Summary
The benefits of immunotherapy by regulatory T (Treg) cells are unpredictable partially due to the uncertainty of their suppressive mechanism. In fact, various suppressive mechanisms have been proposed but each remains controversial. To better understand Treg-mediated suppression, we have investigated factors which may influence the suppressive effects. In an in vitro suppression assay, over-expression of anti-apoptotic Bcl2 enhancing survival of conventional T responder cells (Tconvs) did not subvert Treg-mediated suppression. In contrast, enhancing activation of Tconvs by increasing the potency of calcium signals completely abrogated Treg-mediated suppression. While Tregs were incapable of suppressing already activated Tconvs, they prevented expression of activation markers on naïve Tconvs during activation, thereby indicating that Tregs mediate suppression through controlling early activation stage. Interestingly, IL-2 deprivation or TGF-β, two suppressive mechanisms, did not effectively inhibit Tconv activation and proliferation when applied alone. In contrast, IL-2 deprivation combined with TGF-β suppressed Tconv activation as potently as Tregs. More importantly, in the transwell system, that separates Tregs from Tconvs, TGF-β contributed to Treg suppression under IL-2 depriving condition. In conclusion, these two suppressive mechanisms acting in concert may be necessary to effectively restrain the early activation of Tconvs.
Keywords: IL-2, TGF-β, suppressor mechanism, regulatory T cell
Introduction
While CD4+ T cells play a central role in establishing and maximizing various immune responses, they also contain a unique subpopulation that suppresses these responses. Sakaguchi et al. (1995) identified CD25 (IL-2Rα) as a marker for this CD4+ suppressor cell population, named regulatory T cell (Treg). Their study showed that CD4+CD25+ Tregs prevented CD4+CD25− cell-mediated autoimmune diseases in athymic nude mice [1]. In 2003, the same and other groups demonstrated that the Foxp3 transcription factor was predominantly expressed in CD4+CD25+ cells. The stable expression of Foxp3 is critical for establishing and maintaining Treg lineage [2-5]. Mutations of the Foxp3 gene can result in lethal autoimmunity both in mouse and human [2-5], proving the requirement for Tregs in maintaining self-tolerance.
Given its suppressive nature, it is of great interest to explore the therapeutic effects of Tregs in immune diseases. However, not fully defined suppressive mechanisms of Tregs shroud potential barriers in developing Treg-based immunotherapy. In fact, multiple suppressor mechanisms have been proposed but their exact roles are subjects of intense debates. Tregs suppress conventional T cells (Tconvs) by secreting suppressive cytokines (TGF-β, IL-10, and IL-35) [6-8], by competing with Tconv for IL-2 [9], by expressing suppressive molecules i.e. galectin-1 on their cell surface [10], or by killing effector T cells [11]. In addition, Tregs have been shown to suppress APCs, which in turn fail to activate Tconvs, as Tregs express cell surface molecules (e.g. CTLA-4, LAG-3, CD39, and Nrp-1) that directly influence APC function [8, 12]. Not surprisingly, these increasing number of mechanisms have been associated with numerous controversial issues related to the fact that abrogating only one mechanism may not reverse Treg suppression [13-15]. It is possible and rather likely that two or more timely interacting mechanisms produce effective Treg suppression. Hence, we explored different conditions that may limit inhibitory effects and examined how coordinated suppressive factors may produce optimal suppression.
Difficulties had emerged from observations that Tregs in totally TGF-β1-deficient mice were either protective or nonfunctional in the same colitis model [13-15]. To avoid these complications, Li et al. generated mice with a T-cell specific deletion of TGF-β production, and using these mice demonstrated that TGF-β production by Tregs was absolutely necessary to inhibit the inflammatory bowel disease [6]. More recently, Pesu et al. showed that Tregs deficient in a proprotein convertase furin produced little TGF-β, and that these mice were less protective in preventing colitis [7]. Based on these results, TGF-β produced by Tregs is continuously considered as one of the important suppressor mechanisms. Another important suppressive mechanism is IL-2 deprivation wherein Tregs constitutively expressing IL-2 receptors “consume” local IL-2, thereby inhibiting Tconv responses [9]. Our present study examined the coordinated effects of IL-2 deprivation and TGF-β in Treg-mediated suppression. We have demonstrated that these two mechanisms in concert suppress Tconvs, and that the suppressor effects are achieved through restraining their early activation. Although IL-2 deprivation alone seems insufficient to inhibit an early activation, it provides an important prerequisite for effective TGF-β-mediated suppression.
Materials and methods
Mice
BALB/c, C57BL/6 (B6), C57BL/6-Tg (BCL2)25Wehi/J (Bcl-2 Tg), and B6.129P2-Il2tm1Hor/J (IL-2 KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animals were maintained at the University of Toledo specific pathogen-free animal facility according to institutional guidelines.
Reagents
Anti-CD25-PE (clone; PC61), IL-2 neutralizing antibody (S4B6) and its isotype antibody rat IgG2a were purchased from BD Bioscience (San Jose, CA, USA). Anti-CD25-APC (PC61.5), anti-GITR-PE (DTA-1), and anti-CD4-PE-Cy5 (GK1.5) were obtained from eBioscience (San Diego, CA, USA). TGF-β neutralizing antibody (1D11) and its isotype antibody mouse IgG1, and mouse TGF-β1 and IL-2 DuoSet ELISA kits were purchased from R&D systems (Minneapolis, MN, USA). Human TGF-β-1 (hTGF-β) was obtained from Pepro Tech (Rocky Hill, NJ, USA).
FACS analysis for cell surface molecules and CFSE assay
CD4+CD25− and CD4+CD25+ T cells, sorted from BALB/c mice using a FACSAria cell sorter (Becton Dickinson, San Jose, CA, USA), were used as Tconvs and Tregs, respectively. The purity of cells was determined to be over 97%. Tconvs were suspended in PBS (1 × 106 cells/ml) containing 1 μM CFSE (Invitrogen, CA, USA) and incubated for 12 min at 37°C. After cell wash, 5×104 CFSE-labeled Tconvs were cultured without or with Treg (1:1 ratio) in the presence of 1.5×105 CD3− syngenic splenocytes (APCs) and 0.5 μg/ml soluble anti-CD3 mAb. Some cultures were treated with IL-2 neutralizing antibody or human (h)TGF-β. To determine the cell division and viability, the day-2 or day-3 cultured cells were stained with propidium iodide (PI) followed by flow cytometric analysis. To determine cell surface marker expression, some cultured cells were also stained with anti-GITR-PE and anti-CD25-APC. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson).
Proliferation and Suppression assay
For proliferation assays, 5×104 CD4+CD25− Tconvs from BALB/c mice were stimulated by 1.5×105 CD3− syngenic APCs and 0.5 μg/ml soluble anti-CD3 mAb in 96-well round-bottom plates. Some cultures were treated with various concentration of IL-2 neutralizing mAb, TGF-β neutralizing mAb, isotype control antibodies, or hTGF-β. To determine cell proliferation, cultures were pulsed with 1 μCi of [3H]thymidine for the final 16 h of the 72-h assay and harvested with a Packard harvester. The incorporated radioactivity was determined by a micro-plate scintillation counter (Packard Ramsey, MN).
In vitro Treg function was measured by culturing 5×104 Tconvs with various numbers of Tregs, both of which were isolated from BALB/c mice. 1.5×105 CD3− syngenic APCs and 0.5 μg/ml soluble anti-CD3 mAb were used as stimulators. Some cultures were treated with phorbol 12-myristate 13-acetate (PMA) (3 ng/ml) or ionomycin (750 ng/ml). Where indicated, Tconvs, Tregs, or APCs were also obtained from B6 or BCl-2 Tg mice. Cultures were pulsed with [3H]thymidine and harvested, as described for proliferation assay above.
Transwell experiments
Tconvs (1×105) were isolated from B6 or IL-2 KO mice, and were cultured in the upper chamber of the 24-well transwell plates (Corning, NY, USA) in the presence of 3×105 CD3− syngenic APCs and soluble anti-CD3 (0.5 μg/ml). Tregs (3×105) from B6 mice were placed in the bottom chamber of some transwells, and were activated with Dynabeads Mouse T-activator CD3/CD28 (Invitrogen; bead-to-cell ratio = 1:1) and plate-bound anti-CD3 mAb (4 μg/ml). As a control, both Tconvs and Tregs (1×105) were added into the upper chamber of some transwells. Where indicated, the cultures were added with IL-2 neutralizing antibody or/and TGF-β neutralizing antibody. [3H]thymidine were added to cultures for the final 16 h of the 72-h assay. Cells in the upper chambers were transferred to 96-well plates and harvested with a Packard harvester. The cell proliferation was determined by a scintillation counter.
ELISA
Tconvs were co-cultured with Tregs in transwell plates as indicated above. Culture supernatants were collect from the top chamber of transwells at 48 h after cultivation. The levels of TGF-β1 and IL-2 in culture supernatants were then assessed by ELISA using commercial kits following the manufacturer's instructions.
Statistical analysis
Statistical analysis and p value were calculated using an unpaired, 2-tailed, Student's t test.
Results
Tregs mediate their suppressive function by inhibiting the early activation of Tconvs
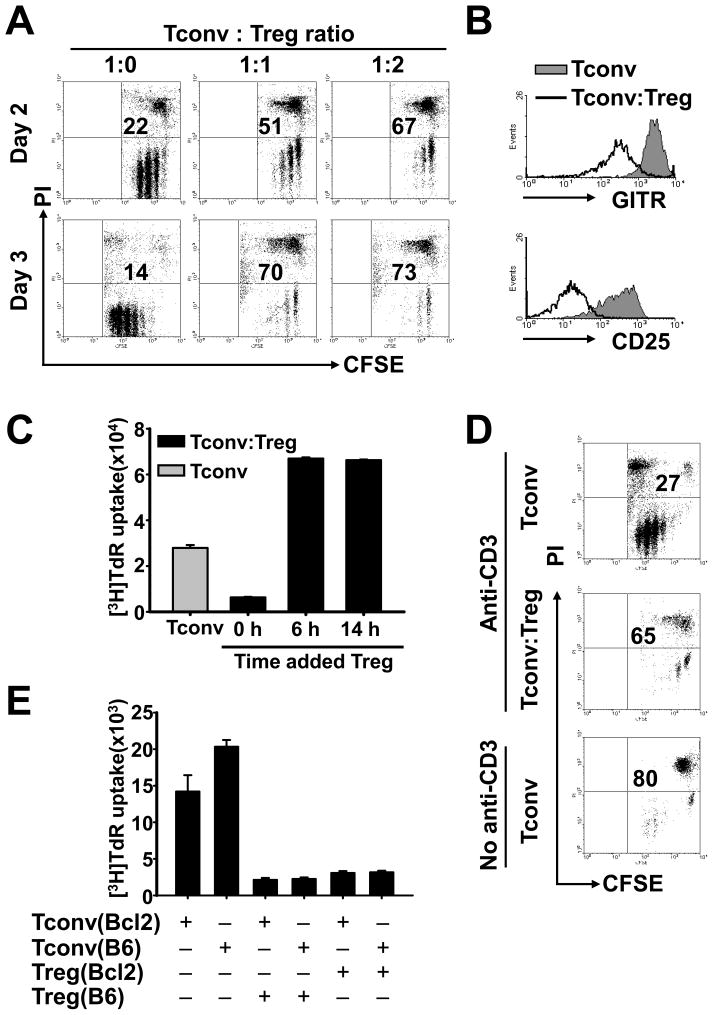
To better understand the molecular mechanisms of Treg suppression, we applied the commonly used in vitro assay in which CD4+CD25− Tconv responder cells co-cultured with or without CD4+CD25+ Treg suppressors (> 90% Foxp3+, data not shown) were stimulated by APCs and soluble anti-CD3 mAb. Comparison of CFSE labeled Tconv cells showed that dramatic suppressive effects were exhibited by Tregs during the first 2 days of cultivation (Fig. 1A). The inhibition was potent as suppressed Tconvs were completely incapable of proliferation between days 2 and 3, whereas during the same time non-suppressed Tconvs (cultured without Tregs) continued an extensive proliferation (Fig. 1A; left panels). We also identified a significant percentage of PI+ dead cells (∼70% on day 3) within the suppressed Tconvs (Figure 1A), which is consistent with other reports [9].
Figure 1. Similar suppressive effects by Tregs on naïve Tconvs and Bcl-2-over-expressing Tconvs but not on activated Tconvs.
Purified CD4+CD25− Tconvs were cultured without or with CD4+CD25+ Tregs at a 1:1 ratio (except in A). Syngeneic APCs and soluble anti-CD3 mAb were used as stimulators (except in D). In A, B, and D, Tconvs were CFSE-labeled before cultivation. (A) Dot plots show CFSE fluorescence vs. PI staining within the CFSE+ Tconv population at culture day 2 and day 3; Tconv:Treg ratios were indicated. Numbers represent the percentage of PI+ Tconvs. (B) Overlay histograms show the expression of GITR or CD25 on CFSE+PI− Tconvs in the day 2 cultures. The black line and the filled gray area represent Tconvs cultured with and without Tregs, respectively. (C) Tregs were added to cultures at the indicated time points. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation. (D) APCs together with (top and middle panels) or without (bottom panel) anti-CD3 mAb were used as stimulators. Dot plots show the frequency of PI+ cells within the CFSE+ Tconv population in the day 3 cultures. (E) As indicated, Tconvs and Tregs isolated from Bcl-2 transgenic or wild-type B6 mice were cultured. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation. More details are described in Materials and Methods. Figures are representative of 3 experiments.
During the same early activation phase Tregs prevented the expression of activation markers, GITR and CD25, on the cell surface of Tconvs in comparison to non-suppressed Tconvs (Fig. 1B). Most importantly, pre-stimulation of Tconvs with APCs plus anti-CD3 mAb for only 6 hrs rendered them resistant to the subsequent suppression by Tregs (Fig. 1C). Thus, Tregs mediate suppression at an early activation phase. Interestingly, enhanced cell proliferation was observed in the co-cultures of Tregs and pre-stimulated Tconvs (Fig. 1C). It is possible that stimulated Tconvs also enhance Treg proliferation [16].
APC alone (without soluble anti-CD3 mAb) is insufficient to activate the majority of T cells (data not shown). About 80% Tconvs were PI+ in the 3-day cultures in which APCs but not anti-CD3 mAb were supplemented (Fig. 1D). These data suggested that insufficient activation of Tconvs in the in vitro cultures may lead to their death. Because Tregs suppress the activation of Tconvs, we speculated that the in vitro death of suppressed Tconvs resulted because of their failed activation (Figure 1A). Indeed, despite over-expressing Bcl-2 in Tconvs (from Bcl-2 Tg mice) to sustain their viability in vitro [17], Tconvs from Bcl-2 Tg mice were susceptible to Treg-mediated suppression (Fig. 1E). Together, our data demonstrate that Tregs suppress early activation of Tconvs. The high frequency of dead cells observed in the suppression assay may be attributed to impaired activation of Tconvs.
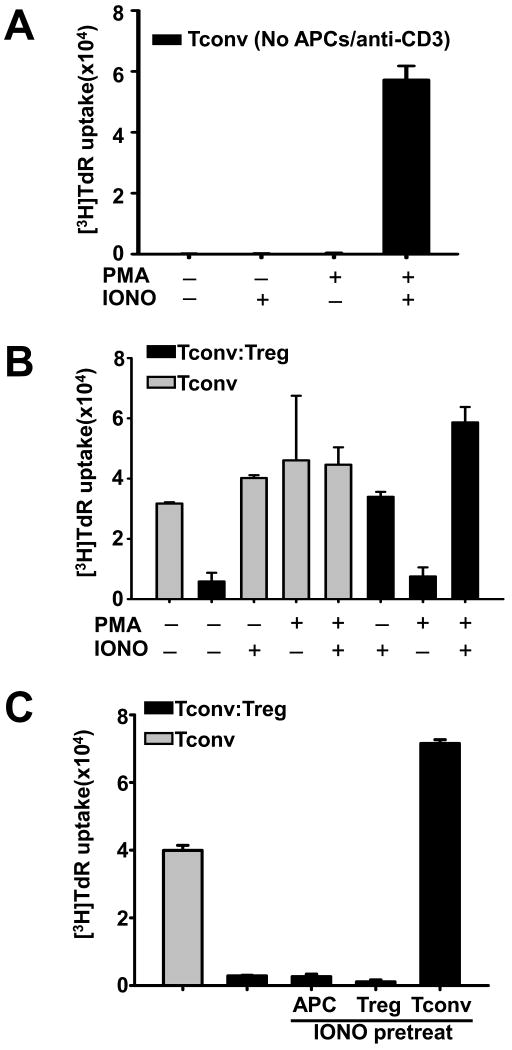
Increasing calcium signaling in Tconvs abrogates Treg-mediated suppression
The above data showed that Tregs suppress the activation of Tconvs. We next investigated whether Tregs can suppress Tconvs upon enhanced activation. Since the amplitude and duration of Ca2+ signals (following TCR engagement) increases the efficiency of gene activation events in T cells [18], we determined whether raising the intracellular Ca2+ influx in Tconvs by ionomycin treatment (data not shown) can render them resistant to Treg suppression. As shown in Figure 2A, 750 ng/ml ionomycin supports Tconv proliferation when combined with 3 ng/ml diacylglycerol analog PMA (Figure 2A; cultures containing only CD4+CD25− cells). However, ionomycin alone was sufficient to completely abrogate Treg suppression (Figure 2B). Furthermore, we pretreated each cell type with ionomycin prior to the suppression assay, and showed that only pre-treating Tconvs, but not Tregs or APCs, caused abrogation of Treg suppression (Figure 2C). Therefore, ionomycin enhances Tconv activation, and thus renders them resistant to Treg suppression.
Figure 2. Increasing calcium signaling in Tconvs abrogates Treg-mediated suppression.
(A) Purified CD4+CD25− Tconvs were cultured alone and were stimulated by PMA and/or ionomycin as indicated. (B-C) CD4+CD25− Tconvs were cultured without (gray bars) or with CD4+CD25+ Tregs at a 1:1 ratio (black bars). Syngeneic APCs and soluble anti-CD3 mAb were used as stimulators. In B, some cultures were supplemented with PMA and/or ionomycin. In C, APCs, Tregs, or Tconvs were pretreated with 750 ng/ml ionomycin for 2 hours, and were washed before culture. As indicated, pretreated APCs, Tregs, or Tconvs were used in cultures, respectively. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation. Figures are representative of 3 experiments.
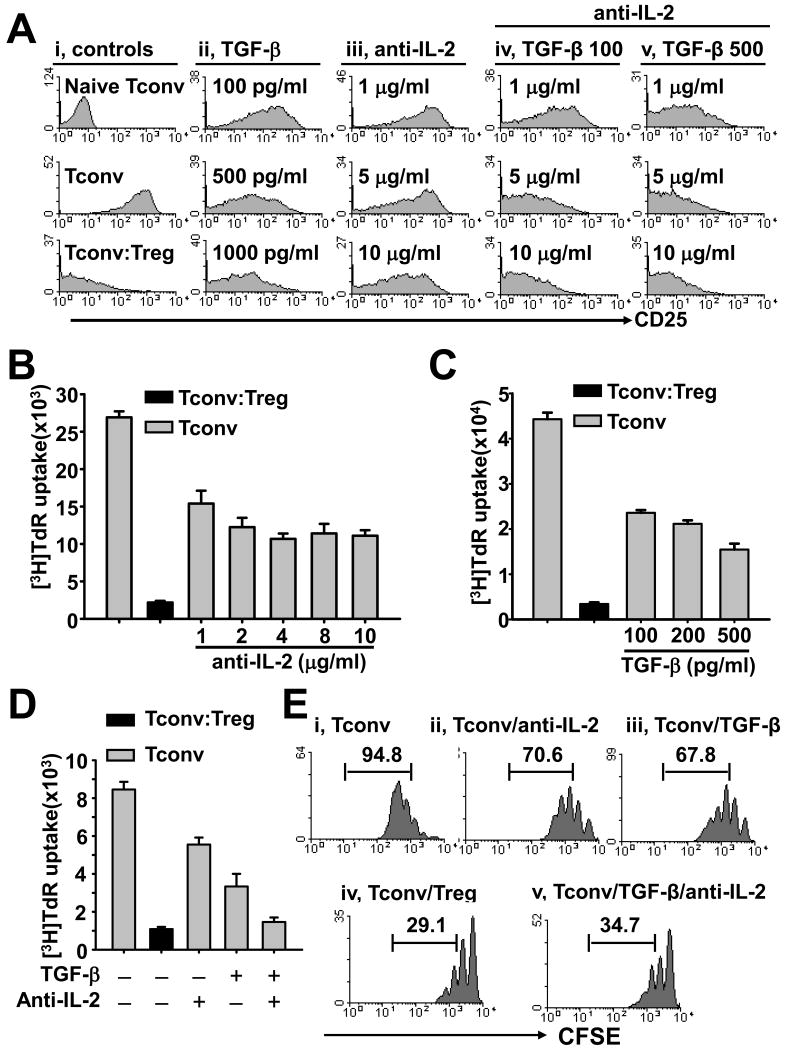
IL-2 deprivation and TGF-β act in concert to potently inhibit activation of Tconvs
Our above data demonstrated that Tregs exert their suppressive function through restraining early Tconv activation. Because of their importance, we examined the impact of two cytokine factors in suppression of Tconvs, namely IL-2 deprivation and addition of TGF-β. Figure 3A shows the expression of activation maker CD25 on Tconvs at day 2 after stimulation by APC and anti-CD3 mAb. While activated Tconvs displayed potent CD25 expression, addition of Tregs almost completely inhibited CD25 expression on Tconvs (Fig. 3A; left column). Although addition of TGF-β (Fig. 3A; second column) or neutralizing anti-IL-2 mAb (Fig. 3A; third column) reduced the expression levels of CD25 on Tconvs, neither of them inhibited Tconv activation as potently as Treg suppression. Interestingly, combination of TGF-β (100 or 500 pg/ml) and neutralizing anti-IL-2 mAb (10 μg/ml) inhibited CD25 expression on Tconvs in a similar pattern as Tregs (Fig. 3A; last two columns). Therefore, deprivation of IL-2 creates a unique environment for TGF-β-mediated suppression during an initial Tconv activation phase.
Figure 3. IL-2 deprivation cooperates with TGF-β to inhibit early activation and proliferation of Tconvs.
CD4+CD25− Tconvs were stimulated by APCs/anti-CD3 mAb. (A) Cultures of CFSE-labeled Tconvs were treated with various concentration of TGF-β (ii), neutralizing anti-IL-2 mAb (iii), or TGF-β (iv, 100 pg/ml; v, 500 pg/ml) together with various concentration anti-IL-2 mAb (as indicated). Tconvs were also cultured without treatment or co-cultured with Tregs at a 1:1 ratio (i, middle or bottom panel, respectively). Histograms show the expression of CD25 on CFSE+PI− Tconvs in the day 2 cultures, or on freshly isolated naïve Tconvs (i, top panel). (B-D) Cultures of Tconvs were treated without or with various concentration of anti-IL-2 mAb or TGF-β as indicated (gray bars). Tconvs co-cultured with Tregs were served as a control (black bars). In D, 500 pg/ml TGF-β and 10 μg/ml anti-IL-2 mAb were used. Cell proliferation was assessed on day 3 by 3H-thymidine incorporation. (E) Cultures of CFSE-labeled Tconvs were treated with anti-IL-2 mAb (ii, 10 μg/ml) or TGF-β (iii, 500 pg/ml), or anti-IL-2 mAb plus TGF-β (v). Tconvs cultured without treatment (i) or co-cultured with Tregs at a 1:1 ratio (iv) served as controls. Histograms show the CFSE fluorescence within the CFSE+PI− Tconv population in the day 3 cultures. Numbers represent the percentage of Tconvs that divided more than once. Figures are representative of 3 experiments.
Very similar patterns have been reproduced by the same two factors when testing to suppress T-cell proliferation (Fig. 3B and 3C). The inhibitory effect of neutralizing anti-IL-2 mAb on Tconv proliferation was also much less potent than Tregs; an increasing anti-IL-2 mAb amount in cultures did not enhance the inhibitory activity (Fig. 3B). These results suggested that Tregs exert their suppressive activity not only through IL-2 consumption. Conversely, addition of TGF-β alone was not sufficient enough to produce suppressive effects comparable to Tregs (Fig. 3C), but combination of anti-IL-2 mAb (10 μg/ml) and TGF-β (500 pg/ml) robustly suppressed Tconvs (Fig. 3D). The same pattern was confirmed by CFSE-labeled Tconvs, as 95% of them divided more than once after stimulation by APCs/anti-CD3 mAb (Fig. 3E; panel i). In cultures deprived of IL-2 or supplemented with TGF-β this number was reduced to 71% and 68%, respectively (Fig. 1E; panels ii and iii). In contrast, addition of Tregs or a combination of neutralizing anti-IL2 mAb and TGF-β suppressed the Tconvs proliferation with only ∼ 30% cells dividing more than once (Fig. 3E; panels iv and v). These collective results showed that two non-redundant suppressive mechanisms are jointly much more effective in inhibiting Tconvs, thereby mimicking effects produced by Tregs.
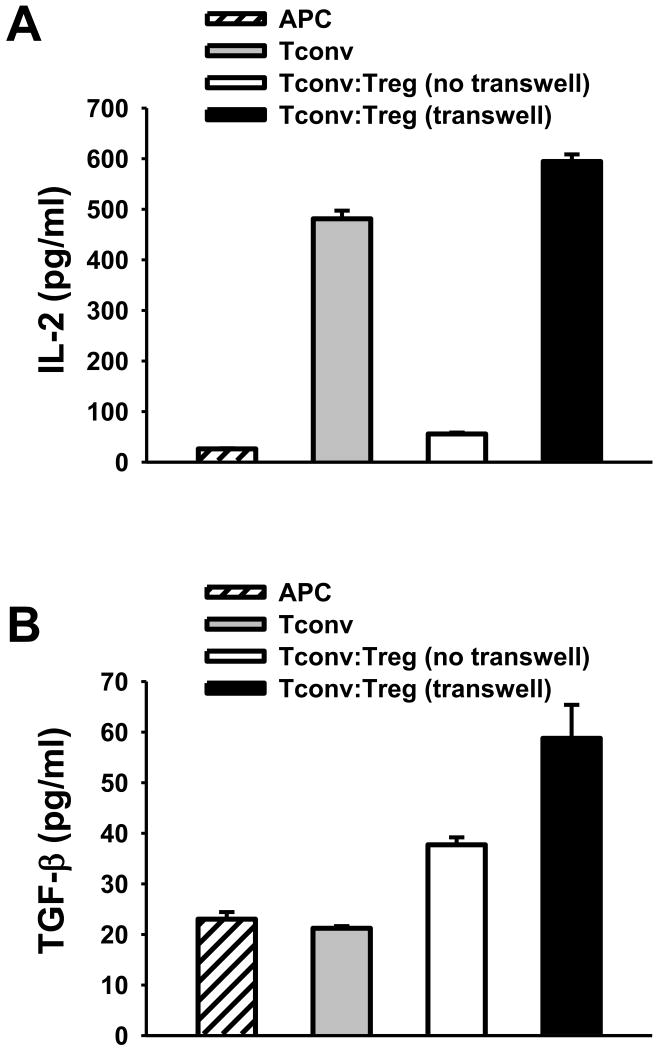
Tregs mediate IL-2-deprivation of Tconvs
A transwell plate system that separates Tregs from Tconvs by a membrane is used to evaluate the role of soluble factors in Treg-mediated suppression. First, we performed an ELISA to measure the levels of TGF-β and IL-2 in transwell cultures of Tregs and Tconvs. As shown in Figure 4A, Tconvs stimulated by APC/anti-CD3 mAb and cultured alone without Tregs produced significant amount of IL-2 (Fig. 4A, gray bar). Mixed culture of Tregs and Tconvs together in the top chamber (but not separated culture of Tregs and Tconvs in two chambers) robustly reduced IL-2 levels (Fig. 4A, white vs. black bars). Therefore, Treg-mediated IL-2 deprivation of Tconvs requires proximity between Tregs and Tconvs. The same cultures examined for TGF-β production showed the elevated TGF-β levels in the presence of Tregs even when Tregs were separately cultured with Tconvs (Fig. 4B). These results indicate that Tregs regulate cytokine environment in cultures.
Figure 4. Tregs mediate IL-2 deprivation of Tconvs.
Tconvs were stimulated by APCs and anti-CD3 mAb. Tconvs were cultured alone (gray bar), or were co-cultured with Tregs either in the same top chamber (white bar, no transwell) or in the different chambers (black bar, transwell) of a transwell. At 48 hours after cultivation, the levels of IL-2 (A) and TGF-β (B) in the culture supernatant were assessed by ELISA assay. Figures are representative of 3 experiments.
IL-2-deprivation creates prerequisite conditions for TGF-β-mediated suppression in transwell system
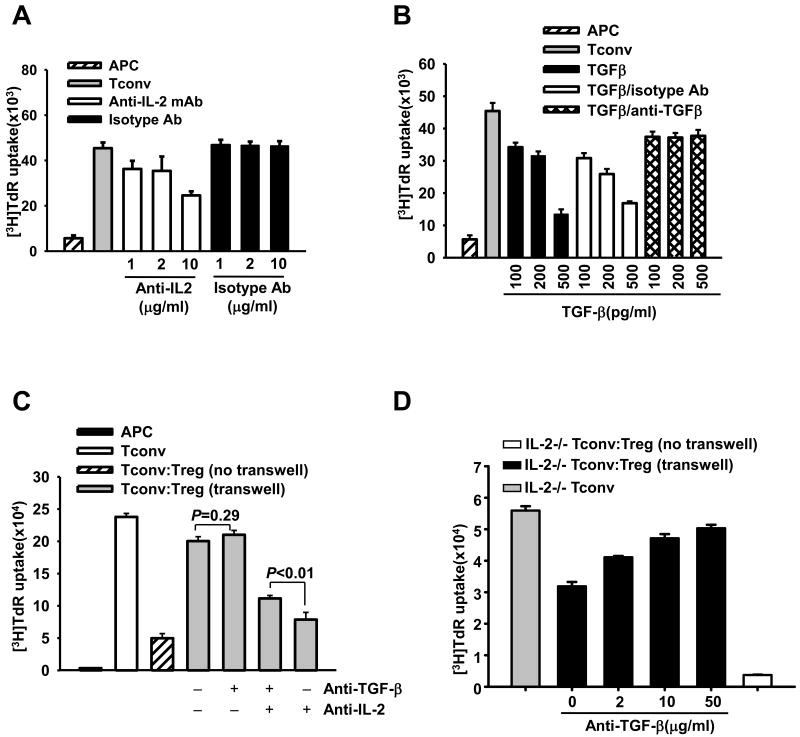
We applied neutralizing mAbs for TGF-β and IL-2 to further investigate the roles of IL-2 deprivation and TGF-β in Treg-mediated suppression. Anti-IL-2 mAb, but not its isotype Ab, decreased Tconv proliferation in concentration-dependent fashion (Fig. 5A). In addition, anti-TGF-β mAb, but not its isotype Ab, abrogated TGF-β-mediated suppression of Tconvs (Fig. 5B). Thus, these neutralizing mAbs effectively prevented the effects of IL-2 or TGF-β on Tconv proliferation.
Figure 5. IL-2 deprivation creates prerequisite condition for TGF-β-mediated suppression by Tregs.
(A-B) Tconvs were stimulated by APCs and anti-CD3 mAb. In A, different amount of anti-IL-2 mAb or isotype control Ab was added into the cultures as indicated. In B, some cultures were added with TGF-β alone, or together with either anti-TGF-β mAb or isotype control Ab. (C-D) Tconvs isolated from wild type B6 (C) or IL-2−/− (D) mice were stimulated by B6 APCs and anti-CD3 mAb. Tconvs were cultured alone (Tconv group), or were co-cultured with Tregs from B6 mice either in the same chamber [Tconv:Treg (no transwell)] or in the different chambers [Tconv:Treg (transwell)] of a transwell. In C, cultures were treated with 10 μg/ml neutralizing anti-TGF-β mAb and/or 10 μg/ml anti-IL-2 mAb as indicated. In D, some cultures were treated with various concentration of anti-TGF-β mAb. Cell proliferation was assessed on day-3 by 3H-thymidine incorporation. Figures are representative of at least 3 independent experiments.
We then demonstrated that separation of Tregs with Tconvs through the membrane in the transwell system almost completely abrogated Treg-mediated suppression (Fig. 5C). Because Treg-mediated IL-2-deprivation could not be reproduced by the two-chamber separation in the transwell system (Fig. 4A), we added neutralizing anti-IL-2 mAb and showed that IL-2-depravation suppressed Tconv proliferation (Fig. 5C; first vs. fourth gray bars). However, this suppression of Tconv proliferation was caused not only by IL-2 deprivation but also by simultaneous TGF-β elevation, because neutralizing anti-TGF-β mAb partially reversed suppression (Fig. 5C; third vs. fourth gray bars; P<0.01). In the absence of anti-IL-2 mAb, neutralizing TGF-β failed to reverse suppression (Fig. 5C; first vs. second gray bars; P=0.29). Thus, IL-2 deprivation creates favorable environment for TGF-β to contribute in Treg-mediated suppression.
To confirm the above findings we have employed IL-2-deficient model wherein IL-2−/− Tconvs served as responders, which in general are less proliferative than wild-type Tconvs (data not shown). In this condition, Tregs suppressed by almost 40% the proliferation of IL-2−/− Tconvs in the transwell system (Fig. 5D; gray and first black bars). Thus, soluble factors contributed to Treg-mediated suppression where Tconvs do not produce IL-2. Since elimination of TGF-β by neutralizing mAb abrogated this suppressive effect in a dose-dependant manner (Fig. 5D; black bars), TGF-β is one of the major soluble factors suppressing IL-2−/− Tconvs in a transwell system. These results support our main conclusion that the IL-2-deprivation conditions create a unique environment for efficient TGF-β-mediated suppression by Tregs.
Discussion
Various cytokines regulate the activation and proliferation of CD4+ T cells. Activated CD4+ T cells themselves produce numerous cytokines to fine-tune the immune responses. Yet, the involvement of cytokines in CD4+Foxp3+ Treg-mediated suppression is undefined and remains controversial [8]. In fact, Tregs produce TGF-β, which is considered a major suppressive cytokine regulating cellular differentiation and proliferation. Tregs also express high-affinity IL-2R to preferentially consume IL-2, a principle T-cell growth factor normally produced by Tconvs during an immune response. Currently, IL-2 consumption and TGF-β have been suggested as suppressive factors of Tregs in some but not in other studies [8]. In this paper, we document that both IL-2 deprivation and TGF-β are non-redundant mechanisms working in concert to suppress Tconvs. We propose that these two factors jointly contribute to produce effective Treg-mediated suppression.
Binding of IL-2 to IL-2R regulates the magnitude and duration of T-cell responses. IL-2 is predominantly produced by Tconvs in response to antigenic stimulation. To deliver an optimal IL-2 signal, Tconvs need express IL-2Rα (CD25; an activation marker) which is undetectable on resting Tconvs but which is readily induced by antigen stimulation. In addition, IL-2 also provides a positive feedback to further increase CD25 expression [19, 20]. Elimination of IL-2 in the proliferation assay moderately reduced the expression of CD25 levels on Tconvs (Fig. 3A). While, a reduced but still significant Tconv proliferation was observed under IL-2 deprivation, an increasing concentration of neutralizing anti-IL-2 mAb failed to further impair Tconv proliferation (Fig. 3B). Therefore, IL-2 independent mechanisms exist to support T-cell growth and expansion. Indeed, while IL-2- and CD25-deficient mice show variable abnormalities in clonal expansion and effector function of Tconvs, both knockout strains are immunocompetent [19, 20].
One of the most important IL-2 function is to maintain Treg homeostasis, as evidenced by the fact that mice deficient of IL-2 or IL-2R display a tenfold reduction in the numbers of peripheral CD4+Foxp3+ Tregs and develop lethal autoimmunities [19]. Although Tregs do not produce IL-2, they represent the only cell subset constitutively expressing high affinity IL-2R containing all three chains, namely IL-2Rα, IL-2Rβ and common γ (γc) chains. Pandiyan et al. have recently indicated that Tregs consume IL-2 causing this cytokine deprivation, which in turn induce apoptosis of activated Tconvs rather than suppress their early activation and proliferation [9]. Indeed, IL-2 deprivation occurred in Treg suppression (Fig. 4A). However, we showed that Tregs suppressed the activation and proliferation of Tconvs (Fig. 1 and 3), and that Treg suppression may not require the induction of Tconv apoptosis as Tregs potently suppressed BCL-2-transgenic Tconvs (Fig. 1E). We further showed that IL-2 deprivation impaired Tconv activation and proliferation (Fig. 3A and 3B). Nevertheless, suppressive effect by IL-2 deprivation is much less potent than the inhibitory effect exhibited by Tregs on Tconvs (Fig. 3B). It is therefore likely that there is another major factor contributing to suppression under IL-2 deprivation conditions.
TGF-β is a potent regulatory cytokine produced by virtually all cell types, including CD4+ cells. It is also confirmed that Tregs produce much more TGF-β in comparison to Tconvs [7, 21]. Consequently, early studies reported that neutralizing TGF-β mAb could reverse Treg-mediated suppression, suggesting that TGF-β is a principle inhibitory factor produced by Tregs [21]. Instead, Piccirillo et al. showed an unimpaired in vitro suppressive effect by TGF-β1−/− Tregs, suggesting that Treg-produced TGF-β might be dispensable [22]. These results were further complicated by work of Fahlen et al. who showed that elimination of TGF-β abrogated the suppressive function even of TGF-β1−/− Tregs, indicating that TGF-β produced by other cells may be essential for Treg-mediated suppression [13]. Recent work by Li et al. used mice with a T-cell specific deletion of the Tgfb1 gene to demonstrate the critical role of Treg-produced TGF-β for Treg function [6]. In support of these data, Pesu et al. confirmed that Tregs with specific deletion of furin had reduced production of TGF-β, resulting in loss of their protective effects in a colitis model [7]. Together, it seems convincing that TGF-β is involved in Treg-mediated suppression, but the exact mechanism of TGF-β in this suppressor mechanism is not fully defined and requires further characterization.
It is also important to know how TGF-β (regardless of its origin) promotes Treg suppression. CD4+ Tconvs from mice that express a dominant negative TGF-β receptor type II cannot respond to TGF-β, and thus escape control by Tregs [13]. We propose that TGF-β contributes to Treg-mediated suppression, at least partially, by targeting early activation steps of Tconv responders (Fig. 3). While TGF-β alone inhibits Tconv proliferation, it exerts a much potent suppressive function when combined with IL-2 deprivation (Fig. 3D and 3E). Hence, IL-2 deprivation and TGF-β have non-redundant functions in suppressing Tconvs.
Limited Treg suppression observed in the transwell system (Fig. 5C) suggests the non-essential role for secreted cytokines in Treg-mediated suppression. However, since cytokines act in a gradient fashion, the proximity between suppressor and responder may be important [8, 9, 23]. Sabatos et al. recently presented evidence that paracrine IL-2 delivery between two activated T cells involves their surface (synapse-based) interactions [23]. In parallel situation, Tregs may not efficiently compete for IL-2 when separated from Tconvs in transwells, as seen by high IL-2 levels in Treg and Tconv transwell cultures (Fig. 4A; black bar). In addition, Tregs also inhibit IL-2 production by Tconvs [8] but they may fail to do so in the transwell system. However, we showed the potent impact of anti-IL-2 neutralizing mAb in the transwell-based Treg suppression, as neutralization of IL-2 inhibited Tconv proliferation. Notably, since the effective inhibition of Tconv proliferation was partially reversed by neutralizing TGF-β, TGF-β produced in transwell cultures contributed to this suppression (Fig. 5C). These latter results have been further strengthened by using Tregs and IL-2-/- Tconvs in the transwell assays. Tregs alone exerted a robust ∼ 40% suppression on the proliferation of IL-2-/-Tconvs in the transwell system, proving the direct evidence for involvement of soluble factors in suppression. The identity of one of them was confirmed by neutralizing of suppression in a dose-dependent fashion by anti-TGF-β mAb (Fig. 5D). These results suggest that interaction of IL-2 deprivation with TGF-β in suppression by Tregs.
In summary, our study coordinated and further clarified the effects of two cytokine factors on Treg-mediated suppression. We show that IL-2 consumption and TGF-β production are non-redundant suppressive mechanisms exhibited by Tregs. Indeed, TGF-β and IL-2 are critically important in Treg development, homeostasis, and suppressor function. Further studies are underway to define the role of IL-2 and TGF-β in T cell biology to unveil the nature of Treg-mediated suppression.
Acknowledgments
This work was supported by NIH grant HL 69723 (S.M.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 5.Khattar M, Chen W, Stepkowski SM. Expanding and converting regulatory T cells: a horizon for immunotherapy. Arch Immunol Ther Exp (Warsz) 2009;57:199–204. doi: 10.1007/s00005-009-0021-1. [DOI] [PubMed] [Google Scholar]
- 6.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–50. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 10.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 11.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–6. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 12.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 13.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, et al. T cells that cannot respond to TGF-beta escape control by CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:737–46. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, et al. TGF-beta1 production by CD4+CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886–95. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 16.Jung YJ, Seoh JY. Feedback loop of immune regulation by CD4+CD25+ Treg. Immunobiology. 2009;214:291–302. doi: 10.1016/j.imbio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–99. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 18.Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- 19.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 20.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–66. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, et al. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–46. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabatos CA, Doh J, Chakravarti S, Friedman RS, Pandurangi PG, Tooley AJ, et al. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29:238–48. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]