Abstract
PURPOSE
To provide preliminary data regarding the safety and efficacy of high-dose intravenous daclizumab (Zenapax; Roche Inc, Nutley, New Jersey, USA) therapy for the treatment of juvenile idiopathic arthritis (JIA)-associated active anterior uveitis.
DESIGN
Interventional case series; open-label prospective, phase II pilot study.
METHODS
Six patients were recruited into the study and received daclizumab therapy at doses of 8 mg/kg at baseline, 4 mg/kg at week 2, and 2 mg/kg every 4 weeks thereafter, for a total of 52 weeks. The study was done at the National Eye Institute between June 29, 2005 and July 9, 2008. The primary outcome was a two-step decrease in inflammation grade assessed at week 12. Primary safety outcome was assessed at weeks 2 and 4. The ocular inflammation was assessed according to the Standardization of Uveitis Nomenclature criteria.
RESULTS
Four of the 6 participants achieved two-step reduction in anterior chamber cells according to Standardization of Uveitis Nomenclature Working Group grading scheme for anterior chamber cells 12 weeks into the study and met the primary efficacy endpoint. One additional patient responded to reinduction whereas 1 patient failed reinduction and was considered an ocular treatment failure. Visual acuity improved from a mean of 68 Early Treatment Diabetic Retinopathy Study letters in the worse eye to a mean of 79.6 letters (2 Snellen lines). Three participants were terminated before 52 weeks: First, because of a rash possibly induced by daclizumab; Second, because of ocular treatment failure; and Last, because of uncontrolled systemic manifestations of JIA.
CONCLUSION
High-dose intravenous daclizumab can help reduce active inflammation in active JIA-associated anterior uveitis; however, patients need to be monitored for potential side effects. Larger randomized trials are needed to better assess treatment effect and safety.
Juvenile Idiopathic Arthritis (JIA) is defined as arthritis of unknown etiology persisting for at least 6 weeks with an onset prior to age 16 years.1 Although patients with systemic JIA have extra-articular features, the most common extra-articular involvement in patients with JIA in general is uveitis that occurs in up to 30% of patients.2,3 JIA may account for up to 75% of all pediatric anterior uveitis cases and is the most common systemic disorder associated with uveitis in childhood.4 Significant structural ocular complications and visual impairment can occur in up to 38% of patients.5–16
Approximately 60% of children with uveitis do not sufficiently respond to corticosteroids and may need additional treatment.17 Recent studies on conventional disease modifying agents have suggested efficacy of second-line agents, and more recently anti-tumor necrosis factor (TNF) agents have shown promise in the treatment of childhood uveitis.18–23
The interleukin-2 (IL-2) receptor system is a well-characterized lymphokine receptor system that plays a central role in the induction of immune responses. Daclizumab (Zenapax; Roche Inc, Nutley, New Jersey, USA) is a humanized blocking monoclonal antibody that is directed against an epitope on the alpha subunit of the IL-2 receptor (CD25), localized on activated T cells and other cells of the immune system. Daclizumab was first shown to be effective in the reduction of acute rejection episodes after renal transplantation and has since been utilized for other solid organ transplants.24,25
Daclizumab has been safely and effectively used at low-doses for the treatment of intermediate and posterior uveitis in adults and to a limited extent in children.26–28 We have previously demonstrated the successful use of intravenous (iv) daclizumab as a glucocorticoid and cyclosporine sparing agent in patients with noninfectious intermediate and posterior uveitis.29 A subsequent study demonstrated that subcutaneous (sc) administration of daclizumab was equally efficacious.30 Most recently we have demonstrated that high-dose daclizumab was efficacious in controlling active intermediate and posterior uveitis.26 While our earlier studies had demonstrated the utility of daclizumab in the treatment of patients with active intermediate and posterior uveitis, there is no information on utility of high-dose daclizumab in active JIA-associated uveitis. The primary purpose of this nonrandomized, open-label, prospective pilot study was to assess the possible efficacy of high-dose daclizumab in the treatment of active JIA-associated uveitis.
METHODS
This study was a prospective, open-label, nonrandomized, phase II pilot study of high-dose iv daclizumab treatment for participants with active JIA-associated uveitis.
INCLUSION AND EXCLUSION CRITERIA
Inclusion criteria included age from 6 to 18 years and a diagnosis of noninfectious active JIA-associated uveitis with at least a grade of 1 + for anterior chamber (AC) cells in at least 1 eye requiring treatment to control the intraocular inflammatory disease with anti-inflammatory medications, systemic and/or topical, at high frequency intervals (≥ to 3 times a day). Participants were required to have visual acuity (VA) in at least 1 eye of 20/640 or better (logarithm minimum angle of resolution [logMAR] < 1.54) and were not anticipated to undergo ocular surgery (ie, cataract extraction) within first 6 months of the study. Participants were required to meet American College of Rheumatology criteria for juvenile rheumatoid arthritis/JIA, and have had systemic treatment for their uveitis. Participants were required to be up to date on all recommended childhood immunizations.
Exclusion criteria included participants younger than 6 years of age; participants who had previously received treatment with an IL-2-directed monoclonal antibody or any other investigational agent that would interfere with the ability to evaluate the safety, efficacy or pharmacokinetics of daclizumab; participants with a history or diagnosis of Behçet disease, with evidence of spondyloarthropathy or enthesopathy or active joint or systemic inflammation requiring immediate addition or increase in systemic anti-inflammatory medications; participants with a significant active infection or history of cancer diagnosed within the past 5 years; participants who used latanoprost (Xalatan; Pfizer Inc, New York, New York, USA) within 2 weeks prior to study enrollment; participants for whom administration of fluorescein dye is medically contraindicated; and participants with a media opacity that precludes assessment of AC inflammation. Also, pregnant or lactating participants, or those who refused to use contraception during the study, were not eligible.
OPHTHALMIC AND MEDICAL EVALUATIONS
At baseline visit patients underwent an ophthalmic examination that included grading of AC cells/flare and vitreous cells/haze, manifest refraction, review of systems, physical examination, fundus photography, and intravenous fluorescein angiography (FA). Angiotensin-converting enzyme and human leukocyte antigen panel were done at baseline. Ophthalmic exams (including VA using the standardized Early Treatment Diabetic Retinopathy Study (ETDRS) refraction protocol at 4 meters, inflammation grading, and dilated fundus exams), height and weight, adverse event assessment, concomitant medication assessment, and complete blood count and differential (CBC/diff) were done at each visit. Patients were evaluated by a pediatric rheumatologist at regular intervals. Standardized joint and functional assessment, complete physical examination, rheumatoid factor, antinuclear antibodies (ANA), IgA, cholesterol, serum protein, lactate dehydrogenase (LDH), uric acid, hematology, liver function tests and urinalysis, pregnancy test (where applicable), and childhood health assessment questionnaire were done at regular intervals. FA was done at baseline and week 52; fundus photography and optical coherence tomography (OCT) were done at baseline, week 24, and week 52 (except Participants 3 and 4). Participants’ serum was also studied with flow cytometry using fluorescence-activated cell sorting at baseline and at weeks 2, 8, 16, 24, and 52.
PRIMARY AND SECONDARY OUTCOME ASSESSMENT
Primary ophthalmic efficacy outcome assessed at week 12 was a two-step reduction (or down to 0 out of a scale of 0 to 4) of AC inflammation according to Standardization of Uveitis Nomenclature (SUN) criteria, while on a topical corticosteroid schedule of less than 3 times a day. Secondary efficacy outcomes included: three-line change in distance best-corrected VA, two-step improvement in vitreous haze and cells from baseline, change in presence or extent of cystoid macular edema and the amount of retinal vascular leakage measured by FA, retinal thickening as measured by OCT, and weighted grading score of concomitant immunosuppressive medications. Secondary outcomes were assessed for participants who continued into maintenance therapy, at 3, 6, and 12 months.
Juvenile idiopathic arthritis outcomes included number of joints with active arthritis, number of joints with limited range of motion, erythrocyte sedimentation rate (ESR), and fever, as well as change in physician’s global assessment and childhood health assessment questionnaires (CHAQ) that were assessed by a pediatric rheumatologist at predetermined intervals. In addition to rheumatologic examination scores, CHAQ included 100-mm visual analog scales for subjective assessment of pain (scale of 0 to 100; 0 indicates no pain), degree to which eye disease affects function (scale of 0 to 100; 0 indicates no effect on function), global evaluation of overall well-being of the child as perceived by the parent(s) (scale of 0 to 100; 0 indicates “very well”), and school attendance (days missed from school because of JIA).
MEDICATION DOSING AND ADMINISTRATION
Intravenous infusion of daclizumab was administered at 8 mg/kg at baseline and 4 mg/kg at week 2 as induction dose and followed by 2 mg/kg infusions at week 4 and every 4 weeks thereafter if the participants met the criteria to continue. The primary safety evaluation was done at week 2 and week 4 and primary efficacy evaluation at 12 weeks. If the induction regimen appeared completely ineffective at 12 weeks (ie, no improvements in the primary or secondary measures are observed), the participant exited the trial. Participants who met the primary ophthalmic efficacy outcome without serious adverse events during the induction treatments had the option to receive additional iv treatments of daclizumab at 4-week intervals for a total of 52 weeks. All patients received their infusions at the pediatric day hospital and were carefully monitored during infusions.
An ophthalmic efficacy assessment was made at 12 weeks, and patients who showed a two-step reduction in their intraocular inflammation, and had not met the safety endpoint, continued with daclizumab therapy. If any participant met the safety failure criterion or had a serious adverse effect attributable to the daclizumab, that participant was terminated from further daclizumab study treatments. According to the protocol these participants were allowed to follow-up and receive standard-of-care alternative treatments with a potentially reduced visit schedule.
During the maintenance phase of the protocol (following week 4), if a participant who met the primary ophthalmic efficacy outcome then presented with an ocular flare-up without meeting the treatment failure criteria, reinduction was instituted. This could be repeated up to twice during the study. After the second induction, if the participant did not meet the primary ophthalmic efficacy outcome, the participant was considered a treatment failure. Schedule creep was not permitted so if treatments could not be made during the ±7-day window as scheduled, they were to be skipped rather than adjust subsequent scheduled treatment dates. Tapering of the concomitant immunosuppressive medications was allowed only after the induction phase was completed, during the maintenance phase of the protocol.
SAFETY ASSESSMENT AND ADVERSE EVENT REPORTING
Safety assessments were made routinely during the study, with a review of the previous visit interval performed at each scheduled visit. Each participant was encouraged to report any apparent adverse events between scheduled visits, and returned for additional evaluations and appropriate treatment between scheduled visits if needed. The development of a sight-threatening ocular inflammation requiring immediate increase in systemic anti-inflammatory therapy or a periocular injection of corticosteroids, greater than three-line drop from baseline in VA at any time during the study in the active eye, two-step increase in inflammation from baseline or an increase to the maximum grade from baseline in the active eye, any severe adverse events, any occurrence of serious infection, and any systemic toxicities required termination from the study.
RESULTS
Six JIA-Associated uveitis patients with active eye disease participated in this pilot study.
DEMOGRAPHIC AND ENROLLMENT CHARACTERISTICS
The demographic information and baseline characteristics of the patients are summarized in the Table. Median age at enrollment was 8.5 years with a range of 6 to 17 years. Types of JIA included pauciarticular (oligoarticular) arthritis in 4 patients (Patients 1, 3, 4, and 5), polyarticular arthritis in 1 patient (Patient 6), and systemic-onset JIA in 1 (Patient 2).
TABLE 1.
Clinical Characteristics, Medications, and Adverse Events in Juvenile Idiopathic Arthritis Patients Receiving High-Dose Daclizumab
| Patient | Age/Gender/Race | JIA Type | ANA/RF | Pre-Study Medications | Medications at Termination |
History of Intolerance to or Failure With |
Significant AE | Joint/Systemic Disease Control |
Comments |
|---|---|---|---|---|---|---|---|---|---|
| 001 | 17/female/white | Pauciarticular JIA | Negative/Negative | None | None | Etanercept Infliximab MTX |
Palpitations, zoster | Yes | Completed study |
| 002 | 6/male/AA | Systemic JIA | Negative/Negative | PF TID OU MTX 15 mg Infliximab 10 mg/kga Methylprednisolone iv 20 mg/kg |
PF BID OU MTX 25 mg Naproxen 250 mg Pred 10 mg |
Etanercept Infliximab |
None | No | Terminated at wk 36 (uncontrolled systemic disease) |
| 003 | 10/female/white | Pauciarticular JIA | Negative/Negative HLA-B27(+) |
PF ×6/day OD, ×3 OS | PF TID OD, BID OS | MMFl Azathioprin MTX |
Rash (resolved with topical steroids) |
Yes | Terminated at wk 40 (daclizumab- related rash) |
| 004 | 6/male/white | Pauciarticular JIA | Negative/Negative | PF QID OU Infliximab 20 mg/kg MTX 25 mg |
MTX 12.5 mg PF TID OU |
MTX Infliximab |
None | Yes | Terminated at wk 24 (failed after reinduction) |
| 005 | 7/female/white | Pauciarticular JIA | Negative/Negative | CsA 100 mg PF QID OD Nevanac BID OD |
CsA 100 mg PF TID OD, BID OS |
MTX Naproxen |
None | Yes | Completed study |
| 006 | 15/female/AA | Polyarticular JIA | Positive/Negative | MTX 20 mg Naproxen 1000 mg Pred 20 mg PF q3h OD, q2h OS |
MTX 20 mg Pred 12.5 mg PF BID OU |
Pred | Lower extremity edema secondary to naproxen |
Yes | Completed study |
AA = African American; AE = adverse events; ANA = anti-nuclear antibody; BID = 2 times a day; CsA = cyclosporine (daily dose); iv = intravenous; JIA = juvenile idiopathic arthritis; MMF = mycophenolate mofetil; MTX = methotrexate (weekly dose); OD = right eye; OS = left eye; OU = both eyes; PF = pred forte; Pred = prednisone (daily dose); QID = 4 times a day; RF = rheumatoid factor; TID = 3 times a day; wk = week.
Infliximab stopped 4 weeks prior to enrollment.
PRIMARY AND SECONDARY OUTCOMES
Using the SUN criteria, grading of AC cells and flare was measured using the National Eye Institute grading system.31 The readings were performed by two ophthalmologists (L.J.F. and S.Y.) who examined the patients independently of the principal investigator. Any differences in observations were adjudicated with the help of a third observer.
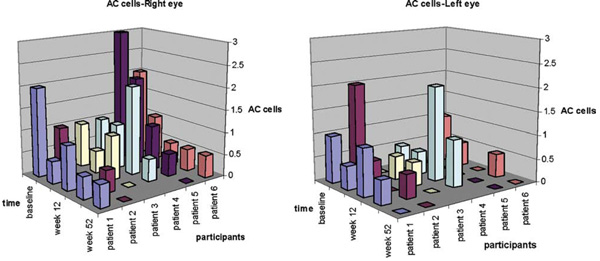
Four of the 6 participants met the primary efficacy endpoint and 1 additional patient (Patient 3) was controlled following reinduction at week 16. The sixth patient (Patient 4) was considered an ocular treatment failure. Patient 3 responded favorably, achieving two-step decrease in inflammation by week 24 (12 weeks into reinduction), but was terminated at week 40 secondary to possible daclizumab-associated rash. Patient 4 showed poor response to reinduction and was terminated from the study at week 24. All of the 4 participants who continued beyond 12 weeks without reinduction maintained quiescence by study termination. Among these 4 participants (Participants 1, 2, 5, and 6) 1 patient (Patient 2) had to terminate the study at week 36 because of uncontrolled systemic JIA despite maintaining quiescence of ocular disease (Figure 1). VA in the eye with worse vision improved from a mean of 68 ETDRS letters to 79.6 letters (2 Snellen lines). Vitreous cells were present in 3 patients at baseline (0.5 to 2+ vitreous cells) (Patients 1, 2, and 5) and improved down to 0 (or by two steps where applicable) in all and AC flare showed an average of one-step improvement at close-out compared to baseline in all participants. None of the patients developed structural ocular complications such as cataract, posterior synechiae, macular edema, epiretinal membrane, optic nerve edema, or hyperemia while in the study. One patient (Patient 3) had macular edema (on OCT and FA) in the right eye at entry that resolved by week 8. One patient (Patient 6) had glaucoma that was treated with Baerveldt shunts in both eyes prior to onset of the study and remained with good IOP control throughout the study.
FIGURE 1.
Anterior chamber (AC) cells over time in patients with juvenile idiopathic arthritis (JIA) receiving high-dose daclizumab. Grading of AC cells by weeks after intravenous daclizumab in each participant’s right (on the left) and left (on the right) eyes are demonstrated. Note that Patient 4 was considered an ocular treatment failure.
Three participants were terminated before 52 weeks; 1 patient (Patient 4) because of treatment failure after reinduction, 1 patient (Patient 3) because of possible daclizumab-related rash, and the other (Patient 2) because of uncontrolled systemic manifestations of JIA.
Joint and systemic disease assessments were done by a pediatric rheumatologist. Only 1 patient (Patient 2) had active joint involvement at baseline (7 joints with decreased range of motion [ROM] on therapeutic assessment) that improved initially but deteriorated at 12 weeks into the study. By week 36 this patient had a systemic disease flare with fever, leukocytosis, and synovitis affecting multiple joints that led to early termination. He was treated with high-dose steroids and increased methotrexate for joint and systemic disease. Physician’s global assessment was “asymptomatic” for all patients both at baseline and on follow-up except for Patient 2 (moderately symptomatic at baseline and week 28). The median CHAQ score was 0 at baseline and at completion of study. Components of CHAQ showed an overall improvement in JIA-related functioning throughout the study period except for Patient 2, who showed an increasing score in all components of the questionnaire consistent with his systemic disease flare. His CHAQ scores were 1.125 at baseline (mild disability), 2.0 at week 12 (severe disability), and 1.5 at week 52 (moderate disability). Patients 2, 4, and 5 had mild anemia early in the study that resolved within 4 weeks into the study. Patient 2, who developed a systemic disease flare at week 36 as mentioned previously, had anemia and an elevated ESR. Patient 5 had mild intermittent leukocytosis throughout the study.
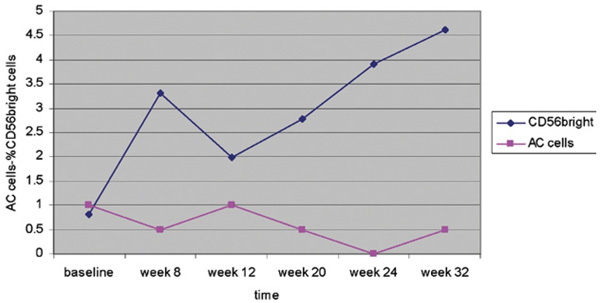
Flow cytometry using fluorescence-activated cell sorting of the peripheral blood samples from 6 patients showed a notable increase in CD56bright cells with daclizumab treatment in all patients but Patient 5, where the increase was minimal. Expansion in CD56bright cell population was consistent with ocular inflammation control in most patients (Figure 2).
FIGURE 2.
CD56bright cells and AC inflammation in a patient with JIA receiving high-dose daclizumab. Grading of AC cells in the right eye of Patient 3 against CD56bright cells (expressed in %) at regular intervals. Note that an expansion in CD56bright cells is associated with a decrease in ocular inflammation whereas a decrease is associated with an increase in intraocular inflammation.
SAFETY ASSESSMENT AND ADVERSE EVENT REPORTING
All patients tolerated the infusions well. Only 1 patient stopped the treatment because of an adverse event that could be directly associated with daclizumab. The majority of the adverse events were mild in severity and considered not related to study therapy. Only 3 events were more than mild (>grade 2) according to Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 and were possibly attributable to study medication: herpes zoster (HZV) rash and palpitations in Patient 1, rash in Patient 3. Palpitations in Patient 1 occurred during daclizumab infusion (11th infusion for this particular patient); the pulse, electro-cardiogram, and cardiac evaluation at the time of the palpitations were unremarkable and she tolerated subsequent infusions without any events. Rash in Patient 3 was biopsied and showed eczematous dermatitis with acanthosis and parakeratosis that was considered to be related to daclizumab. The rash resolved with topical steroids; however, the parents opted to discontinue the study medication. Patient 6 developed lower extremity edema presumably secondary to naproxen that resolved upon discontinuation of naproxen. The Table summarizes all adverse events.
DISCUSSION
Juvenile Idiopathic Arthritis is a disease that can cause both severe arthritis and sight-threatening uveitis. Similar to the uveitis associated with other arthropathies, the severity of the uveitis is unrelated to the severity of the underlying joint disease. Although joint disease often diminishes with age, ocular disease frequently persists into adulthood.12 Visual loss is often multifactorial in children with JIA; however, the visual reduction can be minimized with appropriate anti-inflammatory therapy. A number of immunosuppressive agents have been used to treat severe uveitis associated with JIA.18–23 There is resistance, however, to using many of the cytotoxic agents in children. Additionally, the immunosuppressive medications used to treat uveitis in children may have various side effects, or patients may become refractory to these medications, and thus the development of alternative anti-inflammatory therapies that are both effective and well tolerated continues to be a priority for this pediatric population. Anti-TNF agents have been used for the treatment of JIA-associated uveitis with promising results; experience with infliximab (Remicade; Centocor Inc, Horsham, Pennsylvania, USA) has been particularly encouraging, especially in severe and refractory cases.32,33
The efficacy of daclizumab as a corticosteroid-sparing agent in patients with intermediate and posterior uveitis has been demonstrated previously in several studies. In a phase I/II clinical trial, 8 of 10 patients with intermediate, posterior, or panuveitis were completely tapered off their other immunosuppressive medications while receiving intravenous daclizumab at a dose of 1 mg/kg per infusion.29 Subsequent studies have replicated these findings in a variety of uveitic syndromes.34,35 Similarly, a multicenter series of patients with uveitis treated with subcutaneous daclizumab showed 67% of patients were able to taper their immunosuppressive medications, and a longer-term phase I/II study using daclizumab corroborated similar results, with 70% success.30 Most recently, our group showed that high-dose daclizumab, using same dose protocol used in the current study, reduced inflammation in active intermediate-posterior uveitis.26 Although several studies have demonstrated the safety and efficacy of daclizumab in adult patients, there is very limited information on its utility in childhood uveitis. Gallagher and associates27 reported the use of daclizumab at a lower dose (1 mg/kg, every 2 to 8 weeks) in a retrospective case series. Children who received daclizumab consisted of 5 patients with panuveitis, keratouveitis, and anterior uveitis; however, there were no children with JIA-associated uveitis on daclizumab. They reported that 3 of the 5 children improved on daclizumab therapy and the time to quiescence ranged between 3.4 weeks and 46.3 weeks.
In this study, 4 of the 6 patients showed a decrease in AC inflammation within 12 weeks in the absence of any increase in concomitant immunosuppressives or topical steroids. Two of the 6 patients required reinduction at 12 weeks. One patient (Patient 4) failed to respond to reinduction. Interestingly, one of these 2 patients also had a history of uncontrolled disease while on infliximab treatments. Five patients treated with the induction regimen of high-dose daclizumab demonstrated some decrease in AC cells. The only patient who did not show any evidence of decrease in inflammation was the same patient who did not respond to a reinduction and was considered to be an ocular treatment failure. It is possible that if daclizumab is going to be effective for a particular patient it may be evident in the early phase of the treatment, avoiding futile treatments. We have previously reported that long-term anti-IL2R antibody treatment led to selective expansion of CD56bright cells in adult patients and this expansion may be associated with disease remission in patients with active uveitis treated with daclizumab.36 In this study with young children, we have observed similar induction of selective expansion of CD56bright cells. This increase was consistent with the clinical response in most patients as well. However, the induction of selective expansion of CD56bright cells may not necessarily quantitatively correlate with the disease remission. For example, in Patient 5, despite a consistent favorable clinical response, the increase in CD56bright cells was not dramatic.
Based on the previous experience with high-dose daclizumab in adult uveitis and the theory that a higher initial dose of medication could saturate CD25+ cells even in more sequestered areas such as lymph nodes, we evaluated the use of high-dose daclizumab in this pilot clinical study of JIA-associated active uveitis. The JIA patients with active uveitis were treated initially with a high-dose of intravenous daclizumab followed by a lower dose. The findings from this trial reveal that this therapeutic regimen appears to decrease inflammation in the anterior segment of the eye in most patients. However, the results of this trial need to be interpreted cautiously because of the small number of patients, the heterogeneity of the patient population (such as one participant with systemic JIA, which is very rarely associated with uveitis), and the nonrandomized and unmasked nature of the trial. Daclizumab was well tolerated by most patients in the study; however, an episode of HZV infection and skin rash possibly related to daclizumab is important. The former is likely related to increased risk of infection associated with immunosuppression caused by daclizumab. Our previous experience with rash associated with daclizumab shows that these patients tolerate subsequent infusions well with close monitoring and pretreatment (Faia LJ et al, IOVS 2008;49:ARVO E-Abstract 4725). With this trial and previous evidence, high-dose daclizumab may be an option for children who have uncontrolled disease on other immunosuppressives or are intolerant of conventional treatments. There is an obvious need for larger randomized and masked trials to assess treatment effect and safety and to identify those more likely to benefit.
Acknowledgments
This study was supported by the National Eye Institute intramural program, National Institute of Health, Bethesda, Maryland. Involved in design and conduct of study (G.L.C., R.B.N., K.B., J.R., L.J.F., S.Y., K.H.); collection of data (H.N.S., L.J.F., S.Y.); management (R.B.N.), analysis (H.N.S.), and interpretation of data (H.N.S., R.B.N., Z.L.); and preparation (H.N.S., L.J.F.), review, or approval of the manuscript (H.N.S., L.J.F., R.B.N.). The study was conducted at the National Eye Institute, National Institutes of Health under an Investigational New Drug (IND) application. The study protocol was reviewed and approved by the Institutional Review Board of the National Eye Institute and all procedures conformed to the tenets of the Declaration of Helsinki. Clinical Trials registration: NCT00130637 (NEI protocol no.: 05-EI-0208).
The authors would like to thank Dominic Obiyor, National Eye Institute, Bethesda, Maryland, the study coordinator.
Biography

H. Nida Sen, MD, MHSc, is the Director of the Uveitis and Ocular Immunology Fellowship Program at the National Eye Institute (NEI). Dr Sen received her medical degree from Hacettepe University of Turkey and Masters in Health Sciences in clinical research degree (MHSc) from Duke University, Durham, North Carolina. She completed her ophthalmology residency at George Washington University and her uveitis and ocular immunology fellowship at NEI. Her research interests include pediatric uveitis, biologics, Behçet disease and intraocular lymphoma. Dr Sen has authored a number of peer-reviewed articles, book chapters and has received several research awards including an AUPO research award. Currently, her research is supported by the NEI’s intramural research program.
Footnotes
The authors indicate no financial conflict of interest.
REFERENCES
- 1.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 2.Ravelli A, Felici E, Magni-Manzoni S, et al. Patients with anti-nuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. 2005;52:826–832. doi: 10.1002/art.20945. [DOI] [PubMed] [Google Scholar]
- 3.Kotaniemi K, Kautiainen H, Karma A, Aho K. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Ophthalmology. 2001;108:2071–2075. doi: 10.1016/s0161-6420(01)00773-4. [DOI] [PubMed] [Google Scholar]
- 4.Foster CS. Diagnosis and treatment of juvenile idiopathic arthritis-associated uveitis. Curr Opin Ophthalmol. 2003;14:395–398. doi: 10.1097/00055735-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Chalom EC, Goldsmith DP, Koehler MA, et al. Prevalence and outcome of uveitis in a regional cohort of patients with juvenile rheumatoid arthritis. J Rheumatol. 1997;24:2031–2034. [PubMed] [Google Scholar]
- 6.Zulian F, Martini G, Falcini F, et al. Early predictors of severe course of uveitis in oligoarticular juvenile idiopathic arthritis. J Rheumatol. 2002;29:2446–2453. [PubMed] [Google Scholar]
- 7.Chia A, Lee V, Graham EM, Edelsten C. Factors related to severe uveitis at diagnosis in children with juvenile idiopathic arthritis in a screening program. Am J Ophthalmol. 2003;135:757–762. doi: 10.1016/s0002-9394(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 8.Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol. 2007;143:647–655. doi: 10.1016/j.ajo.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997;104:236–244. doi: 10.1016/s0161-6420(97)30329-7. [DOI] [PubMed] [Google Scholar]
- 10.Wolf MD, Lichter PR, Ragsdale CG. Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology. 1987;94:1242–1248. doi: 10.1016/s0161-6420(87)80007-6. [DOI] [PubMed] [Google Scholar]
- 11.Edelsten C, Lee V, Bentley CR, et al. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. 2002;86:51–56. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg AM, Oen KG. The relationship between ocular and articular disease activity in children with juvenile rheumatoid arthritis and associated uveitis. Arthritis Rheum. 1986;29:797–800. doi: 10.1002/art.1780290615. [DOI] [PubMed] [Google Scholar]
- 13.Chen CS, Roberton D, Hammerton ME. Juvenile arthritis-associated uveitis: visual outcomes and prognosis. Can J Ophthalmol. 2004;39:614–620. doi: 10.1016/s0008-4182(04)80026-7. [DOI] [PubMed] [Google Scholar]
- 14.Key SN, Kimura SJ. Iridocyclitis associated with juvenile rheumatoid arthritis. Am J Ophthalmol. 1975;80:425–429. doi: 10.1016/0002-9394(75)90529-2. [DOI] [PubMed] [Google Scholar]
- 15.Özdal PC, Vianna RN, Deschênes J. Visual outcomes of juvenile rheumatoid arthritis-associated uveitis in adults. Ocul Immunol Inflamm. 2005;13:33–38. doi: 10.1080/09273940590909220. [DOI] [PubMed] [Google Scholar]
- 16.Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840–846. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Chylack LT. The ocular manifestations of juvenile rheumatoid arthritis. Arthritis Rheum. 1977;20:S217–S223. [PubMed] [Google Scholar]
- 18.Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32:362–365. [PubMed] [Google Scholar]
- 19.Kilmartin DJ, Forrester JV, Dick AD. Cyclosporin A therapy in refractory noninfectious childhood uveitis. Br J Ophthalmol. 1998;82:737–742. doi: 10.1136/bjo.82.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tynjälä P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K. Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66:548–550. doi: 10.1136/ard.2006.058248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saurenmann RK, Levin AV, Rose JB, et al. Tumour necrosis factor a inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford) 2006;45:982–989. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 22.Rajaraman RT, Kimura Y, Li S, Haines K, Chu DS. Retrospective case review of pediatric patients with uveitis treated with infliximab. Ophthalmology. 2006;113:308–314. doi: 10.1016/j.ophtha.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Smith JA, Thompson DJ, Whitcup SM, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53:18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 24.Vincenti F, Kirkman R, Light S, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338:161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 25.Bumgardner GL, Hardie I, Johnson RW, et al. Phase III Daclizumab Study Group. Results of 3-year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation. 2001;72:839–845. doi: 10.1097/00007890-200109150-00017. [DOI] [PubMed] [Google Scholar]
- 26.Yeh S, Wroblewski K, Buggage R, et al. High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, non-infectious uveitis. J Autoimmun. 2008;31:91–97. doi: 10.1016/j.jaut.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher M, Quinones K, Cervantes-Castañeda RA, Yilmaz T, Foster CS. Biological response modifier therapy for refractory childhood uveitis. Br J Ophthalmol. 2007;91:1341–1344. doi: 10.1136/bjo.2007.124081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nussenblatt RB, Peterson JS, Foster CS, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology. 2005;112:764–770. doi: 10.1016/j.ophtha.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Nussenblatt RB, Fortin E, Schiffman R, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci U S A. 1999;96:7462–7466. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nussenblatt RB, Thompson DJ, Li Z, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21:283–293. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 31.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saurenmann RK, Levin AV, Rose JB, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford) 2006;45:982–989. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 33.Kahn P, Weiss M, Imundo LF, et al. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113:860–864. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Kiss S, Ahmed M, Letko E, Foster CS. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005;112:1066–1071. doi: 10.1016/j.ophtha.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Papaliodis GN, Chu D, Foster CS. Treatment of ocular inflammatory disorders with daclizumab. Ophthalmology. 2003;110:786–789. doi: 10.1016/S0161-6420(02)01932-2. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: In vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–5191. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]