Abstract
Women must often choose between a vaginal birth after prior cesarean and elective repeat cesarean delivery. Short-term risks of vaginal birth after cesarean can be potentially catastrophic in the setting of uterine rupture. Although randomized controlled trials comparing these two modes of delivery are lacking, observational studies suggest an increased risk of perinatal mortality and hypoxic-ischemic encephalopathy in infants whose mothers undergo a trial of labor. These rare risks compete with more common, albeit less severe, short-term risks associated with elective repeat cesarean delivery with a particular emphasis on increased respiratory morbidities. Further studies are needed to identify potential strategies to improve perinatal outcomes and help guide physicians and patients in choosing optimal methods of delivery.
INTRODUCTION
In many centers across the United States, women with a prior cesarean delivery are offered a trial of labor. Frequently, maternal risks of a failed trial of labor, with uterine rupture recognized as an uncommon but catastrophic complication, and cesarean delivery with its associated operative morbidity, are presented to the mother. More difficult to ascertain, however, are the comparative risks and benefits of vaginal birth after cesarean delivery (VBAC) and elective repeat cesarean delivery (ERCD) to the fetus and neonate. Evaluating these risks is particularly problematic since no randomized controlled trial has compared outcomes between these two modes of delivery.1 However, given that such a trial is unlikely to occur in the near future, we must guide our discussions with women who plan to undertake either VBAC or ERCD with the current observational data that is available. Here we aim to briefly review the current trends and historical influences of VBAC and ERCD and compare the short-term perinatal outcomes of VBAC and ERCD.
CURRENT TRENDS – Are we moving towards “Once a cesarean, always a cesarean”
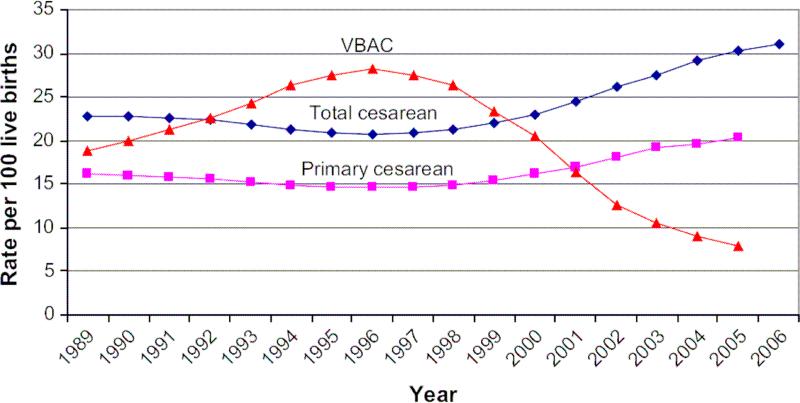
Recent birth data continue to demonstrate an increase in cesarean delivery with a concomitant decrease in vaginal birth after cesarean (VBAC) (Figure 1). Preliminary data for 2007 shows that 31.8% of all US births were by cesarean delivery, marking an increase for the 11th consecutive year and climbing over 50% for the last decade.2-3 Driving part of this increase has been the rise in primary cesarean delivery and decrease in VBAC. Primary cesarean delivery has increased a staggering 39% from 1996 to 2005.4 At the same time, the rate of VBAC has continued to decline with a rate of 7.6% in 2006, an all time low and 9.5% decrease from the prior year.3 Up to 35% of cesareans may be performed because the woman has had previous cesarean delivery.2,5 These trends have continued despite efforts to decrease cesarean delivery (CD) and have been driven, in part, by fears of the perceived risks of VBAC to the mother and her fetus 6 as well as possible litigation pressures.7 Given the current trend of falling VBAC rates and increasing cesarean delivery, women who have a primary cesarean delivery have a greater than 90% chance of having a repeat cesarean delivery.8
Figure 1.
Rates for total cesarean, primary cesarean and vaginal birth after cesarean in the United States from 1989 to 2006. For comparability, 2004 and 2005 primary cesarean and VBAC rates are limited to 37 jurisdictions with unrevised birth certificates, encompassing 69% of 2005 births. (Reprinted with permission.4)
HISTORICAL PERSPECTIVE AND INFLUENCES
In 1980, a National Institutes of Health (NIH) consensus development conference on cesarean delivery concluded that vaginal delivery after a previous low transverse cesarean delivery was a safe and acceptable option.9 This was followed by similar recommendations in 1985 from the National Consensus Conference on Aspects of Cesarean Birth in Canada.10 More recently, the American College of Obstetricians and Gynecologists (ACOG) has published recommendations for a trial of labor in low-risk patients in the appropriate settings.1 The bulletin states that candidates for VBAC include women with one previous low-transverse cesarean delivery, a clinically adequate pelvis and no other uterine scars or previous rupture. In addition, ACOG recommended that physicians be immediately available to monitor labor and perform an emergency cesarean delivery when required. In comparison to the current ACOG guidelines, the early NIH guidelines from 1980 were supported by several studies suggesting that a trial of labor following previous cesarean delivery was safe and not associated with an excess of perinatal mortality or morbidity in comparison to ERCD.11-15 However, as these recommendations took effect and the rate of VBAC rose nationally (Figure 1), concerns arose that the maternal and perinatal risks of VBAC were larger than initially appreciated.6,16 These risks were highlighted in a 1996 study by McMahon and colleagues of 6138 women in Nova Scotia who had undergone a previous cesarean delivery.5 Major maternal complications were almost twice as likely among women whose deliveries were managed with a trial of labor as compared with those who underwent an elective cesarean delivery (odds ratio 1.8, 95% confidence interval (CI) 1.1 to 3.0). Although no maternal deaths were reported, complications included need for hysterectomy, uterine rupture and operative injury.
Coupled with the maternal risks of both VBAC and ERCD are concerns for risks to the fetus and infant. Competing with rare catastrophic risks of perinatal death and HIE in infants whose mothers experience uterine rupture during attempted VBAC, are common short-term morbidities of infants delivered by elective repeat cesarean. Of these short-term risks, neonatal respiratory distress is frequently seen following elective cesarean delivery and has been well-documented in the literature.4,17-22 This is particularly true for infants delivered by cesarean before 39 weeks gestation.23 In the following sections, we further highlight, and where possible, compare the short-term risks of VBAC and ERCD to the fetus and neonate. Several studies we review here have categorized women into two groups: those undergoing an ERCD, either before or after the onset of labor, and those undergoing a trial of labor after prior cesarean delivery (intended VBAC). However, others compare only elective cesarean delivery with vaginal delivery, and caution must be exercised in applying these studies to women undergoing a trial of labor (TOL) or ERCD. Additional categories of interest that we discuss are failed VBAC (with implied emergent cesarean) and operative vaginal deliveries, both of which carry higher risk of neonatal morbidity.
PERINATAL MORTALITY
Perinatal mortality is of obvious concern when considering the risks of VBAC and ERCD, although accurate evaluation is limited by data which involves heterogeneous observational studies. Although we aim to delineate the characteristics of the various studies, we emphasize that methodological limitations may prevent accurate comparisons.
The largest population-based evaluation of perinatal mortality in VBAC and ERCD was performed by Smith and colleagues in 2002.24 The authors compared delivery related perinatal risk of death in infants born by ERCD and those that underwent a trial of labor following previous cesarean delivery (TOL) from a national Scottish linked-record database of 313,238 singleton births with cephalic presentation between 37 weeks and 43 weeks gestation. They found that the rates of delivery related perinatal death were significantly higher in the TOL group than in the ERCD group (rate of perinatal death out of 10,000 births: 12.9 versus 1.1 (95% CIs 7.9 to 19.9 vs 0.0 to 6.1, respectively; p< 0.001). This corresponded to a significantly increased crude odds ratio of 11.6 although the confidence intervals were considerably wide (95% CI 1.6 to 86.7; p = 0.02). The authors also reported that although the overall perinatal death rate was low, there was a marked excess of perinatal deaths due to uterine rupture in the TOL group. A limitation of this study was that the perinatal death rate of planned repeat cesarean delivery, approximately 1.1 per 10,000 women, was lower than other reported studies. Additionally, the authors acknowledged that multivariate statistical analysis becomes more problematic when the number of events is exceedingly small. This is highlighted by the fact that in the above study only one term infant in the ERCD group died and there were no intrapartum deaths.
In a meta-analysis including 39,525 subjects from 11 studies, Mozurkewich and colleagues noted that perinatal mortality was higher in the TOL group when compared to ERCD (odds ratio 1.71, 95% CI 1.28 to 2.28).25 When they excluded perinatal deaths attributable to intrauterine death before labor, lethal anomalies, and prematurity, perinatal mortality remained significantly higher in women undergoing a TOL compared to ERCD (odds ratio 2.05, 95% CI 1.17 to 3.57). Similar results were reported by Landon and colleagues in a 2004 prospective multi-center cohort of 33,699 women at 19 academic medical centers in the United States who either underwent a TOL following previous cesarean delivery or an ERCD.26 They reported increased stillbirth and a trend towards increased neonatal death in women who attempted a TOL versus women who underwent an ERCD. (Neonatal death odds ratio 1.82, 95% CI 0.73 to 4.57; p=0.19); antepartum stillbirth 37-38 weeks odds ratio 2.93, 95% CI 1.27 to 6.75; p=0.008; antepartum stillbirth ≥39 wk odds ratio 2.70, 95% CI 0.99 to 7.38; p=0.07). In this cohort, the neonatal mortality in cases of uterine rupture was 1.8%.
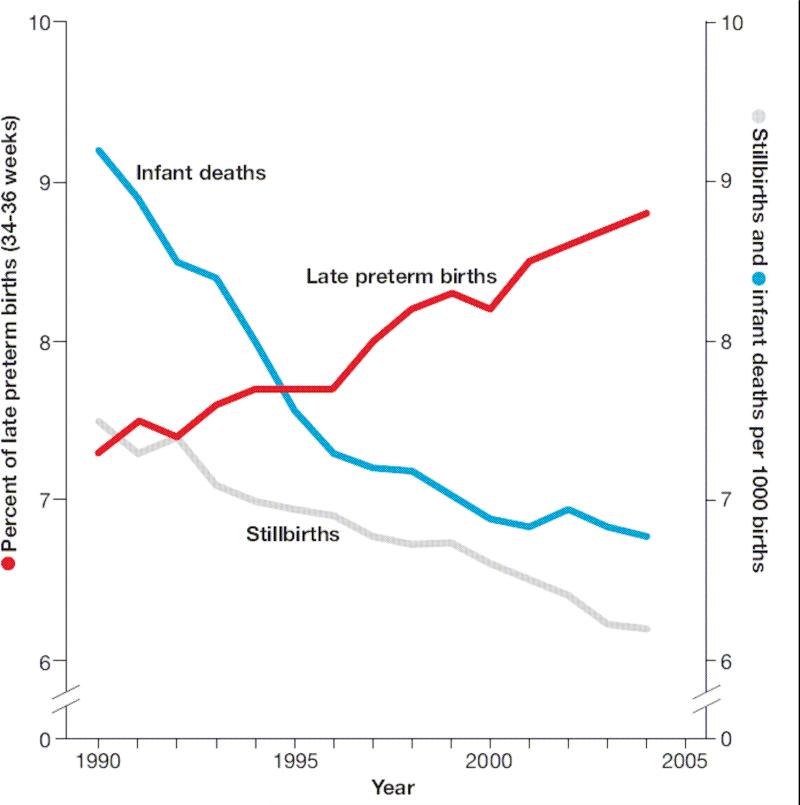
Further complicating the relative risks of VBAC and ERCD on perinatal mortality is the prospect that elective repeat cesarean delivery may have additional benefits in the reduction of stillbirths. Indeed, the significant decrease in perinatal mortality over the last two decades has been, in part, due to the continued decline of stillbirths (Figure 2).27 Hankins and colleagues demonstrate this effect by suggesting that offering elective cesarean delivery (ECD) to all women in the United States at 39 weeks EGA would prevent two fetal deaths per 1000 living fetuses.28 To account for the competing risks of neonatal death after ECD and stillbirth in pregnancies that would have been managed expectantly, Signore and colleagues published results of a decision analysis model to compare the predicted outcomes among 1 million ECD and 1 million planned vaginal deliveries.29 This model predicted higher rates of stillbirth in those women that were managed expectantly (1118 per million planned deliveries) and, as expected, none in those who underwent ECD. In contrast, their model predicted increased neonatal death from ECD (804 per million) as compared to expectant management (378 per million). Their overall predicted perinatal death rate was 804 per million ECD at 39 weeks compared with 1496 per million deliveries managed expectantly. The preponderance of stillbirth in women managed expectantly after 39 weeks is supported by Smith and colleagues finding that the majority of perinatal mortality in women undergoing a trial of labor was seen at ≥ 39 weeks gestation.24
Figure 2.
Trends in late preterm birth, stillbirth, and infant mortality, United States, 1990-2004 Left axis shows trends in stillbirth and infant mortality rates per 1000 total births. Right axis shows trends in late preterm birth (34-36 weeks) per 100 live births. (Reprinted with permission.27)
Although these recent studies suggest an increased risk of perinatal mortality in women undergoing a TOL, possibly through both uterine rupture and increased stillbirth, the overall risks of mortality remain low. In a review of 142,075 attempted TOL from 72 published studies, Chauhan and colleagues determined the perinatal mortality was 0.4 per 1,000 TOL.30 When only hospitals in the United States were evaluated, the perinatal mortality decreased to 0.3 per 1000 TOL. To put this in context, the rate of uterine rupture in the review was 6.8 per 1000 TOL. When considering the competing risks of ERCD and TOL, it is important to remember that the overall risks of perinatal mortality are low and that the majority of women who undergo a TOL deliver successfully.1
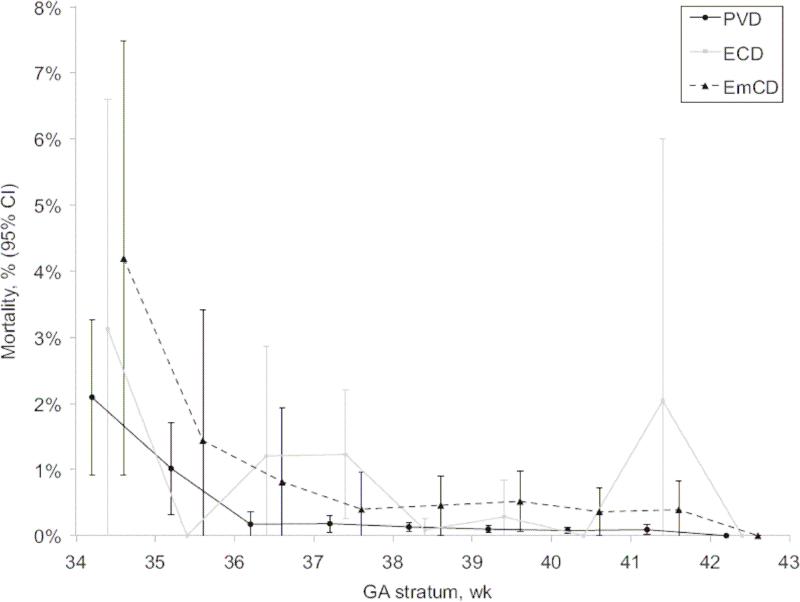
While not as relevant to the more direct comparison we present between ERCD and VBAC, it is helpful to view this information against the backdrop of mortality data from studies comparing elective cesarean delivery (ECD) and planned vaginal delivery (PVD). A recent such study by De Luca and colleagues from Switzerland reported outcomes of 56,549 prospectively recorded late-preterm and term deliveries.31 Their results suggest a significantly higher mortality in ECD when compared to infants delivered by PVD (figure 4). Similarly, a study by MacDorman and colleagues evaluated neonatal mortality for primary ECD and vaginal delivery to low-risk women using U.S live births and infant deaths from 1999 to 2002.8 Their adjusted neonatal mortality rate for ECD with no labor complications or procedures was 2.4 times that for planned vaginal deliveries. The small risk of uterine rupture notwithstanding, one is left to wonder why vaginal birth after a single prior cesarean delivery carries such a higher risk to the fetus than a planned first or second vaginal delivery.
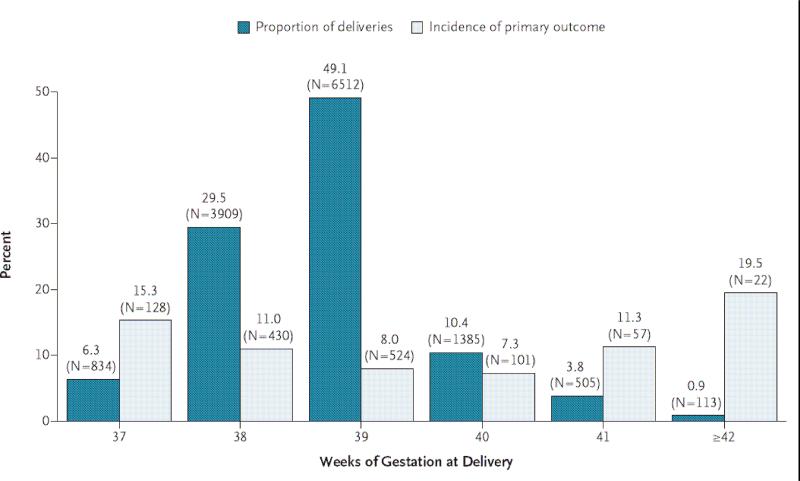
Figure 4.
Timing of ERCD and the incidence of the primary outcome according to the number of completed weeks of gestation. Primary outcome composite of neonatal death and any of several adverse events, including respiratory complications, treated hypoglycemia, newborn sepsis, and admission to the neonatal intensive care unit (ICU). (Reprinted with permission.23)
SHORT-TERM MORBIDITY
Respiratory morbidity
Respiratory morbidity following cesarean delivery is well recognized. Neonates delivered by cesarean, particularly without the onset of labor, have increased risks of transient tachypnea of the newborn (TTN), respiratory distress syndrome (RDS) and persistent pulmonary hypertension of the newborn (PPHN).17 These infants are deprived of the maturational benefits of labor mediated by changes in endogenous steroids and catecholamines as well as the decreased active clearance of fetal lung fluid by amiloride-sensitive sodium channels (ENaC).17,32-33
A study by Hansen and colleagues evaluated a cohort of 34,458 pregnancies and found that neonates delivered by elective caesarean section at 37 to 39 weeks gestation have a two to four times increased risk of respiratory morbidity compared with neonates delivered by intended vaginal delivery (37 weeks: odds ratio 3.9, 95% CI 2.5 to 6.5; 38 weeks: odds ratio 3.0, 95% CI 2.1 to 4.3; 39 weeks: odds ratio 1.9, 95% CI 1.2 to 3.0). Of more concern is that the risk of serious respiratory morbidity (oxygen therapy for more than two days, nasal continuous positive airway pressure or need for mechanical ventilation) was even higher, particularly at earlier gestations (37 weeks: odds ratio 5.0, 95% CI 1.6 to 16; 38 weeks: odds ratio 4.2, 95% CI 1.6 to 11; 39 weeks: odds ratio 2.4, 95% CI 0.5 to 12). The risk of respiratory morbidity in early term infants (37 to 39 weeks) was confirmed by Tita and colleagues in an early 2009 study evaluating a cohort of 13,258 women who underwent an ERCD (Figure 3).23 They noted that incidence of respiratory distress syndrome (RDS) was between 0.8 to 3.7% and significantly higher at the extremes of term gestation following elective repeat cesarean delivery. Similarly, the incidence of transient tachypnea of the newborn was 2.5% to 6.2% and also higher at the extremes of gestation. The authors suggested that iatrogenic respiratory morbidity could be reduced by performing ERCD after 39 weeks gestation.
Figure 3.
Intra-partum and pre-discharge mortality by mode of delivery. ECD, elective cesarean delivery. EmCD, emergency cesarean delivery. PVD, planned vaginal delivery (PVD). (Reprinted with permission.31)
Although a large number of infants with respiratory distress after ERCD will have a short course of TTN without sequelae, a critical number will develop respiratory failure due to RDS and/or PPHN and go on to require ECMO. A study by Keszler and colleagues found that babies delivered by ERCD, when compared to vaginal delivery, were more likely to develop RDS, PPHN or amniotic fluid aspiration that went on to require ECMO (p<0.05).34 Levine and colleagues, in a retrospective review of 29,669 consecutive deliveries in Illinois, found that PPHN was almost 5 times more likely in women who delivered by elective CD compared with vaginal delivery (odds ratio 4.6, 95% CI 1.9 to 11; P<0.001).35 They also noted increased risk of TTN (odds ratio 2.8, 95% CI 2.1 to 3.8; p<0.001) but no-significant difference in RDS (Odds ratio 1.3, 95% CI 0.5 to 3.8; p=0.18) in women delivered by ECD compared to vaginal delivery. In addition to need for ECMO, neonates that develop RDS after ERCD have prolonged hospital stays. In a 1993 study, Parilla and colleagues found that the incidence of respiratory distress syndrome was 0.41% (5 of 1270 births) in women who underwent elective repeat cesarean delivery without labor and these neonates had an average NICU stay of 11.2 days.36
Kamath and colleagues, in a recently published retrospective cohort study, note that neonates born by intended cesarean have an increased incidence of oxygen requirement after admission to the NICU when compared to those with intended VBAC (5.8% vs 2.4%; p< .028).37 Of concern, however, was the finding that neonates born by failed VBAC required the greatest amount of resuscitation and respiratory support. To identify predictors of failed VBAC, the authors performed a subanalysis and found that chorioamnionitis (adjusted odds ratio 5.58 (95% CI 2.08 to 14.99) and induction of labor (adjusted odds ratio 2.53, 95% CI 1.35 to 4.78) were both predictive of failure in women who underwent a TOL. They additionally stratified respiratory outcomes by gestational age and noted that infants at 37 weeks, regardless of their mode of delivery, had the highest rates of oxygen required in the delivery room and admissions to the NICU. These findings again corroborate ACOG recommendations that, in general, women at less than 39 weeks gestation should not be delivered by ERCD.
Asphyxia and Birth Depression
Asphyxia related injury attributable to VBAC typically occurs following uterine rupture. The incidence ranges from 1/2500 to 1/5000 trials of labor.11,13,38 Landon and colleagues demonstrated an increased incidence of uterine rupture during attempted VBAC compared with ERCD (0.7% vs 0%; p<0.001) with an associated increase in the incidence of hypoxic-ischemic encephalopathy (HIE) (0.08% vs 0%; p<0.001).26 However, the absolute risks of perinatal asphyxia were small. In a secondary analysis of the previous study, Spong and colleagues evaluated the risk of uterine rupture in women with a prior cesarean delivery and found the incidence to be 0.74% in the TOL group. Only a fraction of these infants (0.08%) developed hypoxic ischemic encephalopathy..39. In the previously mentioned large review by Chauhan and colleagues, fetal acidosis (umbilical artery pH <7.0) was seen in 2.0/1000 trials of labor in the United States.30 However, they did not evaluate for HIE. Bujold and colleagues reviewed 23 cases of uterine rupture following 2233 trials of labor after a previous low transverse cesarean delivery. 40 Thirty nine percent of neonates had severe metabolic acidosis (pH < 7.0) and one-third of these infants developed HIE. In a study by Hook and colleagues, infants delivered after a TOL were more likely to have Apgar scores ≤6 at 1 minute (10% vs 4%; p<0.002) when compared to ERCD but both groups had no statistically significant difference at 5 minutes (1.2% vs 0.6%; p=NS).41 The timing and impact of birth resuscitation will be discussed later and further data on long-term outcomes, including HIE, will be discussed in another paper in this issue.
Birth Trauma
Birth trauma is a commonly recognized complication of both vaginal and cesarean delivery. Types of birth trauma are specific to the mode of delivery, although it is unclear if either mode confers a protection to all types of birth trauma. Puza and colleagues suggest that overall reductions in birth trauma may be related to increases in cesarean delivery, although this effect appears partially due to improvements in surgical technique.42 Here we focus on fetal lacerations and trauma to the head and neck.
Fetal Lacerations
Common to cesarean deliveries are fetal lacerations, which have been reported as high as 3%.43-44 However, when comparing the risks of fetal laceration due to ERCD, the effect of a failed TOL resulting in CD must be accounted for. In a study by Alexander and colleagues with the NICHD Maternal-Fetal Medicine Units Network, fetal skin lacerations were seen in 0.73% of all cesarean deliveries.45 They were more common in failed VBAC than in ERCD (0.9% vs 0.4%). Additionally, the overall rates of fetal injury were higher in failed VBAC when compared with elective repeat cesarean (1.2% vs 0.5%; p< 0.001).
Head and Neck Injury
In contrast to fetal lacerations, vaginal deliveries are associated with higher rates of injury to the head and neck. Hughes and colleagues evaluated head and neck trauma in a review of 9310 deliveries at a single institution.46 Although they did not specifically evaluate VBAC, they noted that vaginal delivery, particularly those requiring operative assistance, were predisposing risk factors of birth trauma specific to the head and neck. Head and neck trauma, including intracranial hemorrhage and nervous injury to the face and brachial plexus are likely influenced by both need for operative vaginal delivery as well an underlying abnormal course of labor.
Intracranial hemorrhage
Intracranial hemorrhage is an uncommon but serious complication in term infants and one that is feared in operative vaginal birth.47 In a study of 583,340 live-born singleton infants between 2500 and 4000 grams born to nulliparous women from a linked California database, the lowest rate of intracranial hemorrhage was seen in cesarean delivery prior to the start of labor.48 The incidence of intracranial hemorrhage was 9 times higher after a failed attempt to deliver vaginally with forceps or vacuum extraction when compared to cesarean prior to onset of labor (1 of 334 infants compared to 1 of 2750 infants). Although subdural and cerebral hemorrhage were the most common types of intracranial hemorrhage in term infants, intraventricular (IVH) and subarachnoid (SAH) hemorrhage were also seen in both vaginal and cesarean deliveries. When operative vaginal deliveries were excluded, women who underwent an elective cesarean delivery prior to onset of labor and those who underwent a spontaneous vaginal delivery had similar cumulative rates of overall intracranial hemorrhage (5.3 cases per 10,000 in women with spontaneous delivery compared to 4.9 cases per 10,000 in women undergoing cesarean delivery without labor). The authors of the study suggested that a substantial portion of the morbidity may be due to underlying abnormal labor. Supporting this claim were the high rates of intracranial hemorrhage in women who underwent cesarean delivery during labor, particularly in the case of a failed vaginal delivery. Caution must be used when applying these data to women undergoing a TOL given their risks are different than nulliparous women and candidates for a TOL may be more carefully selected and undergo earlier emergent cesarean delivery than nulliparous counterparts.
Facial Nerve Injury and Brachial Plexus Injury
In the previously mentioned trial by Towner and colleagues, facial nerve injury was more common in operative vaginal deliveries, particularly in forceps-assisted deliveries where the incidence was 0.45% and odds ratio 13.6 when compared to spontaneous vaginal delivery (95% CI 10.0 to 18.4).48 However, the incidence of facial-nerve injury in spontaneous vaginal delivery (0.033%) was much less common and relatively similar when compared with cesarean delivery during labor without attempt at vaginal delivery (0.028%) or without labor (0.049%). Similar trends were seen with brachial plexus injury, where the incidence was highest in infants delivered with the use of both vacuum and forceps (incidence 0.46%). Although the incidence of brachial plexus injury was much lower in spontaneous vaginal delivery (0.077%), it remained higher than either cesarean delivery during labor (0.016%) or without labor (0.041%).
Sepsis
Sepsis is a frequent indication for admission to the NICU. In a 1997 study by Hook and colleagues of infants ≥37 weeks born to 1007 mothers with prior cesarean delivery, infants delivered after a TOL had higher rates of both suspected (5% vs 2%; p <0.004) and proven sepsis (1% vs 0.1%; p<0.02).41 This may be explained, in part, by higher risk factors for sepsis in the TOL group. Both maternal fever (8% in TOL vs 0% in ERCD; p <0.0002) and prolonged rupture of membranes greater than 18 hours (6% in TOL vs 1% in ERCD; p<0.0002) were more common in the TOL group. In the same study, neonates who failed VBAC and required cesarean delivery had significantly higher rates of suspected sepsis (12% vs 2%; p<0.0001) which correlated with increased rates of admission to the NICU (7% vs 2%; p<0.007). As discussed previously, characteristics of abnormal labor such as chorioamnionitis, likely play an important role in influencing the success of VBAC. In a more recent study by Kamath and colleagues, the incidence of chorioamnionitis was significantly higher in the failed VBAC group as compared to the successful VBAC and ERCD with labor groups (16.5%, 4.1% and 3.9% respectively; p<0.001).37 The lowest rate of chorioamnionitis, as expected, was seen in the ERCD without labor group (1.7%).
Need for resuscitation
Neonates delivered by ERCD (with or without labor) appear to need more mild to moderate resuscitation efforts (blow-by oxygen and mask CPAP) while those with VBAC, and particularly failed VBAC, are more likely to need bag-mask ventilation (BMV) and intubation in the delivery room. This is highlighted by two previously discussed studies. The first, by Hook and colleagues, noted that infants born by a TOL had higher rates of BMV (7% vs 2%; p<0.001) and intubation for ventilation (2% vs 0.4%; p<0.02) when compared to ERCD.41 In the second study, Kamath and colleagues compared infants delivered by ERCD and VBAC.37 Those that underwent ERCD had higher rates of oxygen use during delivery room resuscitation (41% vs 23%) and mask CPAP (9% vs 3.9%) while those that underwent VBAC were more likely to need BMV (3.3% vs 2.3%) and intubation (2.4% vs 0.6%). The increased need for BMV and intubation in the VBAC group was attributable to women who failed VBAC, where need for BMV and intubation was seen in 9.4% and 5.9%, respectively, of delivered infants. Only one infant in the study required cardiopulmonary resuscitation and/or medications, making comparison of more extensive resuscitative efforts difficult.
Timing of resuscitation
Prompt intervention in cases where fetal distress is detected during a trial of labor is associated with reduced neonatal morbidity. Leung and colleagues evaluated outcomes of 106 cases of uterine rupture at a single institution and found that significant neonatal morbidity occurred when 18 or more minutes elapsed between the onset of prolonged deceleration and delivery.49 However, in the setting of prolonged deceleration preceded by intermittent late deceleration, perinatal asphyxia occurred as early as 10 minutes between the onset of prolonged deceleration and delivery. This highlights the importance of ACOG guidelines to ensure hospitals that perform VBAC are equipped with personnel to rapidly intervene in situations of fetal distress.1,50
NICU admission
Kamath and colleagues compared a cohort of 672 women with a single prior cesarean delivery and 9.3% of infants born by ERCD were admitted to the NICU versus 4.9% of infants delivered by VBAC (p=0.025) (Table 1).37 This is likely explained by the increased need for delivery room oxygen and CPAP in neonates born by intended cesarean as compared with intended VBAC in the study. While the study by Tita and colleagues did not report outcomes after VBAC or NSVD for comparison, their NICU admission rates in ERCD of 4.8 to 14.2%, again particularly high at the extremes of gestation, further confirmed this finding.23 Jain and Dudell compared neonatal outcomes after ERCD and VBAC and found significantly higher NICU admissions in ERCD (Table 2).17 Hook and colleagues also evaluated neonatal admission to a level 3 nursery in women undergoing a TOL compared with ERCD and found no significant differences (incidence of 3% in TOL compared with 2% in ERCD; p=NS).41 However, failed TOL requiring CD was associated with much higher NICU admission (7% compared with 2%; p<0.007). In the case of uterine rupture, NICU admissions are expectedly high. In a study by Landon and colleagues, 40.4% of neonates were admitted to the NICU after the occurrence of uterine rupture during a TOL.26
Table 1.
Adjusted odds ratio of admission to NICU by mode of delivery
| Mode of Delivery | Adjusted Odds Ratio | 95% CI | p |
|---|---|---|---|
| ERCD, no labor | 2.93 | 1.28-6.72 | 0.011 |
| ERCD, with labor | 2.26 | 0.85-6.00 | 0.100 |
| Failed VBAC | 1.91 | 0.66-5.58 | 0.235 |
| Successful VBAC | 1 |
ERCD, elective repeat cesarean delivery; VBAC, vaginal birth after cesarean. CI, confidence interval. Data from Kamath, BD, Todd, JK, Glazner, JE, et al, Neonatal outcomes after elective cesarean delivery. Obstet Gynecol. 113:1231-1238, 2009.
Table 2.
Neonatal morbidity after ERCD compared with VBAC.
| Morbidity | ERCD (n= 15,212) | VBAC (n=8,336) | p |
|---|---|---|---|
| RDS | 318 (2.1%) | 119 (1.4%) | 0.0003 |
| TTN | 630 (4.1%) | 156 (1.9%) | 0.0001 |
| NICU Admission | 1682 (11.1%) | 626 (7.5%) | 0.0001 |
| Oxygen | 673 (4.4%) | 212 (2.5%) | 0.0001 |
| Ventilator | 192 (1.3%) | 63 (0.8%) | 0.0003 |
Data from the The National Institute of Child Health and Human Development (NICHD) created the Maternal Fetal Medicine Units (MFMU) Network registry. ERCD, elective repeat cesarean delivery. VBAC, vaginal birth after cesarean. RDS, respiratory distress syndrome. TTN, transient tachypnea of newborn. NICU, neonatal intensive care unit. Data from Jain, L and Dudell, GG, Respiratory transition in infants delivered by cesarean section. Semin Perinatol. 30:296-304, 2006.
Length of stay and hospital costs
Both length of stay and hospital costs are significantly higher in infants with intended cesarean delivery compared to those with intended VBAC. Villar and colleagues reported that in infants delivered by ERCD, the risk for an extended length of stay in the NICU of 7 days or more was significantly higher (odds ratio 1.9, 95% CI 1.6 to 2.3).20 However, total costs due to failed VBAC are higher than those of both successful VBAC and intended cesarean delivery (Table 3). Despite this, infants born with intended elective repeat cesarean delivery maintain higher median facility, physician and total costs for both the mother and neonate than the intended VBAC group, total costs $8,268 versus $6,647 (p<0.001).
Table 3.
Total neonatal hospital costs and length of stay by mode of delivery
| Hopsital Costs and LOS | Intended CD (n=343) | Intended VBAC (n=244) | Failed VBAC (n=85) | p |
|---|---|---|---|---|
| Total Cost ($USD) | $2,099 | $1,526 | $2,129 | <0.01 |
| LOS (days) | 4.0 | 3.0 | 4.0 | <0.01 |
VBAC, vaginal birth after cesarean. CD, cesarean delivery. LOS, length of stay. P values are listed for comparison of intended cesarean and intended VBAC groups. Data from Kamath, BD, Todd, JK, Glazner, JE, et al, Neonatal outcomes after elective cesarean delivery. Obstet Gynecol. 113:1231-1238, 2009.
Factors influencing failure of VBAC
Approximately twenty to forty percent of women attempting VBAC will fail.1,51 Given that many of the aforementioned short-term risks are increased in neonates after failed VBAC, selection of appropriate candidates for VBAC is essential. As previously discussed, candidates for a TOL are outlined in ACOG recommendations.1 However, predicting failure in women that fall outside these guidelines can be more difficult. In a retrospective multicenter cohort study by Srinivas and colleagues, several factors such as cephalopelvic disproportion and induction of labor were associated with VBAC failure although they were not able to reliably predict VBAC failure.51 Other candidates that may be eligible for a TOL, but whose risks may differ from the ideal candidate, include macrosomic infants and twin gestations.
For macrosomic infants (weight > 4,000 grams), success rates may be <50% if the woman has never had a prior vaginal delivery, particularly if cephalopelvic disproportion is present.52 However, in the same study by Elkousy and colleagues, women who had a successful prior vaginal delivery had more favorable chances of a successful TOL. In a study by Cahill and colleagues, women with twin gestations are less likely to attempt a VBAC (adjusted odds ratio 0.3, 95% CI 0.2 to 0.4) despite no apparent increase in failure (adjusted odds ratio 1.1, 95% CI 0.8 to 1.6).53
Vaginal delivery after two prior cesarean deliveries
The outcome data for the relative risks of VBAC after two previous cesarean deliveries is less clear. In 2009, Tahseen and Griffiths published a systematic review of VBAC after two previous cesarean deliveries (VBAC-2) and noted that although maternal risks of VBAC-2 are greater than after a single previous cesarean delivery (VBAC-1), the neonatal outcomes of asphyxial injury and perinatal death (attributable to mode of delivery) were similar (0.09% in VBAC-2 vs 0.05% in VBAC-1; p=0.35).54 When they compared VBAC-2 to a third repeat elective cesarean (CS3), the rates of asphyxial injury and perinatal death were higher in VBAC-2, but did not reach statistical significance (0.09% with VBAC-2 and 0.01% with CS3; p=0.14). The rates of admission to the NICU in these groups were similar (8.85% in VBAC-2 vs 8.49% in CS3; p=0.57)
Conclusion
Over the last decade, the dramatic rise in ERCD has underscored the importance of offering appropriate candidates a TOL, given most of these women will go on to deliver successfully. Recommendations for VBAC have been based on numerous large observational studies, since no randomized controlled trial has compared the neonatal outcomes of VBAC and ERCD. We are therefore left with current data to guide our risk assessments and counsel potential patients. When presenting the option of a TOL to women, the uncommon but increased risk of perinatal death and birth asphyxia in women who undergo a TOL needs to be evaluated with consideration of the more frequent but less serious neonatal risks associated with ERCD. These include increased respiratory morbidity, hospital costs and length of stay in neonates who undergo ERCD. While the majority of women who undergo a TOL will be successful, women who fail VBAC are at higher risk of birth injury, need for resuscitation and sepsis. Better prediction of those women who will fail VBAC may lead to more careful selection of candidates for a TOL and improved perinatal outcomes. Personnel able to rapidly intervene in cases of failed VBAC requiring emergent cesarean should be available, given the benefit of prompt intervention on neonatal morbidity. Further studies are needed to help clarify the short-term risks of VBAC and better guide physicians and patients who are presented with the choice of an attempted VBAC or ERCD. Future studies will also need to assess interventions with the promise of improving neonatal outcome after ERCD. One such intervention is the use of antenatal steroids prior to an ERCD. While studies such as the one by Stuchfield and colleagues suggest a reduction in NICU admissions and risk of neonatal respiratory distress with a relatively small number needed to treat, practitioners await further proof of the concept as well as long term outcome data before exposing a large number of women and their unborn fetuses to yet another intervention! Meanwhile, since normal spontaneous vaginal birth in women with no prior history of CD continues to have the best overall outcome, efforts continue to bring back the rates of primary CD to a level which would yield the best outcomes for a mother and her baby.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.ACOG Practice Bulletin #54: vaginal birth after previous cesarean. Obstet Gynecol. 2004;104:203–212. [PubMed] [Google Scholar]
- 2.Hamilton BE, MJ, Ventura SJ. Births: Preliminary data for 2007. National vital statistics reports, Web release. National Center for Health Statistics. 2009;57:23. [Google Scholar]
- 3.Martin JA, HB, Sutton PD, Ventura SJ, et al. Births: Final data for 2006. National vital statistics reports. National Center for Health Statistics. 2009;57:102. [Google Scholar]
- 4.Ramachandrappa A, Jain L. Elective cesarean section: its impact on neonatal respiratory outcome. Clin Perinatol. 2008;35:373–393. vii. doi: 10.1016/j.clp.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon MJ, Luther ER, Bowes WA, Jr., et al. Comparison of a trial of labor with an elective second cesarean section. N Engl J Med. 1996;335:689–695. doi: 10.1056/NEJM199609053351001. [DOI] [PubMed] [Google Scholar]
- 6.Landon MB. Vaginal birth after cesarean delivery. Clin Perinatol. 2008;35:491–504. ix–x. doi: 10.1016/j.clp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Yang YT, Mello MM, Subramanian SV, et al. Relationship between malpractice litigation pressure and rates of cesarean section and vaginal birth after cesarean section. Med Care. 2009;47:234–242. doi: 10.1097/MLR.0b013e31818475de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008;35:293–307. v. doi: 10.1016/j.clp.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Cesarean Childbirth. National Institutes of Health. 1981;82-2067:351–374. [Google Scholar]
- 10.Indications for cesarean section: final statement of the panel of the National Consensus Conference on Aspects of Cesarean Birth. CMAJ. 1986;134:1348–1352. [PMC free article] [PubMed] [Google Scholar]
- 11.Flamm BL, Goings JR, Liu Y, et al. Elective repeat cesarean delivery versus trial of labor: a prospective multicenter study. Obstet Gynecol. 1994;83:927–932. doi: 10.1097/00006250-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Flamm BL, Lim OW, Jones C, et al. Vaginal birth after cesarean section: results of a multicenter study. Am J Obstet Gynecol. 1988;158:1079–1084. doi: 10.1016/0002-9378(88)90224-4. [DOI] [PubMed] [Google Scholar]
- 13.Flamm BL, Newman LA, Thomas SJ, et al. Vaginal birth after cesarean delivery: results of a 5-year multicenter collaborative study. Obstet Gynecol. 1990;76:750–754. doi: 10.1097/00006250-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Paul RH, Phelan JP, Yeh SY. Trial of labor in the patient with a prior cesarean birth. Am J Obstet Gynecol. 1985;151:297–304. doi: 10.1016/0002-9378(85)90290-x. [DOI] [PubMed] [Google Scholar]
- 15.Rosen MG, Dickinson JC, Westhoff CL. Vaginal birth after cesarean: a meta-analysis of morbidity and mortality. Obstet Gynecol. 1991;77:465–470. [PubMed] [Google Scholar]
- 16.Sachs BP, Kobelin C, Castro MA, et al. The risks of lowering the cesarean-delivery rate. N Engl J Med. 1999;340:54–57. doi: 10.1056/NEJM199901073400112. [DOI] [PubMed] [Google Scholar]
- 17.Jain L, Dudell GG. Respiratory transition in infants delivered by cesarean section. Semin Perinatol. 2006;30:296–304. doi: 10.1053/j.semperi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Hansen AK, Wisborg K, Uldbjerg N, et al. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008;336:85–87. doi: 10.1136/bmj.39405.539282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen AK, Wisborg K, Uldbjerg N, et al. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstet Gynecol Scand. 2007;86:389–394. doi: 10.1080/00016340601159256. [DOI] [PubMed] [Google Scholar]
- 20.Villar J, Carroli G, Zavaleta N, et al. Maternal and neonatal individual risks and benefits associated with caesarean delivery: multicentre prospective study. BMJ. 2007;335:1025. doi: 10.1136/bmj.39363.706956.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hack M, Fanaroff AA, Klaus MH, et al. Neonatal respiratory distress following elective delivery. A preventable disease? Am J Obstet Gynecol. 1976;126:43–47. doi: 10.1016/0002-9378(76)90462-2. [DOI] [PubMed] [Google Scholar]
- 22.Yee W, Amin H, Wood S. Elective cesarean delivery, neonatal intensive care unit admission, and neonatal respiratory distress. Obstet Gynecol. 2008;111:823–828. doi: 10.1097/AOG.0b013e31816736e7. [DOI] [PubMed] [Google Scholar]
- 23.Tita AT, Landon MB, Spong CY, et al. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360:111–120. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GC, Pell JP, Cameron AD, et al. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287:2684–2690. doi: 10.1001/jama.287.20.2684. [DOI] [PubMed] [Google Scholar]
- 25.Mozurkewich EL, Hutton EK. Elective repeat cesarean delivery versus trial of labor: a meta-analysis of the literature from 1989 to 1999. Am J Obstet Gynecol. 2000;183:1187–1197. doi: 10.1067/mob.2000.108890. [DOI] [PubMed] [Google Scholar]
- 26.Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351:2581–2589. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 27.Ananth CV, Gyamfi C, Jain L. Characterizing risk profiles of infants who are delivered at late preterm gestations: does it matter? Am J Obstet Gynecol. 2008;199:329–331. doi: 10.1016/j.ajog.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Hankins GD, Clark SM, Munn MB. Cesarean section on request at 39 weeks: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. Semin Perinatol. 2006;30:276–287. doi: 10.1053/j.semperi.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Signore C, Klebanoff M. Neonatal morbidity and mortality after elective cesarean delivery. Clin Perinatol. 2008;35:361–371. vi. doi: 10.1016/j.clp.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan SP, Martin JN, Jr., Henrichs CE, et al. Maternal and perinatal complications with uterine rupture in 142,075 patients who attempted vaginal birth after cesarean delivery: A review of the literature. Am J Obstet Gynecol. 2003;189:408–417. doi: 10.1067/s0002-9378(03)00675-6. [DOI] [PubMed] [Google Scholar]
- 31.De Luca R, Boulvain M, Irion O, et al. Incidence of early neonatal mortality and morbidity after late-preterm and term cesarean delivery. Pediatrics. 2009;123:e1064–1071. doi: 10.1542/peds.2008-2407. [DOI] [PubMed] [Google Scholar]
- 32.Jain L. Alveolar fluid clearance in developing lungs and its role in neonatal transition. Clin Perinatol. 1999;26:585–599. [PubMed] [Google Scholar]
- 33.Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006;30:34–43. doi: 10.1053/j.semperi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Keszler M, Carbone MT, Cox C, et al. Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extracorporeal membrane oxygenation. Pediatrics. 1992;89:670–672. [PubMed] [Google Scholar]
- 35.Levine EM, Ghai V, Barton JJ, et al. Mode of delivery and risk of respiratory diseases in newborns. Obstet Gynecol. 2001;97:439–442. doi: 10.1016/s0029-7844(00)01150-9. [DOI] [PubMed] [Google Scholar]
- 36.Parilla BV, Dooley SL, Jansen RD, et al. Iatrogenic respiratory distress syndrome following elective repeat cesarean delivery. Obstet Gynecol. 1993;81:392–395. [PubMed] [Google Scholar]
- 37.Kamath BD, Todd JK, Glazner JE, et al. Neonatal outcomes after elective cesarean delivery. Obstet Gynecol. 2009;113:1231–1238. doi: 10.1097/AOG.0b013e3181a66d57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller DA, Diaz FG, Paul RH. Vaginal birth after cesarean: a 10-year experience. Obstet Gynecol. 1994;84:255–258. [PubMed] [Google Scholar]
- 39.Spong CY, Landon MB, Gilbert S, et al. Risk of uterine rupture and adverse perinatal outcome at term after cesarean delivery. Obstet Gynecol. 2007;110:801–807. doi: 10.1097/01.AOG.0000284622.71222.b2. [DOI] [PubMed] [Google Scholar]
- 40.Bujold E, Gauthier RJ. Neonatal morbidity associated with uterine rupture: what are the risk factors? Am J Obstet Gynecol. 2002;186:311–314. doi: 10.1067/mob.2002.119923. [DOI] [PubMed] [Google Scholar]
- 41.Hook B, Kiwi R, Amini SB, et al. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics. 1997;100:348–353. doi: 10.1542/peds.100.3.348. [DOI] [PubMed] [Google Scholar]
- 42.Puza S, Roth N, Macones GA, et al. Does cesarean section decrease the incidence of major birth trauma? J Perinatol. 1998;18:9–12. [PubMed] [Google Scholar]
- 43.Dessole S, Cosmi E, Balata A, et al. Accidental fetal lacerations during cesarean delivery: Experience in an Italian level III university hospital. American Journal of Obstetrics and Gynecology. 2004;191:1673–1677. doi: 10.1016/j.ajog.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Wiener JJ, Westwood J. Fetal lacerations at caesarean section. J Obstet Gynaecol. 2002;22:23–24. doi: 10.1080/01443610120101655. [DOI] [PubMed] [Google Scholar]
- 45.Alexander JM, Leveno KJ, Hauth J, et al. Fetal injury associated with cesarean delivery. Obstet Gynecol. 2006;108:885–890. doi: 10.1097/01.AOG.0000237116.72011.f3. [DOI] [PubMed] [Google Scholar]
- 46.Hughes CA, Harley EH, Milmoe G, et al. Birth trauma in the head and neck. Arch Otolaryngol Head Neck Surg. 1999;125:193–199. doi: 10.1001/archotol.125.2.193. [DOI] [PubMed] [Google Scholar]
- 47.Harer WB., Jr. Vaginal birth after cesarean delivery: current status. JAMA. 2002;287:2627–2630. doi: 10.1001/jama.287.20.2627. [DOI] [PubMed] [Google Scholar]
- 48.Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709–1714. doi: 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- 49.Leung AS, Leung EK, Paul RH. Uterine rupture after previous cesarean delivery: maternal and fetal consequences. Am J Obstet Gynecol. 1993;169:945–950. doi: 10.1016/0002-9378(93)90032-e. [DOI] [PubMed] [Google Scholar]
- 50.Flamm BL. Vaginal birth after caesarean (VBAC). Best Pract Res Clin Obstet Gynaecol. 2001;15:81–92. doi: 10.1053/beog.2000.0150. [DOI] [PubMed] [Google Scholar]
- 51.Srinivas SK, Stamilio DM, Stevens EJ, et al. Predicting failure of a vaginal birth attempt after cesarean delivery. Obstet Gynecol. 2007;109:800–805. doi: 10.1097/01.AOG.0000259313.46842.71. [DOI] [PubMed] [Google Scholar]
- 52.Elkousy MA, Sammel M, Stevens E, et al. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188:824–830. doi: 10.1067/mob.2003.186. [DOI] [PubMed] [Google Scholar]
- 53.Cahill A, Stamilio DM, Pare E, et al. Vaginal birth after cesarean (VBAC) attempt in twin pregnancies: is it safe? Am J Obstet Gynecol. 2005;193:1050–1055. doi: 10.1016/j.ajog.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 54.Tahseen S, Griffiths M. Vaginal birth after two caesarean sections (VBAC-2)-a systematic review with meta-analysis of success rate and adverse outcomes of VBAC-2 versus VBAC-1 and repeat (third) caesarean sections. BJOG. 2009 doi: 10.1111/j.1471-0528.2009.02351.x. [DOI] [PubMed] [Google Scholar]