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lovibond P, Preston G, Mackintosh N. Context specificity of conditioning, extinction, and latent inhibition. J Exp Psych: Anim Behav Processes. 1984;10:360–375. [Google Scholar]
- 85.Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, Kida S. Brain regionspecific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 88.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 91.McNally GP, Cole S. Opioid receptors in the midbrain periaqueductal gray regulate prediction errors during pavlovian fear conditioning. Behav Neurosci. 2006;120:313–323. doi: 10.1037/0735-7044.120.2.313. [DOI] [PubMed] [Google Scholar]
- 92.Mercer TR, Dinger ME, Mariani J, Kosik KS, Mehler MF, Mattick JS. Noncoding RNAs in Long-Term Memory Formation. Neuroscientist. 2008;14:434–445. doi: 10.1177/1073858408319187. [DOI] [PubMed] [Google Scholar]
- 93.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 94.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in conceit. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 95.Milanovic S, Radulovic J, Laban O, Stiedl O, Henn F, Spiess J. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 1998;784:37–47. doi: 10.1016/s0006-8993(97)01266-3. [DOI] [PubMed] [Google Scholar]
- 96.Miller RR, Matzei LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 51–92. [Google Scholar]
- 97.Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Putting fear in its place: remapping of hippocampal place cells during fear conditioning. J Neurosci. 2004;24:7015–7023. doi: 10.1523/JNEUROSCI.5492-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 99.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 100.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 101.Nijholt IM, Dolga AM, Ostroveanu A, Luiten PG, Schmidt M, Eisel UL. Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell Signal. 2008;20:1715–1724. doi: 10.1016/j.cellsig.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 102.O’Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Clarendon Press; 1978. [Google Scholar]
- 103.Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci USA. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Papa M, Pellicano MP, Welzl H, Sadile AG. Distributed changes in c-Fos and c-Jun immuno-reactivity in the rat brain associated with arousal and habituation to novelty. Brain Res Bull. 1993;32:509–515. doi: 10.1016/0361-9230(93)90299-q. [DOI] [PubMed] [Google Scholar]
- 106.Pavlov I. Conditioned reflexes. Oxford: Oxford University Press; 1927. [Google Scholar]
- 107.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 108.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 109.Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Learning about pain: the neural substrate of the prediction error for aversive events. Proc Natl Acad Sci U S A. 2000;97:9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of re-exposure duration. Learn Mem. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J Neurosci. 1998;18:7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Radulovic J, Tronson NC. Protein synthesis inhibitors, gene superinduction and memory: too little or too much protein? Neurobiol Learn Mem. 2008;89:212–218. doi: 10.1016/j.nlm.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- 114.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 115.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 116.Rescorla RA. Pavlovian conditioned inhibition. Psychol Bull. 1969;72:77–94. [Google Scholar]
- 117.Rescorla RA. Effect of US habituation following conditioning. J Comp Physiol Psychol. 1973;82:137–143. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
- 118.Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 119.Rizley RC, Rescorla RA. Associations in second-order conditioning and sensory preconditioning. J Comp Physiol Psychol. 1972;81:1–11. doi: 10.1037/h0033333. [DOI] [PubMed] [Google Scholar]
- 120.Rossato JI, Beviiaqua LR, Lima RH, Medina JH, Izquierdo I, Cammarota M. On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience. 2006;143:15–23. doi: 10.1016/j.neuroscience.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 121.Ryu J, Futai K, Feliu M, Weinberg R, Sheng M. Constitutively active Rap2 transgenic mice display fewer dendritic spines, reduced extracellular signal-regulated kinase signaling, enhanced long-term depression, and impaired spatial learning and fear extinction. J Neurosci. 2008;28:8178–8188. doi: 10.1523/JNEUROSCI.1944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, Tsai LH. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanderson DJ, Good MA, Skeiton K, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Enhanced long-term and impaired short-term spatial memory in GluAl AMPA receptor subunit knockout mice: evidence for a dual-process memory model. Learn Mem. 2009;16:379–386. doi: 10.1101/lm.1339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schimanski LA, Wahlsten D, Nguyen PV. Selective modification of short-term hippocampal synaptic plasticity and impaired memory extinction in mice with a congenitally reduced hippocampal commissure. J Neurosci. 2002;22:8277–8286. doi: 10.1523/JNEUROSCI.22-18-08277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmajuk NA, DiCarlo JJ. A neural network approach to hippocampal function in classical conditioning. Behav Neurosci. 1991;105:82–110. doi: 10.1037//0735-7044.105.1.82. [DOI] [PubMed] [Google Scholar]
- 128.Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus) J Comp Physiol Psychol. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- 129.Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 130.Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ON. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seiden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversiye conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–50. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 132.Sheth A, Berretta S, Lange N, Eichenbaum H. The amygdala modulates neuronal activation in the hippocampus in response to spatial novelty. Hippocampus. 2008;18:169–181. doi: 10.1002/hipo.20380. [DOI] [PubMed] [Google Scholar]
- 133.Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- 134.Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- 135.Sokolov EN, Vinogradova OS. Neuronal mechanisms of the orienting reflex. Hillsdale, N.J: L. Erlbaum Associates; 1975. [Google Scholar]
- 136.Solomon PR. Role of the hippocampus in blocking and conditioned inhibition of the rabbit’s nictitating membrane response. J Comp Physiol Psychol. 1977;91:407–417. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- 137.Sorayia LM, Heinrichs M, Aerni A, Maroni C, Schilling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in Humans. Proc Natl Acad Sci USA. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontalamygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- 139.Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse, brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- 140.Stiedl O, Palve M, Radulovic J, Birkehfeld K, Spiess J. Differential impairment of auditory and contextual fear conditioning by protein synthesis inhibition in C57BL/6N mice. Behav Neurosci. 1999;113:496–506. doi: 10.1037//0735-7044.113.3.496. [DOI] [PubMed] [Google Scholar]
- 141.Strange B, Dolan R. Functional segregation within the human hippocampus. Mol Psychiatry. 1999;4:508–511. doi: 10.1038/sj.mp.4000593. [DOI] [PubMed] [Google Scholar]
- 142.Sweatt JD. Hippocampal function in cognition. Psychopharmacology (Berl) 2004;174:99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- 143.Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMK1I in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- 144.Taubenfeld SM, Riceberg JS, New AS, Aiberini CM. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biol Psychiatry. 2009;65:249–257. doi: 10.1016/j.biopsych.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tronson NC, Schrick C, Fischer A, Sananbenesi F, Pages G, Pouyssegur J, Radulovic J. Regulatory mechanisms of fear extinction and depression-like behavior. Neuropsychopharmacology. 2008;33:1570–1583. doi: 10.1038/sj.npp.1301550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci. 2009;29:3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vansteenwegen D, Francken G, Vervliet B, De Clercq A, Eelen P. Resistance to extinction in evaluative conditioning. J Exp Psychol Anim Behav Process. 2006;32:71–79. doi: 10.1037/0097-7403.32.1.71. [DOI] [PubMed] [Google Scholar]
- 148.Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fearmotivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci US A. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- 150.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdaia infusions of D-cyclpserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, Spencer RL. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology. 2009;150:749–61. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context pre-exposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci. 2001;115:26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]
- 153.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yamaguchi S, Hale LA, D’Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–24. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 156.Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108:19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]