Abstract
Background and Purpose
Despite the critical importance of the corpus callosum (CC) to the connection between brain hemispheres, little is known about the independent contribution of degenerative and vascular processes to regional changes in the microstructural integrity of the CC. Here, we examine these changes in subjects with mild cognitive impairment (MCI), Alzheimer's disease (AD), and in cognitively normal elderly adults.
Methods
We used three-dimensional brain MRI with diffusion tensor imaging in 47 AD, 77 MCI, and 107 cognitively normal subjects, and calculated mean fractional anisotropy (FA) values for four CC regions corresponding to four homologous regions of cortical gray matter (GM). To assess vascular and degenerative processes, we also measured cortical GM and white matter hyperintensity (WMH) volume in corresponding regions, along with evaluation of their vascular risk.
Results
We found that GM volume in anterior and posterior regions was significantly related to FA findings in the corresponding regions of the CC for all three diagnostic groups. Independent of GM volume, frontal WMH volume was also associated with FA values in the corresponding CC regions, but posterior WMH volume was not. Vascular risk was associated with FA of most CC regions, while diagnosis for cognitive state was associated only with FA of the anterior and posterior CC regions.
Conclusions
We found differential region-specific associations between degenerative and vascular processes and the structural integrity of the CC across the spectrum of cognitive ability. Based on these results, we propose a model to explain regional disruption in the interhemispheric connection.
Keywords: Alzheimer's disease, cerebrovascular disorders, mild cognitive impairment, corpus callosum, diffusion tensor imaging
The corpus callosum (CC) is the major white matter structure involved in interhemispheric cortico-cortical communication and critical to multiple cognitive functions.1 The fibers of the CC arise predominantly from large pyramidal neurons in cortical layers III and V.2 These same neuron populations are selectively affected by Alzheimer disease (AD) pathologies.3,4 In support of this relation, several MRI studies have reported microstructural alteration of the CC as measured by fractional anisotropy (FA) derived from diffusion tensor imaging (DTI) in AD and even mild cognitive impairment (MCI),5,6 a transitional state between normal cognitive function and dementia, especially AD. Knowledge about region-specific relationships between CC microstructural alteration and cortical degenerative process in AD and MCI, however, remains limited.
Vascular processes are also hypothesized to affect the integrity of the CC in normal aging, MCI and AD.6-8 Subcortical vascular white matter damage, grossly reflected by white matter hyperintensities (WMH) seen on MRI, is common to aging, MCI and AD, as well as cerebrovascular disease (CVD).7 Such white matter damage could also alter CC integrity through direct injury to subcortical axonal fibers. Nevertheless, little is known about regional associations between cerebrovascular pathology and CC alteration in normal aging or cognitive impairment.
Previous work in our laboratory has found regional patterns in WMH7 and white matter FA6 that are distinct for both CVD and AD processes suggesting that both processes might independently affect CC integrity. Given the importance of the CC to integrative cognitive processes, further investigation of the influence of AD and CVD processes on CC integrity may lead important insights relating to the pathobiology of cognitive impairment due to these two pathologies individually or in combination.
This report, therefore, seeks to further delineate the complicated region-specific associations between both degenerative and vascular processes and CC microstructural integrity. To accomplish this, we first investigated the neuroanatomical relationships of regional cortical gray matter (GM) and WMH volumes with homologous CC subregional FA amongst a group of subjects with normal cognition, MCI and AD. Secondly, the associations of diagnosis [cognitively normal (CN), MCI and AD] and vascular risk with each of regional cortical GM volume, WMH volume, and CC FA were evaluated. Combining the results from these two steps of investigation, we proposed a possible patho-anatomical model to explain regional CC disruptions by both AD degenerative and vascular processes.
Methods
Subjects
Subjects included 47 AD, 77 MCI, and 107 CN individuals. The AD group consisted of 78.7% patients with probable AD, 19.1% patients with possible AD, and 2.1% patients with AD and sufficient CVD defined as two or more stroke at least one of which is outside cerebellum on MRI, or single stroke with clearly documented temporal relationship to the onset or aggravation of cognitive impairment9 for the diagnosis of mixed dementia. 83.6 % of MCI patients are amnestic and 16.4% are non-amnestic subtype. No subjects except the mixed dementia cases had clinical stroke. The diagnosis of AD was made according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria.10 The diagnosis of mixed dementia was according to the criteria of the State of California Alzheimer's Disease Diagnostic and Treatment Centers.9 MCI was diagnosed according to current consensus criteria.11 CN was diagnosed if there was no clinically significant cognitive impairment.
Subjects were recruited from the Alzheimer's disease Center at the University of California, Davis. All participants received a comprehensive clinical evaluation and neuropsychological testing with a standardized test battery.12 In addition, all subjects received a standardized MRI scan of the brain at the baseline evaluation. The institutional review boards at all participating institutions approved this study, and subjects or their legal representatives gave written informed consent.
Vascular risk assessment
The presence or absence of stroke, diabetes, hyperlipidemia, transient ischemic attack, hypertension, and coronary artery disease was systematically assessed to create a composite score for vascular risks that was the sum of the factors present ranging 0 to 6 and reported as a percentage.6
Image acquisition and processing
Image acquisition
All brain imaging was performed at the University of California at Davis Imaging Research Center on a 1.5T GE Signa Horizon LX Echospeed system. Three sequences were employed: a three-dimensional T1-weighted coronal spoiled gradient-recalled echo acquisition (3D T1-SPGR), a FLAIR sequence, and a DTI sequence. DTI was performed according to previously reported methods.6
Regions of Interest
For determination of regional gray matter, WMH volumes, and CC FA, we drew three different sets of regions of interest (ROIs) on a minimal deformation template (MDT)13 (see online only figure 1). The first set consisted of four gray matter ROIs labeled as GM I, II, III, and IV, corresponding to prefrontal cortex, premotor and supplementary motor cortex, sensory-motor cortex, and parieto-temporo-occipital cortex without post-central gyrus, respectively. GM IV was additionally divided into three sub-regions for more detailed comparison (IV-p for parietal cortex, IV-t for temporal cortex, and IV-o for occipital cortex). Both left and right GM of each region were combined to create each GM ROI. The second set consisted of two white matter ROIs, i.e., frontal white and posterior white matter ROI. The cerebral white matter was divided into frontal and posterior white matter ROI by an arbitrary curved plane containing the bilateral central sulci. The third set included four CC sub-region ROIs specifically identified as homologous to the four cortical regions as previously determined.14 The CC was outlined on midsagittal and four adjacent sagittal slices (i.e., two right and two left slices to the midsagittal one: one slice thickness = 0.938 mm), and divided into four sub-region ROI, i.e., CC I, II, III, and IV. The proportions for division were determined according to a recently proposed CC subdivision based on cytoarchitectonic cortical topography and advanced DTI tractography.14
Image analysis
Image analysis was performed to obtain subject tissue volumes and FA values amongst the predetermined ROIs drawn on the MDT. This was accomplished in four stages.
Image registration
The method of image registration has been previously described.6 Native space T1 SPGR brain images were first stripped of the skull using an in-house semi-automatic algorithm. Next they were linearly aligned to the template image using a 12-parameter affine transformation. These aligned images were then warped onto the template using a high-dimensional B-spline deformation.
Four-tissue image segmentation
Segmentation of gray matter, white matter, WMH, and CSF was performed on native space T1 SPGR images by an in-house computer program using Bayesian maximal-likelihood EM computation.15 Tissue probabilities used a combination of Gaussian intensity distributions combined with a Markov Random Field (MRF) component for modeling the tissue classification of voxel neighborhoods. Two in-house enhancements included 1) automatic initialization of the EM step via a high dimensional B-spline warp in which template-based tissue probability maps are fitted to the native T1 SPGR images; and 2) edge detection to dictate the appropriate neighborhood clique structure of the MRF for locations in homogeneous tissue or at tissue boundaries. The segmentation of WMH was determined by WMH maps derived from subject FLAIR images according to an in-house procedure which has been previously described.7,16
Automatic fitting of template ROIs
The linear alignment followed by B-spline warp, done to register the native T1 SPGR image with the template, were reversed in order to fit each ROI back into native space. Each ROI was transformed using the inverse of the warp, followed by the inverse of the affine transformation. Accurate ROI tissue volumes can then be achieved in conjunction with the native space tissue segmentations.
ROI Volume and Tissue Calculation
Volumetric calculations were made using ROIs transformed onto native space images. For every CC region mask on a subject native brain, volume and mean FA were calculated. Mean FA values were obtained by averaging FA values over every voxel within the mask. Gray matter and WMH volumes inside an ROI were obtained by counting gray matter or WMH voxels of the segmented image falling within the desired ROI.
Statistical analysis
Demographic, clinical, and overall MRI volume data for the three cognitive groups were compared by one-way analysis of variance (ANOVA) with Tukey post-hoc comparisons. In order to investigate independent, region-specific association of GM and WMH volume with CC FA, we first tested multiple linear regression models with CC FA as the dependent variable, and corresponding GM and WMH volume (frontal WMH volume for CC I and II FA, and posterior WMH volume for CC III and IV FA), and age as independent variables for each CC region across AD, MCI, and CN groups. The associations of diagnosis and vascular risk with any regional brain volume or FA were tested by analyses of covariance (ANCOVAs) with age as a covariate using Tukey post hoc comparison. Values with p < 0.05 were regarded as significant when otherwise not specified.
Results
Subject characteristics
Demographic, clinical, and overall MRI volume description of all the subjects are summarized in Table 1.
Table 1. Subject Characteristics.
| CN | MCI | AD | P value | |
|---|---|---|---|---|
| No. (F/M) | 107 (69/38) | 77 (42/35) | 47 (28/19) | - |
| Age, y | 74.2 ± 7.3 | 74.5 ± 7.2 | 76.9 ± 8.9 | 0.114 |
| Education, y | 12.3 ± 4.8 | 12.0 ± 5.6 | 10.9 ± 5.1 | 0.275 |
| MMSE | 27.9 ± 2.3 | 25.2 ± 4.1 | 20.0 ± 5.6 | <0.001*†‡ |
| Vascular risk score, % | 24.3 ± 22.0 | 27.1 ± 21.4 | 31.7 ± 21.0 | 0.186 |
| Brain vol., % TCV | 77.62 ± 2.34 | 76.37 ± 2.43 | 74.77 ± 2.40 | <0.001*†‡ |
| CSF vol., % TCV | 22.38 ± 2.34 | 23.62 ± 2.43 | 25.23 ± 2.39 | <0.001*†‡ |
| WMH vol., % TCV§ | -0.432 ± 0.446 | -0.239 ± 0.458 | -0.165 ± 0.509 | 0.001*‡ |
Data presented as means ± SD. MRI volume measures corrected for head size (% TCV). Group comparison by ANOVA. Post-hoc comparison of significant group differences:
CN vs AD,
MCI vs AD,
CN vs MCI.
Log transformed to normalize variance
CN = cognitively normal; MCI = mild cognitive impairment; AD = Alzheimer's disease; MMSE = Mini-Mental State Examination; CSF = cerebrospinal fluid; WMH = white matter hyperintensities; TCV = total cranial volume
Regional GM and WMH volumes and CC FA
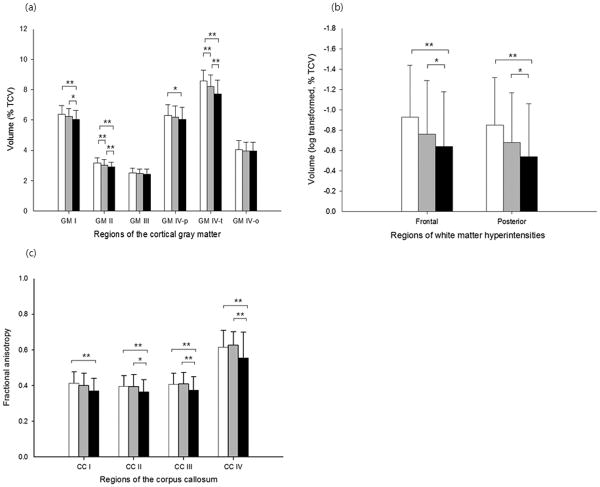
Before investigating the relationships amongst the target variables, we first compared regional cortical GM and WMH volumes and CC FA between diagnostic groups. There were significant diagnostic group differences in volume for each of GM I, II, and IV-t (F=6.92, p=0.001 for GM I; F=14.73, p<0.001 for II; F=28.31, p<0.001 for IV-t; Figure 1a). A strong trend toward significant group differences was also found for GM IV-p (F=2.93, p=0.054). We also found a significant group difference in whole GM IV volume (F=9.80, p<0.001). In contrast, there were no significant diagnostic group differences in GM III and IV-o volume. Furthermore, there were significant diagnostic group differences in both frontal and posterior WMH volume (F=5.85, p=0.003 for the frontal WMH; F=4.13, p=0.017 for the posterior WMH; Figure 1b). The results of post-hoc comparisons for each analysis are presented in Figure 1 (a and b). Significant group differences were found for FA measures in all CC regions (F=3.90, p=0.022 for CC I; F=3.90, p=0.022 for II; F=4.18, p=0.017 for III; F=6.87, p=0.001 for IV; Figure 1c). Post-hoc analyses showed that AD patients had significantly lower FA values in all the CC regions as compared to CN individuals (Figure 1c). Effect size (i.e., Cohen's d17) for the difference of FA between CN and AD was 0.591 for CC I, 0.530 for CC II, 0.507 for CC III, and 0.583 for CC IV indicating that the greatest group differences were at the genu and splenium of the CC. AD also had significantly lower FA in the CC II, III, and IV than MCI (Figure 1c). There were, however, no significant differences of regional CC FA values between CN and MCI.
Figure 1.
Graphic displays of regional volume and fractional anisotropy (FA) according to diagnostic group. (a) Regional gray matter (GM) volume; (b) regional white matter hyperintensity volume; (c) FA of sub-regions of the corpus callosum (CC). White columns = cognitively normal older individuals; gray columns = patients with mild cognitive impairment; black columns = patients with Alzheimer's disease. TCV indicates total cranial volume. Error bars indicates SD. *p<0.05, **p <0.01 by Tukey post-hoc diagnostic group comparison.
Region-specific association of GM and WMH volume with CC FA
Among four multiple regression models explaining regional CC FA by corresponding GM and WMH volume that included all subjects, the models for CC I, II, and IV FA were significant, but the model for CC III FA was not (Table 2). The results for individual independent variables were quite different amongst each of the significant models. In case of the model for CC I FA, both corresponding GM and WMH volume were statistically significant, while only GM volume was significant in the model for CC IV FA. In the model CC II FA, GM volume was significant and WMH volume was marginally significant.
Table 2. Multiple regression models explaining regional corpus callosum FA.
| Dependent variable | Independent variable | beta | p-value | R2 |
|---|---|---|---|---|
| CC region I FA | <0.001 | 0.194 | ||
| Age | -0.177 | 0.007 | ||
| GM region I vol. | 0.276 | <0.001 | ||
| Frontal WMH vol. | -0.161 | 0.014 | ||
| CC region II FA | 0.014 | 0.046 | ||
| Age | 0.003 | 0.968 | ||
| GM region II vol. | 0.162 | 0.014 | ||
| Frontal WMH vol. | -0.121 | 0.088 | ||
| CC region III FA | 0.078 | 0.030 | ||
| Age | 0.002 | 0.976 | ||
| GM region III vol. | 0.129 | 0.065 | ||
| Posterior WMH vol. | -0.084 | 0.264 | ||
| CC region IV FA | <0.001 | 0.121 | ||
| Age | -0.040 | 0.575 | ||
| GM region IV vol. | 0.310 | <0.001 | ||
| Posterior WMH vol. | -0.066 | 0.357 |
FA = fractional anisotropy; CC = corpus callosum; CC region I = most anterior part of the CC; CC region II = middle anterior part of the CC; CC region III = middle posterior part of the CC; CC IV = most posterior part of the CC; GM = gray matter; WMH = white matter hyperintensities
Additionally, to identify any differences in GM and WMH volume vs. CC FA relationship among AD, MCI and CN groups, both GM volume by diagnosis and WMH volume by diagnosis interaction effects were tested with main effects of age, GM volume, WMH volume, and diagnosis for each regional CC FA using general linear model. For CC IV FA, there was an additionally significant posterior WMH volume by diagnosis interaction (F=6.33, p=0.002). We, therefore, tested individual associations between posterior WMH volume and CC IV FA again for each diagnostic group controlling age and GM IV volume. Posterior WMH volume had a significant negative association with CC IV FA only in AD group, but not in CN and MCI groups, while GM IV volume was significantly associated with CC IV FA in all three groups (Table 3 and online only figure 2). No such WMH volume by diagnosis interactions were found for CC I, II, and III FA. In addition, there were no GM volume by diagnosis interactions for all regional CC FA.
Table 3. Multiple regression models for corpus callosum region IV FA in each diagnostic group.
| Variables | CN (n=107) |
MCI (n=77) |
AD (n=47) |
|
|---|---|---|---|---|
| GM IV vol. | beta | 0.283 | 0.260 | 0.328 |
| ΔR2 | 0.074 | 0.059 | 0.129 | |
| P-value | 0.005 | 0.036 | 0.017 | |
| Posterior WMH vol. | beta | 0.053 | 0.109 | -0.406 |
| ΔR2 | 0.002 | 0.009 | 0.156 | |
| P-value | 0.621 | 0.416 | 0.008 | |
| Age | beta | -0.044 | -0.121 | 0.020 |
| ΔR2 | 0.002 | 0.010 | <0.001 | |
| P-value | 0.677 | 0.400 | 0.889 | |
| Overall model | R2 | 0.081 | 0.095 | 0.271 |
| P-value | 0.033 | 0.064 | 0.004 |
FA = fractional anisotropy; CN = cognitively normal; MCI = mild cognitive impairment; AD = Alzheimer's disease; GM = gray matter; WMH = white matter hyperintensities;
Independent association of diagnosis and vascular risk with regional GM and WMH volume and CC FA
Independent of vascular risk, diagnosis was significantly associated with volumes of GM I, II, and IV, but not with that of GM III. Vascular risk was not significantly associated with regional GM volumes (Table 4). In contrast, both diagnosis and vascular risk were significantly associated with frontal and posterior WMH volume. Contribution of vascular risk to regional WMH volumes, however, was relatively greater in the frontal region than in the posterior region (Table 4). Finally, with regard to regional CC FA, diagnosis was significantly related only with CC I and CC IV FA showing relatively greater contribution to CC IV compared to CC I. In contrast, vascular risk showed significant association with CC I, II, III FA, and marginally significant association with CC IV FA.
Table 4. Independent effect of age, vascular risk, and diagnosis on regional gray matter volume, white matter hyperintensity volume, and corpus callosum FA.
| Dependent variable | Independent variable | Statistics | Subregions | |||
|---|---|---|---|---|---|---|
| GM volume | GM I | GM II | GM III | GM IV | ||
| Diagnosis | F | 9.90 | 3.49 | 0.77 | 20.40 | |
| P-value | <0.001 | 0.032 | 0.465 | <0.001 | ||
| Vascular Risk | F | 0.14 | 0.21 | <0.01 | 1.85 | |
| P-value | 0.713 | 0.650 | 0.962 | 0.175 | ||
| Age | F | 11.04 | 2.06 | 15.95 | 14.72 | |
| P-value | 0.001 | 0.153 | <0.001 | <0.001 | ||
| WMH volume | Frontal WMH | Posterior WMH | ||||
| Diagnosis | F | 4.96 | 5.10 | |||
| P-value | 0.007 | 0.007 | ||||
| Vascular Risk | F | 7.73 | 3.89 | |||
| P-value | 0.006 | 0.049 | ||||
| Age | F | 40.31 | 69.19 | |||
| P-value | <0.001 | <0.001 | ||||
| CC FA | CC I | CC II | CC III | CC IV | ||
| Diagnosis | F | 3.58 | 2.35 | 2.45 | 6.10 | |
| P-value | 0.030 | 0.098 | 0.089 | 0.003 | ||
| Vascular Risk | F | 5.79 | 12.24 | 4.42 | 3.76 | |
| P-value | 0.017 | 0.001 | 0.037 | 0.054 | ||
| Age | F | 14.03 | 0.05 | <0.01 | 2.24 | |
| P-value | <0.001 | 0.820 | 0.956 | 0.136 | ||
Data are F and p-values by ANCOVA including diagnosis as a factor, and age and vascular risk as covariates.
CC = corpus callosum; GM = gray matter; WMH = white matter hyperintensities FA = fractional anisotropy.
Discussion
Our ROI-based approach revealed that microstructural integrity, as measured by FA, differed significantly by degree of cognitive impairment in all CC sub-regions with greater effect sizes in the anterior (i.e., CC I) and posterior regions (IV) compared to the middle region (II and III). The volume of cortical GM I, II, and IV was significantly related to the FA of topographically homologous CC subregions across the spectrum of cognitive ability. Independent of GM volume, frontal WMH was also associated with corresponding CC FA, but posterior WMH was not. Conversely, clinical diagnosis was associated with CC I and CC IV FA, while vascular risk contributed to reduced FA across the entire CC. As far as we know, this is the first report to show differential, region-specific influences of degenerative and vascular processes on CC integrity across the spectrum of cognitive ability.
We found significant AD-associated FA changes in all CC regions, whereas other ROI-based studies reported significant FA deficits only in the splenium, but not in the genu of the CC.5 Our analytical method may be one reason for the differences. While most previous studies focused only on the genu and splenium utilizing relatively small ROIs,5 we investigated regional FA across the entire CC based on cytoarchitectonic cortical topography.14 In support of our findings, a voxel-based study18 reported prominent FA differences between AD and CN in the anterior CC, encompassing both the genu and anterior body.
To delineate regional patho-anatomical associations between degenerative and vascular processes and CC integrity, we first investigated region-specific associations between cortical GM and WMH volumes and CC FA using topographically corresponding ROI pairs.14 Although one previous study reported correlation between GM change and regional CC integrity in AD, it focused only on small regions in the genu and splenium and did not take into consideration the topographical organization of the entire CC.19 The FA of the CC I, II, and IV were significantly associated with the volume of the corresponding cortical GM regions within the entire subject group. These findings suggest that CC microstructural integrity is closely associated with secondary degenerative changes of axonal fibers due to primary GM injury. This is further supported by significant diagnosis-CC FA and diagnosis-GM volume associations in similar regions. Interestingly, however, a similar GM-CC association was also observed in CN, as well as AD and MCI.(Table 3). Although this is a preliminary finding from a secondary analysis, it does suggest the possibility that some of these elderly individuals may have considerable, but asymptomatic AD pathology,20 and this requires further study, particularly with direct pathological examination and longitudinal evaluation.
When analyzed across diagnostic groups, the regional WMH-CC FA association was significant only in the most anterior CC subregion (CC I). In contrast, vascular risk had meaningful associations with most CC regions. This regional discrepancy between WMH-CC and vascular risk-CC relationship appears to be related to relatively different etiologies of WMH between the frontal and posterior WMH.7 As shown in Table 4, posterior WMH are most strongly related to degenerative processes, while frontal WMH are equally related to both vascular process and degeneration. When we analyzed each diagnostic group individually, the posterior CC was associated with posterior WMH in AD group, but not in CN and MCI groups. This may also be explained by strong association of WMH with AD degeneration in this region.
Based on the results of the current study, we propose an explanatory model of patho-anatomical mechanism for the microstructural disruption of the CC in CN, MCI, and AD (Figure 2). Briefly, both AD degenerative and vascular processes contribute to a similar degree to the alteration of the anterior CC (genu and anterior body) (Figure 2a), whereas the influence of degenerative process is prominent compared to vascular process for the posterior CC (splenium) (Figure 2c). In case of the middle CC (middle and posterior body), vascular impact remains substantial, but the impact of degenerative process appears less (Figure 2b). In this model, subcortical WM damage, grossly measured by WMH, mediates not only the direct impact of vascular injury process but also the indirect influence of cortical GM degeneration that, in combination, contributes to the microstructrual alteration of the CC. Future work will examine the cognitive consequences of these CC differences.
Figure 2.
Proposed patho-anatomical explanatory model for the microstructural alterations of the corpus callosum (CC) subregions. Sub-models for (a) the anterior; (b) the middle; (c) posterior CC alteration. Width of the arrows reflect the strength of the association. See text for detailed descriptions of the model.
Summary
We found differential, region-specific associations of degenerative and vascular processes with CC microstructural alteration in a cohort of subjects with a spectrum of cognitive ability. Based on the results of the current study, a patho-anatomical explanatory model for regional disruptions of the interhemispheric connection is proposed. Our results are also consistent with the evolving literature that suggests that both AD and CVD additively affect brain structure leading to increased risk for late-life dementia.
Supplementary Material
Acknowledgments
This study was supported by NIH P30 AG 10129, R01 AG010220, and R01 AG021028.
Footnotes
Disclosure statement for authors: We have no conflicts of interest to disclose. We have no contracts relating to our research with any organization that could benefit financially from our research. There are no agreements that involve any financial interest in our work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloom JS, Hynd GW. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- 2.Innocenti GM, Aggoun-Zouaoui D, Lehmann P. Cellular aspects of callosal connections and their development. Neuropsychologia. 1995;33:961–987. doi: 10.1016/0028-3932(95)00033-y. [DOI] [PubMed] [Google Scholar]
- 3.Giannakopoulos P, Hof PR, Bouras C. Selective vulnerability of neocortical association areas in Alzheimer's disease. Microsc Res Tech. 1998;43:16–23. doi: 10.1002/(SICI)1097-0029(19981001)43:1<16::AID-JEMT3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: A review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- 6.Lee DY, Fletcher E, Martinez O, Ortega M, Zozulya N, Kim J, Tran J, Buonocore M, Carmichael O, DeCarli C. Regional pattern of white matter microstructural changes in normal aging, MCI, and AD. Neurology. 2009;73:1722–1728. doi: 10.1212/WNL.0b013e3181c33afb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delano-Wood L, Bondi MW, Jak AJ, Horne NR, Schweinsburg BC, Frank LR, Wierenga CE, Delis DC, Theilmann RJ, Salmon DP. Stroke risk modifies regional white matter differences in mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the state of california Alzheimer's disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the nincds-adrda work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The uniform data set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 13.Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P. Regional spatial normalization: Toward an optimal target. J Comput Assist Tomogr. 2001;25:805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. 2009;30:3172–3187. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempster AP, L NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society Series B (Methodological) 1977;39:1–38. [Google Scholar]
- 16.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 18.Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- 19.Sydykova D, Stahl R, Dietrich O, Ewers M, Reiser MF, Schoenberg SO, Moller HJ, Hampel H, Teipel SJ. Fiber connections between the cerebral cortex and the corpus callosum in Alzheimer's disease: A diffusion tensor imaging and voxel-based morphometry study. Cereb Cortex. 2007;17:2276–2282. doi: 10.1093/cercor/bhl136. [DOI] [PubMed] [Google Scholar]
- 20.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.