Abstract
Excess activation of glutamatergic neurotransmission in the cerebral cortex following ethanol withdrawal is considered to contribute to significant behavioural disturbances, and to alcohol craving. Astrocytes may play a role in these manifestations because astrocytes are essential in the regulation of released glutamate and its conversion to glutamine through the enzyme glutamine synthetase (GS). However, it is unclear if withdrawal from free-choice ethanol drinking causes changes in the numbers of astrocytes expressing GS or the cytoskeletal protein of astrocytes glial fibrillary acidic protein (GFAP). Alcohol-preferring (P) rats exposed to free-choice ethanol drinking were either maintained without forced interruption of ethanol drinking, subjected to a 3-day withdrawal period at the end of 2 months, or subjected to three 3-day withdrawal periods along 6 months. At 2 months, P rats were also compared with alcohol-naïve alcohol non-preferring rats (NP) rats. Packing density of GS and GFAP-immunoreactive (IR) astrocytes was measured in sections from the prelimbic cortex (PLC) using the optical disector probe. An alcohol deprivation effect was observed in P rats with withdrawals during a 6-month ethanol drinking period. Ethanol withdrawal significantly increased the packing density of GS- and GFAP-IR astrocytes in the PLC of P rats as compared with P rats with continuous access to ethanol. In addition, there was a positive correlation between the pre-withdrawal ethanol consumption and the packing density of GS-IR astrocytes. The present results suggest the involvement of astrocytes in the regulation of the glutamatergic activation associated with withdrawal from free-choice ethanol consumption and point to differential adaptations of GS and GFAP to prolonged alcohol drinking in the PLC of P rats.
INTRODUCTION
Withdrawal from prolonged intake of alcoholic beverages results in increased excitation of cortical and subcortical neurons (De Witte et al., 2003). This excitation has been proposed to contribute importantly to the behavioural and motor pathology that accompanies withdrawal from ethanol drinking. Various lines of evidence support that this increased excitation is mediated by excess glutamatergic activation brought about by upregulation of glutamate receptors induced during the period of prolonged ethanol consumption (Tsai, 1998). Another effect related to withdrawal from alcohol drinking is the alcohol deprivation effect (ADE), a temporary increase (as compared with the pre-withdrawal period) in alcohol intake after a period of alcohol deprivation (Rodd et al., 2004). Some lines of evidence also implicate altered glutamatergic neurotransmission in the manifestations of ADE (Backstrom et al., 2004; Schroeder et al., 2005; Vengeliene et al., 2005).
The effects of ethanol intake on glutamatergic neurotransmission are not limited to changes in the abundance of glutamate receptors. Alcohol also causes changes in presynaptic terminals that eventually result in increased levels of glutamate release during withdrawal from alcohol (Dahchour and De Witte, 1999; Rossetti et al., 1999). The levels of released glutamate in the extracellular compartment are tightly regulated by astrocytes (Anderson and Swanson, 2000; Gadea and Lopez-Colome, 2001). In order for glutamate to be reused by neurons, astrocytes rapidly convert glutamate into glutamine by the action of glutamine synthetase (GS) (Norenberg and Martinez-Hernandez, 1979; Hallermayer and Hamprecht, 1984; Hertz and Zielke, 2004). Glutamine is then transferred to neurons, where it is converted into glutamate once again. Astrocytes expressing GS are thus a crucial element in ensuring the rapid recycling of taken-up glutamate for its use in synaptic transmission, and are likely to contribute critically to the regulation of glutamatergic excitation during and after ethanol deprivation.
In culture, ethanol exposure produces a reduction in GS activity (Davies and Vernadakis, 1984). Ethanol by itself inhibits the proliferation of astrocytes in culture (Kane et al., 1996). In addition, the extracellular levels of glutamate affect the activity and the expression of astrocytic GS (Tiffany-Castiglioni et al., 1989; Mearow et al., 1990; Rauen and Wiessner, 2000; Shen et al., 2004). Since astrocytes are the origin of GS involved in the conversion of glutamate to glutamine, changes in the packing density of GS containing astrocytes may influence the regulation of glutamate conversion and eventually the levels of excitation in the surrounding neuropil. The packing density of astrocytes in cortex might also influence the ability to limit the effects of excess activation of glutamate receptors. In adult alcohol-preferring (P) rats, a line of rats from Indiana University that become dependent on alcohol even when offered a free-choice between water and aqueous ethanol solutions (McBride and Li, 1998; Rodd et al., 2004), the packing density of glial-fibrillary-acidic-protein immunoreactive (GFAP-IR)-astrocytes in the prelimbic cortex is significantly lower than in alcohol non-preferring (NP) or in Wistar rats (Miguel-Hidalgo, 2005) and it is possible that this is paralleled by a lower packing density of GS-IR astrocytes. In addition, given the involvement of GS-IR astrocytes in the regulation of the perisynaptic glutamatergic cycle, withdrawal may affect the density of GS-IR astrocytes in the prelimbic cortex of P rats, a cortical area with strong connections to subcortical nuclei heavily involved in the regulation of responses to reward stimuli and substances of abuse (Pinto and Sesack, 2000; Killcross and Coutureau, 2003; Zavala et al., 2003; Kalivas et al., 2005).
The present study aimed to ascertain whether withdrawal from free-choice alcohol drinking in P rats is associated with changes in the packing density of GS and GFAP-immunoreactive astrocytes and whether those changes correlated with the amount of alcohol consumed before the withdrawal period. We also sought to ascertain whether the packing density of GS-immunoreactive astrocytes was lower in P rats than in alcohol-naïve NP rats.
MATERIALS AND METHODS
Animals
Inbred alcohol-preferring (P) rats in the 40th and 41st generations and inbred alcohol non-preferring (NP) rats in the 44th generation from the Alcohol Research Center at Indiana University were used throughout the study and were fed with standard rat chow (Rodent Diet 8640, Harlan, Indianapolis, IN) ad libitum. Upon arrival, all animals were housed in cages with three animals per cage and drank only water for 1 week. Later, animals were singly caged and continued drinking liquid ad libitum (24-h continuous availability). P rats randomly tagged for alcohol drinking spent 4 days with only one bottle of 10% ethanol and then they were given two bottles according to the protocols below. Rats were separated into several groups and were subjected to one of the following drinking protocols: Groups 1 and 2: P rats offered two bottles per cage containing only water for 2 (n = 15, Group 1) or 6 (n = 8, Group 2 months) months; Groups 3 and 4: animals with one bottle with water and another bottle with 10% (vol:vol) ethanol diluted in water for 2 (n = 10, Group 3) or 6 (n = 5, Group 4) months of continuous access; Group 5: animals with one water bottle and a 10% ethanol bottle for 2 months that were withdrawn from ethanol (that is, they had two bottles with water) the last 3 days before sacrifice (n = 9); Group 6: animals with one water bottle and a 10% ethanol bottle for 6 months that were withdrawn from ethanol during 3 days at 60 and 150 days after single-caging and for 3 days before sacrifice (n = 8); Group 7: NP rats, which had two bottles, both with water, for 2 months (n = 6). Two times per week, the volume of liquid consumed from each bottle was measured. At the end of each measuring session bottles were refilled and their content measured again. Measurements of liquid intake were always made between 9 and 12 am and the last measurement was made on the morning of the day the animals were sacrificed and perfused. In those groups with withdrawal periods, measurements of liquid intake were always taken in the day before the start of withdrawal.
To rule out a bias towards drinking from a particular bottle simply because of its position, the drinking spouts and the position of the bottles were changed during the refilling sessions. At the end of the drinking schedules rats were perfused through the heart with 50 ml of phosphate buffered saline solution and 250 ml of 4% paraformaldehyde. Dissected brains were postfixed overnight and submerged sequentially into 10 and 30% sucrose. Coronal sections at a thickness of 30 µm that included the rat prelimbic cortex were obtained with a vibratome, and stored in an antifreeze solution at −20°C. From each brain three coronal sections through the PFC containing the prelimbic region and separated by ~1 mm (approximately levels 6, 8 and 10 of the atlas of Paxinos and Watson (Paxinos and Watson, 1998) were preincubated in a solution of 0.1 M Tris–HCl buffer, pH 7.4, containing 1% BSA, and 200 µg/ml Triton X (incubation solution, used throughout all incubations) and then stored overnight in the same solution containing a monoclonal antibody to GFAP (Chemicon, Temecula, CA, USA) at 1:1000 dilution. Three other adjacent, equally spaced sections were incubated overnight at 4°C with a monoclonal antibody to GS (Chemicon, Temecula, CA, USA) also at 1:1000. After incubation in the first antibody sections were washed for 30 min in Tris–HCl and incubated for 90 min at room temperature with a biotinylated anti-mouse secondary antibody (1:200, Vector Laboratories, Burlingame, CA). After another 30-min wash in Tris–HCl, sections were incubated for 60 min at room temperature with the ABC complex (Vector Laboratories, Burlingame, CA). Immunoreactivity was visualized in a solution with 0.05% 3′-3′-diaminobenzidine, 0.6% and nickel ammonium sulphate, and 0.01% H2O2 in 0.1M Tris–HCl at pH 7.6. Possible variability of immunostaining due to varying experimental conditions between experiments was minimized by immunostaining equal numbers of samples from each of the four 2-month treatment groups in every single experiment while using aliquots of the same washing, antibody, and chromogenic reaction solutions. The sections from the animals with 6 months of treatment were also processed simultaneously although at a different time from the 2-month survival experiments. In the immunostained PFC sections, the prelimbic region (PLC) was delineated aided by an adjacent section stained with Cresyl Violet. Identification of PLC was performed according to the cytoarchitectonic criteria described by Uylings et al. (Van Eden and Uylings, 1985a, b; Uylings and Van Eden, 1990; Uylings et al., 2003) for the rat brain. Omission of the primary antibody, the biotinylated secondary antibody or the ABC complex resulted in the absence of specific immunoreactivity either in the neuropil, in cell bodies or in processes.
Packing density
Packing density of GS and GFAP-immunoreactive astrocytes was measured in three sections per immunostaining procedure under a microscope with a 40× objective with 1.3 numerical aperture. The counting procedure involved a motorized stage and the use of the Stereo Investigator software (Microbrightfield, Inc.) according to the method described in Miguel-Hidalgo (2005). The technician counting cells worked with coded slides and was blind both to the group and the actual animal identification. To obtain an estimate of the packing density of GS-IR or GFAP-IR astrocytes in each of the three sections per brain, the total number of GS-IR or GFAP-IR astrocytes counted was divided into the total volume of the counting frames sampled. Values of packing density in the three sections were averaged and the averages taken as values of the packing density variable used for statistical comparisons.
Volume of the frontal lobe
In the rats from the four groups with 2-month treatment the outline of the left frontal lobe was drawn in Nissl-stained coronal sections 360 µm apart (8–10 sections per brain) from the rostral tip of the PFC to the beginning of the corpus callosum, and the area within each outline was measured using the area measurement capabilities of the Stereo Investigator Software (Microbright-field, Inc.). In each brain, the sum of all areas from a brain was multiplied by the distance in between the sections measured (360 µm) to obtain an estimate of the volume of the frontal lobe.
Statistics
Differences in the packing density of immunoreactive astrocytes and in the volume of the frontal cortex were analysed using Analysis of Variance (ANOVA). If ANOVA showed a significant effect of the group on any of the dependent variables the Tukey–Kramer test was used for post hoc multiple comparisons. For comparisons of alcohol intake before and after the withdrawal, the paired Student’s t-test was applied. Correlation between the packing density of immunoreactive astrocytes and the ethanol consumption in the days preceding withdrawal or sacrifice in Groups 1 and 3 were performed with Pearson correlation analysis.
RESULTS
Qualitative observations
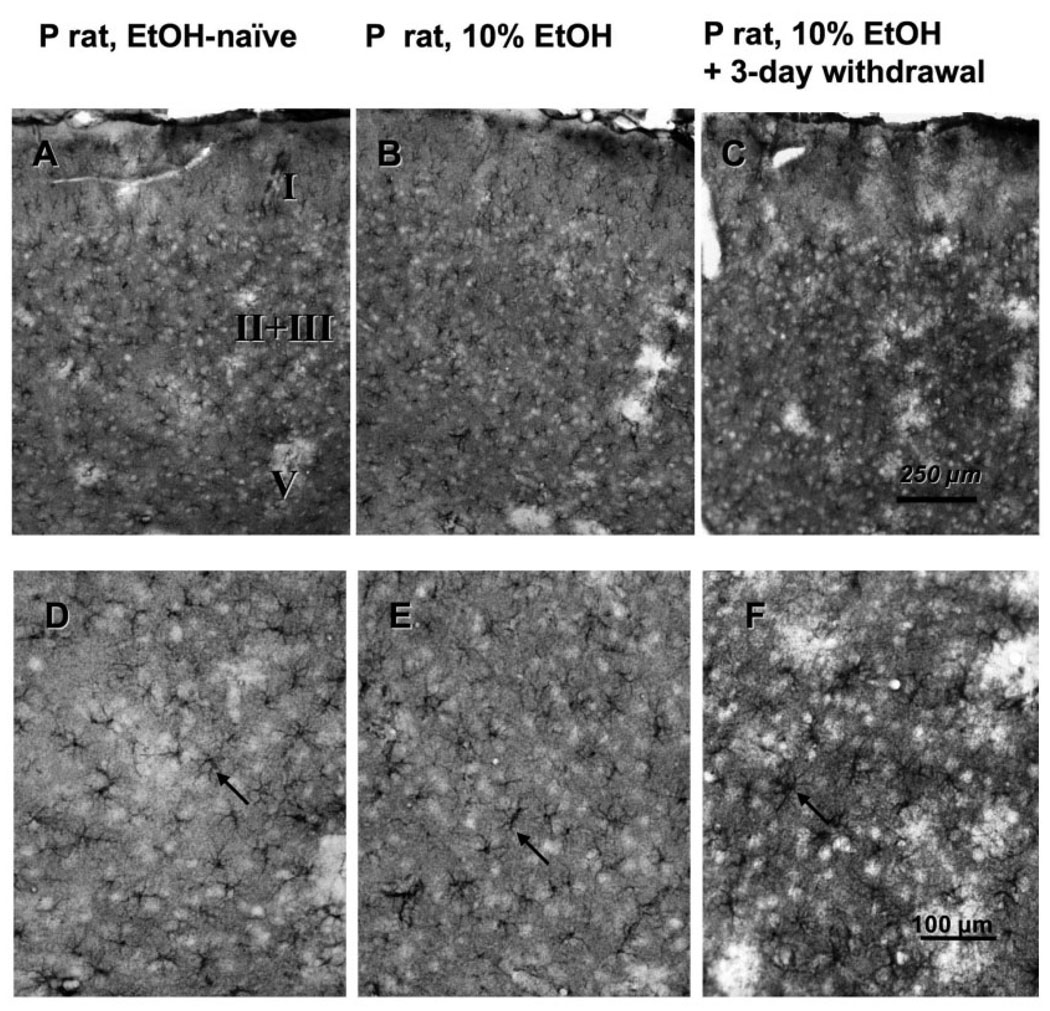
The immunostaining for both GS and GFAP allowed distinguishing individual astrocytes. GFAP immunostaining was restricted to solidly immunoreactive processes and virtually without diffuse staining in the neuropil. This immunostaining pattern allowed identification of astrocytes by the several positive processes clearly diverging from a common centre, although the cell bodies were not fully filled with GFAP labelling. GS immunoreactivity was considerably more widespread and was more diffusely distributed in the neuropil than GFAP immunoreactivity. In addition, the cell bodies of astrocytes were filled with GS immunoreactivity, the initial segments of their processes being easily identifiable (Fig. 1). Due to a more conspicuous GS staining of the cell bodies of astrocytes, more individual astrocytes were routinely identified and counted in GS-immunostained sections than in GFAP-immunostained sections.
Fig. 1.
Micrographs of the glutamine synthetase (GS) immunoreactivity in the prelimbic cortex of alcohol-naïve P rats (A and D), alcohol-drinking P rats (B and E) (2-month free-choice drinking) and alcohol-drinking P rats (C and F) with 2-month free-choice alcohol drinking and a 3-day period of withdrawal. The top three panels show the overall pattern of immunoreactivity across the cortical layers and the bottom panels allow identifying individual GS-immunoreactive astrocytes (arrows).
Packing density of GS and GFAP-immunoreactive astrocytes
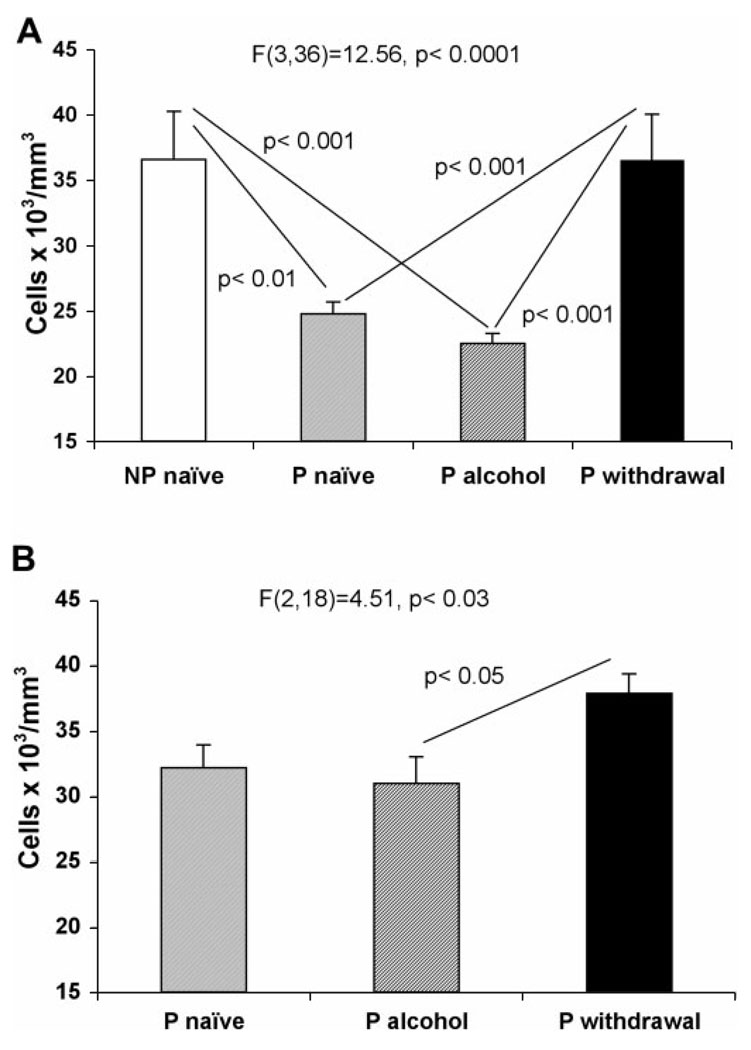
The packing density of GS-immunoreactive astrocytes in the prelimbic cortex was significantly different among the four experimental groups with 2 months treatments F(3,36) = 12.56, P < 0.0001 (Fig. 2A). Post hoc comparisons revealed that packing density was significantly higher in ethanol-withdrawn P (WP) (Group 5) rats than in naïve (Group 1) (q = 6.11, P < 0.001) or in non-withdrawn ethanol drinking (EP) (group 3) (q = 6.68, P < 0.001) P rats. In NP rats (Group 7) the density of GS-IR astrocytes was not different from that in WP rats although it was significantly higher than in naïve P rats (q = 5.34, P < 0.01) and in EP rats (q = 5.95, P < 0.001).
Fig. 2.
Graphs of the average packing density of GS-immunoreactive astrocytes in the prelimbic cortex of NP rats (Group 7, n = 6), alcohol naïve P rats (Group 1, n = 15; Group 2, n = 8), alcohol-drinking P rats (Group 3, n = 10 and Group 4, n = 5) and alcohol-drinking P rats with withdrawals after 2-month (Group 5, n = 9) or 6-month treatments (Group 6, n = 8). In (A) the values correspond to 2-month treatments in NP, P naïve, P drinking and P withdrawn from ethanol (Groups 7, 1, 3, and 5, respectively). In (B) the values correspond to 6-month treatments in P naïve, P ethanol-drinking rats and P rats withdrawn from ethanol (Groups 2, 4, and 6, respectively). Experiments with 6-month exposure only included P rats. Whiskers represent the standard error of the mean.
The density of GS-immunoreactive astrocytes was also significantly different among the groups of animals with 6 months treatments F(2,18) = 4.51, P < 0.03) (Fig. 2B). Again, rats with three withdrawal periods (Group 6) had a higher density of GS or GFAP-immunoreactive astrocytes than 6-month EP rats (Group 4) (q = 4.05, P < 0.05). The density in 6-month WP rats (Group 6) was also higher than in naïve P rats (Group 2), but the difference was not significant (q = 2.93). The density in 6-month naïve (Group 2) and 6-month EP (Group 4) rats appeared increased as compared with the density in the groups of rats with 2-month treatments.
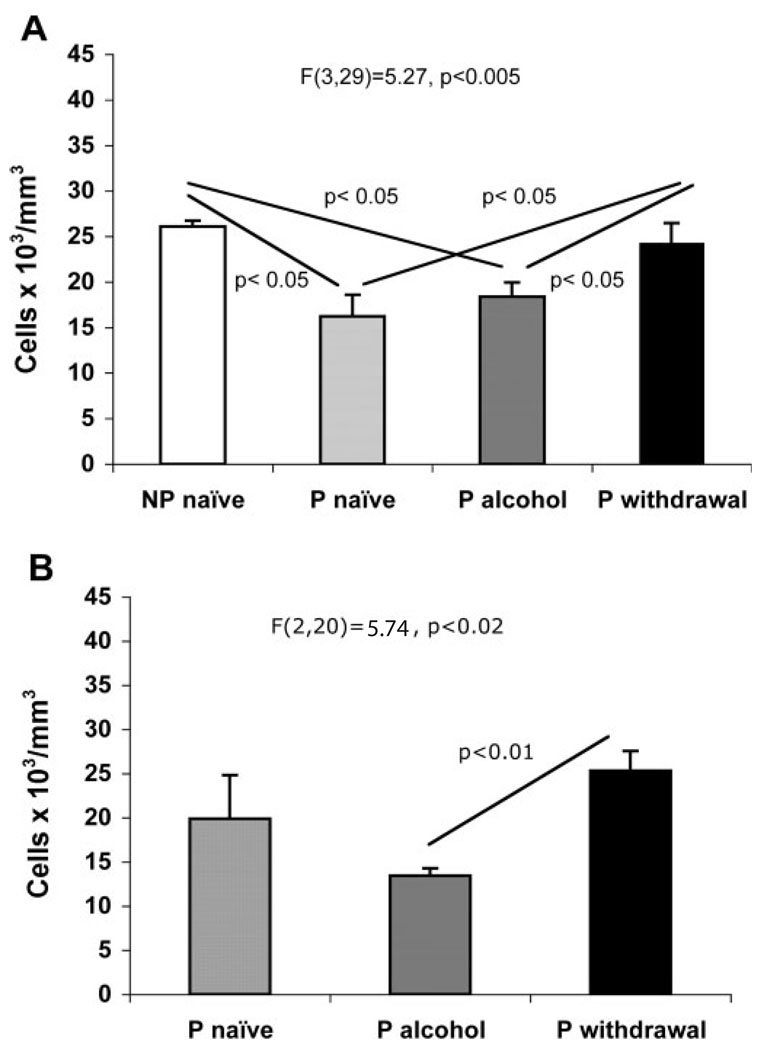
The results concerning the density of GFAP-immunoreactive astrocytes were similar to those of GS-immunoreactive astrocytes. Differences between groups at 2 and 6 months were significant [F(3,29) = 5.27, P < 0.005, 2 months; F(2,20) = 5.74, P < 0.02, 6 months]. At 2 months (Fig. 3A), the density of GFAP-immunoreactive astrocytes was significantly higher in animals with withdrawal periods than in naïve or EP rats but was not significantly different from NP rats. At 6 months (Fig. 3B), the density of GFAP-IR astrocytes was highest in the group with three withdrawals, and in this group that density was significantly higher than in the group with continuous access to 10% ethanol (q = 4.781, P < 0.01).
Fig. 3.
Graphs of the average packing density of GFAP-immunoreactive astrocytes in the prelimbic cortex of NP rats, alcohol naïve P rats, alcohol-drinking P rats, and alcohol drinking P rats with withdrawal after 2-month (A) or 6-month treatments (B). The experiment with 6-month exposure only included P rats. The identification of groups is as in Fig. 2. Whiskers represent the standard error of the mean.
At 2 months of survival the volume of the prefrontal cortex comprised between the rostral most tip of the cortex and the beginning of the corpus callosum was not different among NP rats, alcohol-naïve P rats, alcohol-drinking P rats without withdrawal, and alcohol-drinking P rats with a terminal 3-day withdrawal period.
Alcohol deprivation effect on ethanol intake in rats with three withdrawal periods
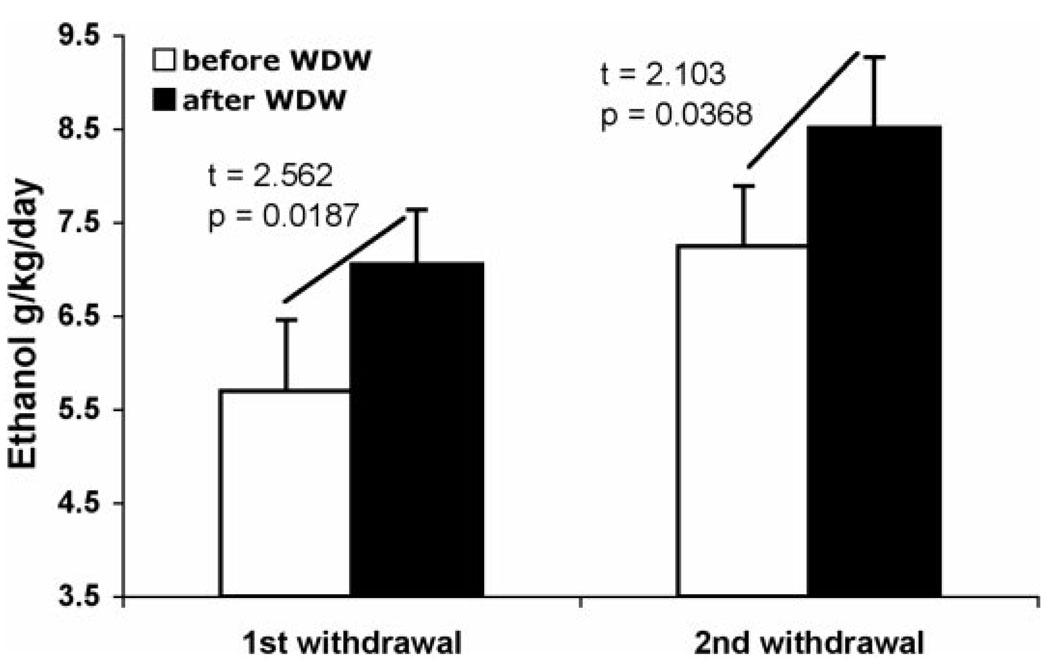
Analysis of the drinking patterns immediately before and after the two first 3-day withdrawal periods in rats exposed to alcohol drinking for 6 months revealed that, in the week after the withdrawal, rats drink more alcohol than in the week immediately preceding the withdrawal (Fig. 4), both in the first (t = 2.562, P = 0.019) and the second withdrawal (t = 2.103, P = 0.037). At those days corresponding to the days of withdrawal of WP rats (Group 7), EP rats (continuous 6-month ethanol drinking, Group 6) did not show significant differences in the levels of ethanol intake.
Fig. 4.
Bar graphs illustrating an alcohol deprivation effect on ethanol intake after 3 days of withdrawal at two different times during the period of 6-months of alcohol drinking in P rats. ‘Before’ denotes during the week immediately preceding the withdrawal period. ‘After’ denotes during the week following the withdrawal period. WDW = withdrawal.
Correlation of pre-withdrawal ethanol intake and density of immunoreactive astrocytes
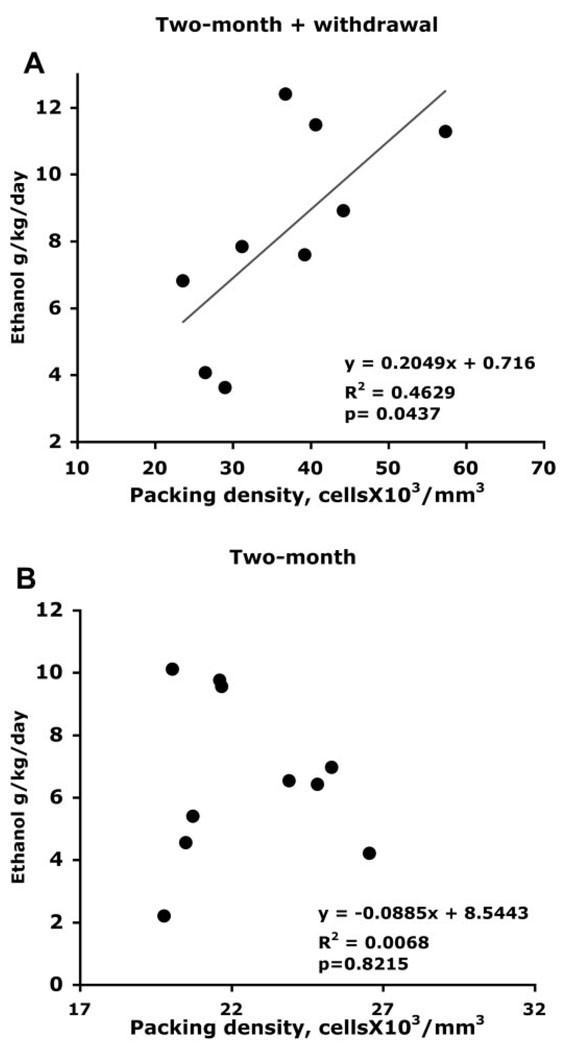
In order to explore whether the levels of alcohol drinking before withdrawal may predict the packing density of GS or GFAP-immunoreactive astrocytes measured after withdrawal, we examined the correlation between the average ethanol consumption (expressed in daily grams of ethanol per kilogram of body weight) in the 4 days preceding the initiation of withdrawal and the packing density of GS-IR or GFAP-IR astrocytes after the last withdrawal (3 days duration before sacrifice) in animals with 2-month ethanol exposure. We found a significant positive correlation (R2 = 0.469, P = 0.044) between the packing density of GS-IR astrocytes and the pre-withdrawal level of alcohol consumption (Fig. 5A). However, there was no correlation between the density of GFAP-IR astrocytes and the pre-withdrawal ethanol consumption (R2 = 0.039, P = 0.446). In animals that were not withdrawn from ethanol there was no correlation of ethanol consumption (in the same days as those in the pre-withdrawal period of the withdrawn animals) with the density of either GS-IR (R2 = 0.0068, P = 0.822) (Fig. 5B) or GFAP-IR (R2 = 0.1002, P = 0.2007) cells.
Fig. 5.
Scatter plots illustrating the correlation of levels of ethanol consumption (during the last 4 days of ethanol drinking in animals with 2 months of survival) with the values of packing density of GS-immunoreactive astrocytes in the prelimbic cortex of alcohol-withdrawn (A), and alcohol-drinking rats without withdrawal (B).
DISCUSSION
The present results support a relationship between withdrawal from free-choice ethanol intake and increases in the density of GS- and GFAP-immunoreactive astrocytes in the rat prefrontal cortex. The present study does not distinguish whether the increase in detectable GS-IR astrocytes is due to alterations in the turnover of astrocytes, to an increase in the numbers of identifiable astrocytes because of an increase in GS synthesis, or to both phenomena simultaneously. The magnitude of the effect, however, appears to be larger for GS-IR astrocytes than for GFAP-IR astrocytes. In fact, a stronger positive relationship between the detection of GS-IR astrocytes and the magnitude of pre-withdrawal ethanol consumption is underscored by the significant positive correlation of ethanol consumption in the 4 days preceding the withdrawal with the packing density of GS-IR (but not GFAP-IR) astrocytes measured 3 days into the final withdrawal. The regulation of GS-IR astrocytes during withdrawal is consistent with the glutamatergic activation that has been measured by other authors in the brain during withdrawal (Hoffman, 1995; Dahchour and De Witte, 1999; Rossetti et al., 1999). In addition, such regulation indicates that astrocytes, through the involvement of GS, may play an important role in regulating the effects of putative glutamatergic activation in the prefrontal cortex of alcohol-preferring rats.
In vitro experiments have shown that alcohol inhibits the activity of GS (Davies and Vernadakis, 1984) and the proliferation of cultured astrocytes (Davies and Vernadakis, 1984; Kane et al., 1996). A change in the population of immunoreactive astrocytes caused by alcohol drinking is not evident in our experiments at 2 or 6 months of continuous free-choice ethanol intake. Although binge ethanol produces a reduction in cell proliferation in the hippocampus, chronic ethanol intake (30 days) appears to result in compensatory mechanisms that restore proliferation in the dentate gyrus to normal levels (Rice et al., 2004). If such compensatory mechanisms occur in the PLC, this may partly explain the absence of changes in density of GS immunoreactivity in ethanol-drinking P rats. On the other hand the higher density of GS-IR astrocytes observed after withdrawal from free-choice ethanol intake may be due to increased proliferation of GS-immunoreactive astrocytes caused by withdrawal itself. Recently, withdrawal from chronic binge alcohol intake has been found associated with an increase in cell proliferation in the hippocampus that appears to be independent of the severity of withdrawal (Nixon and Crews, 2004). Further experimentation is needed to determine if the increased number of GS- and GFAP-IR astrocytes in the prelimbic cortex of the P rat is related to withdrawal-induced cell proliferation.
The present data of lower density of GS-IR (and GFAP-IR) astrocytes in the PLC of alcohol-naïve P rats as compared with NP rats are also in line with our previous study showing a significantly lower number of GFAP-IR astrocytes in P rats as compared with NP and Wistar rats (Miguel Hidalgo, 2005). In addition, the withdrawal-related increase in the packing density of GS- and GFAP-immunoreactive astrocytes appears to occur concomitantly with a significant ADE (observed in the two first periods of withdrawal in animals with 6-month alcohol-drinking and three withdrawal periods). This coincidence may link the ADE to the increased GS immunoreactivity. Clearly, experiments with longer periods of ethanol deprivation (at times when the alcohol deprivation can still be detected) are needed to determine whether the number of GS-IR astrocytes remains elevated or returns to lower values beyond 3 days of abstinence.
The initially higher packing density of GS-IR astrocytes in NP rats than in alcohol-naïve P rats would be consistent with an influence of lower GS-IR astrocytes in the initiation of high alcohol drinking. However, a simple correlation between a higher density of GS-IR astrocytes and lower ingestion of ethanol is not straightforward because, after withdrawal, animals show both ADE (increased alcohol intake) and a higher average packing density of GS-IR astrocytes. It is also possible that the packing density of GS-IR astrocytes is linked to neural processes in the prelimbic cortex that influence the initial response to the ingestion of substances of abuse, and, upon initiation of such ingestion and development of dependence in P rats, compensatory changes due to withdrawal from free-choice ethanol drinking are more important in driving the expression of GS in astrocytes, while the increased ethanol drinking that defines ADE might be unrelated to the withdrawal-induced increase in GS-IR astrocytes.
In summary, the present results suggest that a relatively low (as compared with NP rats) packing density of GS-IR astrocytes in P rats is increased after withdrawal from free-choice ethanol drinking, but that the density of GS-IR astrocytes at 3 days of withdrawal is dependent on the levels of pre-withdrawal alcohol drinking. Further experiments are needed to determine if changes in GS-IR astrocytes are due to increases in the synthesis of GS, or to changes in the survival and proliferation of astrocytes.
Acknowledgements
The author gratefully acknowledges the excellent technical help of Valerie Wanzo and Bouchra Koussih. Supported by RR017701 from the National Center for Research Resources.
REFERENCES
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, et al. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clinical and Experimental Research. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Davies DL, Vernadakis A. Effects of ethanol on cultured glial cells: proliferation and glutamine synthetase activity. Brain Research. 1984;318:27–35. doi: 10.1016/0165-3806(84)90059-2. [DOI] [PubMed] [Google Scholar]
- De Witte P, Pinto E, Ansseau M, et al. Alcohol and withdrawal: from animal research to clinical issues. Neuroscience and Biobehavioral Reviews. 2003;27:189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine and GABA I. Glutamate transporters. Journal of Neuroscience Research. 2001;63:453–460. doi: 10.1002/jnr.1039. [DOI] [PubMed] [Google Scholar]
- Hallermayer K, Hamprecht B. Cellular heterogeneity in primary cultures of brain cells revealed by immunocytochemical localization of glutamine synthetase. Brain Research. 1984;295:1–11. doi: 10.1016/0006-8993(84)90810-2. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends in Neuroscience. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metabolic Brain Disease. 1995;10:73–79. doi: 10.1007/BF01991784. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Berry A, Boop FA, et al. Proliferation of astroglia from the adult human cerebrum is inhibited by ethanol in vitro. Brain Research. 1996;731:39–44. doi: 10.1016/0006-8993(96)00456-8. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Critical Reviews in Neurobiology. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Mearow KM, Mill JF, Freese E. Neuron-glial interactions involved in the regulation of glutamine synthetase. Glia. 1990;3:385–392. doi: 10.1002/glia.440030510. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clinical and Experimental Research. 2005;5:766–772. doi: 10.1097/01.alc.0000164378.92680.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Research. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pinto A, Sesack SR. Limited collateralization of neurons in the rat prefrontal cortex that project to the nucleus accumbens. Neuroscience. 2000;97:635–642. doi: 10.1016/s0306-4522(00)00042-7. [DOI] [PubMed] [Google Scholar]
- Rauen T, Wiessner M. Fine tuning of glutamate uptake and degradation in glial cells: common transcriptional regulation of GLAST1 and GS. Neurochemistry International. 2000;37:179–189. doi: 10.1016/s0197-0186(00)00021-8. [DOI] [PubMed] [Google Scholar]
- Rice AC, Bullock MR, Shelton KL. Chronic ethanol consumption transiently reduces adult neural progenitor cell proliferation. Brain Research. 2004;1011:94–98. doi: 10.1016/j.brainres.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, et al. Recent advances in animal models of alcohol craving and relapse. Pharmacology, Biochemistry and Behavior. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-d-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PubMed] [Google Scholar]
- Shen F, Chen B, Danias J, et al. Glutamate-induced glutamine synthetase expression in retinal Muller cells after short-term ocular hypertension in the rat. Investigative Ophthalmology and Visual Science. 2004;45:3107–3112. doi: 10.1167/iovs.03-0948. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Roberts JA, Sheeler-Gough LV. Reduction of glutamine synthetase specific activity in cultured astroglia by ferrous chloride. Journal of Neuroscience Research. 1989;24:508–516. doi: 10.1002/jnr.490240408. [DOI] [PubMed] [Google Scholar]
- Tsai G. Glutamatergic neurotransmission in alcoholism. Journal of Biomedical Sciences. 1998;5:309–320. doi: 10.1007/BF02253441. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Progress in Brain Research. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioral Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. Journal of Comparative Neurology. 1985a;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Postnatal volumetric development of the prefrontal cortex in the rat. Journal of Comparative Neurology. 1985b;241:268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, et al. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, et al. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Research. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]