Abstract
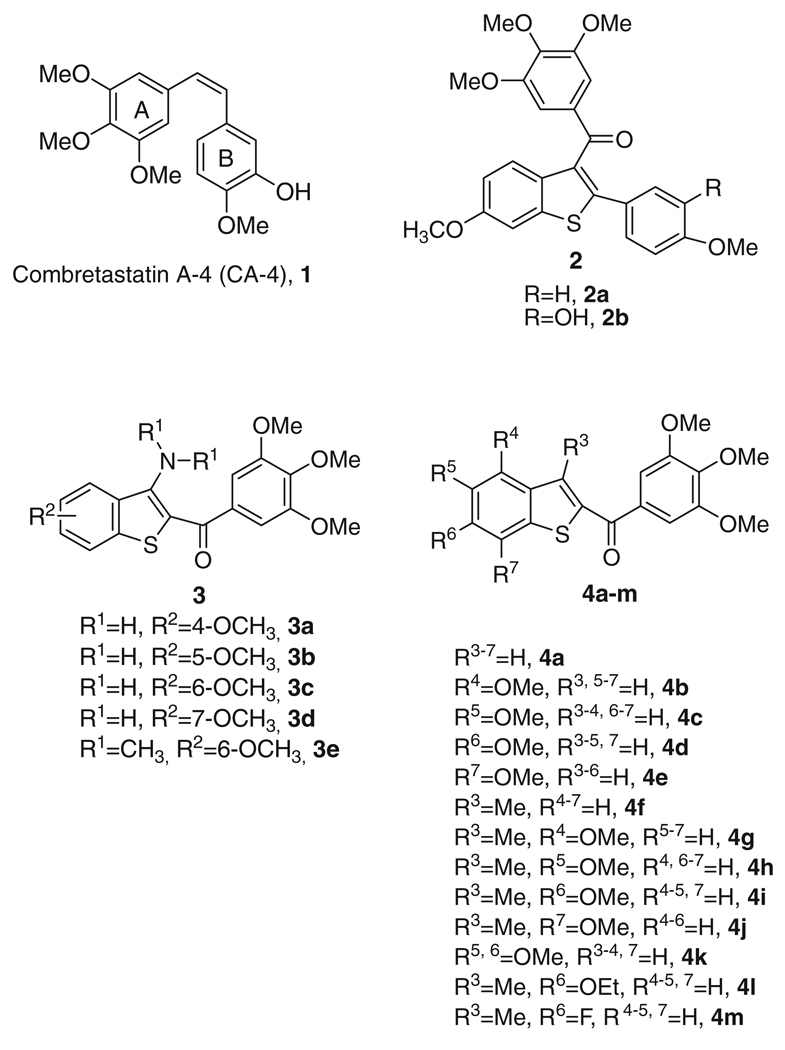
The central role of microtubules in cell division and mitosis makes them a particularly important target for anticancer agents. On our early publication, we found that a series of 2-(3′,4′,5′-trimethoxybenzoyl)-3-aminobenzo[b]thiophenes exhibited strong antiproliferative activity in the submicromolar range and significantly arrested cells in the G2–M phase of the cell cycle and induced apoptosis. In order to investigate the importance of the amino group at the 3-position of the benzo[b]thiophene skeleton, the corresponding 3-unsubstituted and methyl derivatives were prepared. A novel series of inhibitors of tubulin polymerization, based on the 2-(3,4,5-trimethoxybenzoyl)-benzo[b]thiophene molecular skeleton with a methoxy substituent at the C-4, C-5, C-6 or C-7 position on the benzene ring, was evaluated for antiproliferative activity against a panel of five cancer cell lines, for inhibition of tubulin polymerization and for cell cycle effects. Replacing the methyl group at the C-3 position resulted in increased activity compared with the corresponding 3-unsubstituted counterpart. The structure–activity relationship established that the best activities were obtained with the methoxy group placed at the C-4, C-6 or C-7 position. Most of these compounds exhibited good growth inhibition activity and arrest K562 cells in the G2–M phase via microtubule depolymerization.
Keywords: Antiproliferative agents, Tubulin polymerization inhibitors, Microtubules, Antitumor
1. Introduction
Besides being critical for cell architecture, the microtubule system of eukaryotic cells is essential for cell division, since micro-tubules are key components of the mitotic spindle.1 Microtubules also play a crucial role in a wide number of essential cellular processes, such as maintenance of cellular shape, regulation of motility, cell signaling, secretion and intracellular transport.2 In recent years, there has been an intense effort to identify and develop chemically diverse substances, many of which are derived from natural products, that inhibit tubulin assembly for treatment of cancer.3
Among the microtubule depolymerizing agents, combretastatin A-4 (CA4, 1; Chart 1) is one of the more studied compounds. CA4, isolated from the bark of the South African tree Combretum caffrum,4 strongly inhibits the polymerization of tubulin by binding to the colchicine site.5 The activity and structural simplicity of CA-4 have stimulated extensive studies to examine the structure–activity relationship (SAR) of this compound and its analogues.6
Chart 1.
Inhibitors of tubulin polymerization.
Among the synthetic inhibitors of tubulin polymerization, there are limited examples of small molecules based on the benzo[b]thio-phene molecular skeleton as the core structure. Pinney and co-workers described a series of tubulin binding agents with general structure 2, which incorporates the 3-(3′,4′,5′-trimethoxybenzoyl)-6-methoxy benzo[b]thiophene ring system.7 Previous studies have shown that the 6-methoxy substituent significantly contributes to maximize the activity of these compounds, presumably as a mimic of the 4-methoxy group in the B-ring of CA-4.6
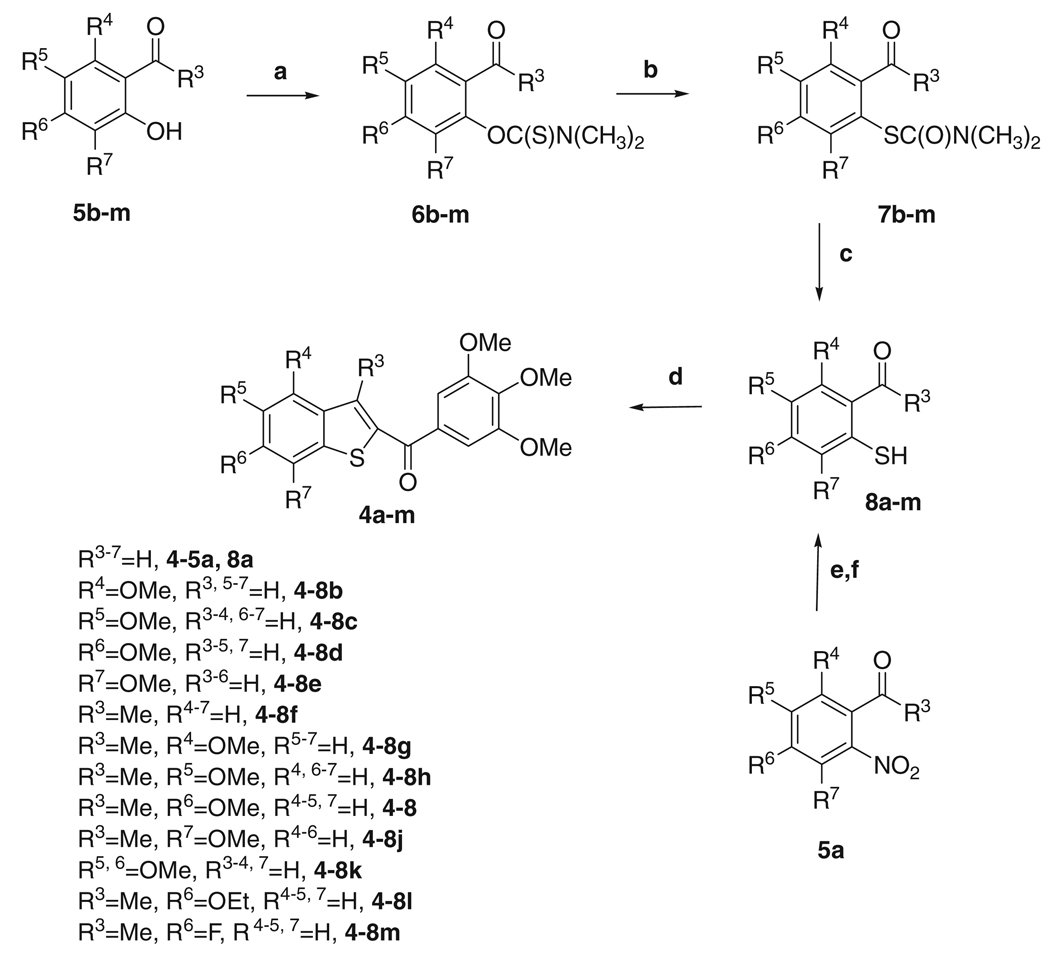
We have recently reported the biological evaluation of a series of derivatives with general structure 3, based on the 2-(3′,4′,5′-trimethoxybenzoyl)-3-aminobenzo[b]thiophene molecular skeleton. These compounds inhibited the growth of cancer cell lines and of tubulin polymerization by binding to the colchicine site of tubulin. As expected, these compounds caused cells to arrest in the G2/M phase of the cell cycle.8 Potent activity appears to be highly dependent upon the presence and position of a fourth methoxy substituent on benzo[b]thiophene system. A fairly dramatic difference was observed between C-4/5 and C-6/7-substituted compounds (3a–b and 3c–d, respectively). The greatest activity occurred when the methoxy group was located at the C-6 or C-7 position, the least when located at the C-4 or C-5 position (IC50 >10 µM). The slight reduction in potency of the 3-dimethylamino derivative 3e, which did not differ significantly from that of the 3-amino counterpart 3c, allowed us to verify that an intramolecular hydrogen bond between the unsubstituted 3-amino group and the carbonyl oxygen of the 2-trimethoxybenzoyl moiety is not required for activity. These encouraging results prompted us to study this class of compounds in more detail. We herein describe the synthesis and SAR of additional 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]thiophene derivatives in continuation of our search for new potent antitubulin agents.
In the present investigation, the 3′,4′,5′-trimethoxyphenyl of the 2-benzoyl moiety was kept unchanged because it is the characteristic structural requirement for activity in numerous inhibitors of tubulin polymerization, such as colchicine, CA4 and podophyllotoxin.6 In the current studies we examined the importance of the 3-position of the benzo[b]thiophene skeleton by studying the effects of replacing the amino or dimethylamino substituents of compounds with general structure 3 with a hydrogen or a methyl group, to furnish derivatives with general structure 4. The SAR was elucidated with electron-donating methoxy substitution at the C-4, C-5, C-6 or C-7 position on the benzo[b]thiophene ring.
In the series of compounds 4a–j, the 3-amino group of the benzo[b]thiophene system was replaced by hydrogen (4a–e) or methyl (4f–j). These molecules possessed either no substituent (4a and 4f) or a methoxy group at each of the four possible positions on the benzene ring (compounds 4b–e and 4g–j). Keeping the C-6 methoxy group intact, compound 4k was prepared with the aim of evaluating the effect on biological activity of one additional methoxy substituent at the C-5 position. Finally, the 6-methoxy position (corresponding to the 4-methoxy group in the B-ring of CA4) of 4j was further studied by synthesizing the corresponding 6-ethoxy (4l) and 6-fluorine (4m) analogues.
2. Chemistry
The 2-(3′,4′,5′-trimethoxybenzoyl)benzo[b]thiophene derivatives 4a–m were synthesized by the four step synthesis shown in Scheme 1. Starting from the variously substituted salicylaldehydes 5b–e and 5k or 2-hydroxyacetophenones 5f–j and 5l–m, the reaction with N,N-dimethyl thiocarbamoyl chloride in N,N-dimethylformamide (DMF) in the presence of DABCO yielded the O-arylthiocarbamates 6b–m in good yields. These latter were submitted to Newman–Kwart rearrangement9 by heating under microwave irradiation (MW) in the absence of solvent, to furnish the corresponding S-arylthiocarbamates 7b–m. The subsequent hydrolysis using a 3 M aqueous sodium hydroxide solution furnished the desired 2-thiophenols 8b–m, which were used without further purification for the next reaction. 2-Mercaptobenzaldehyde 8a was obtained by S-debenzylation of 2-benzylthiobenzaldehyde with aluminum chloride. This latter compound was obtained by condensation of 2-nitrobenzaldehyde 5a with the potassium salt of phenyl methanethiol in cold aqueous DMF.10
Scheme 1.
Reagents and conditions. (a) ClC(S)N(CH3)2, Dabco, DMF, 50 °C, 5 h; (b) no solvent, heating of fused substrates, microwaves; (c) NaOH, EtOH/H2O,65 °C; (d) (3,4,5-trimethoxyphenyl)-2-bromo-ethanone, K2CO3, (CH3)2CO, rx; (e) C6H5CH2SH, KOH, DMF; (f) AlCl3, C6H6.
Finally, the 2-(3′,4′,5′-trimethoxybenzoyl)benzo[b]thiophene derivatives 4a–m were synthesized by a ‘one-step’ condensation, followed by intramolecular cyclization, of 8a–m with 2-bromo-1-(3,4,5-trimethoxyphenyl)ethanone using anhydrous potassium carbonate in refluxing acetone.
3. Biological results and discussion
Table 1 summarizes the growth inhibitory effects of 2-(3′,4′,5′-trimethoxy)benzo[b]thiophene derivatives 4a–m against murine leukemia (L1210), murine mammary carcinoma (FM3A), human T-lymphoblastoid (Molt/4 and CEM) and human cervix carcinoma (HeLa) cells, with 3-amino-2-(3′,4′,5′ -trimethoxy)benzo[b]thiophene counterparts 3a–d and CA4 (1) as reference compounds. Five compounds (4b, 4e, 4g, 4i and 4j) displayed strong growth inhibitory activity, with IC50 values lower than 100 nM against all 5 cell lines. In addition, 4f, 4l and 4m yielded IC50 values below 100 nM in one to three cell lines. The 3-methyl-4-methoxy derivative 4g possessed the highest potency, inhibiting the growth of L1210, FM3A, Molt/4, CEM and HeLa cells with IC50 values of 19, 23, 18, 19 and 16 nM, respectively. These values were similar to those obtained with CA4 against FM3A and Molt4 cells, while 4g was 5–10-fold less active than CA-4 against L1210, CEM and HeLa cells. Compound 4g also showed at least 100-fold greater activity than its 4-methoxy-3-amino-2-(3′,4′,5′-trimethoxy)benzo[b]thiophene counterpart 3a.
Table 1.
In vitro inhibitory effects of compounds 3a–d, 4a–m and CA4 against the proliferation of murine leukemia (L1210), murine mammary carcinoma (FM3A), human T-lymphocyte (Molt/4 and CEM) and human cervix carcinoma (HeLa) cells
| Compound | IC50a (nM) | ||||
|---|---|---|---|---|---|
| L1210 | FM3A/0 | Molt4/C8 | CEM/0 | Hela | |
| 3a | >10,000 | >10,000 | >10,000 | >10,000 | n.d. |
| 3b | >10,000 | >10,000 | >10,000 | >10,000 | n.d. |
| 3c | 39 ± 16 | 46 ± 12 | 10 ± 7 | 7.7 ±2.9 | n.d. |
| 3d | 33 ± 29 | 27 ± 13 | 8.5 ± 1.4 | 8.9 ±2.0 | n.d. |
| 4a | 490 ± 40 | 860 ± 16 | 500 ± 31 | 510 ± 17 | 1100 ± 100 |
| 4b | 87 ± 5 | 99 ± 0 | 75 ± 6 | 83 ± 9 | 75 ± 5 |
| 4c | 580 ± 15 | 790 ± 18 | 2100 ± 300 | 3000 ± 100 | 900 ± 23 |
| 4d | 1200 ± 200 | 1500 ± 0 | 550 ± 14 | 1700 ± 40 | 590 ± 32 |
| 4e | 84 ± 5 | 85 ± 4 | 27 ± 2 | 46 ± 7 | 24 ± 1 |
| 4f | 140 ± 20 | 190 ± 30 | 93 ± 5 | 94 ± 5 | 99 ± 1 |
| 4g | 19 ± 2 | 23 ± 7 | 18 ± 0 | 19 ± 1 | 16 ± 1 |
| 4h | 600 ± 10 | 550 ± 10 | 430 ± 10 | 500 ± 50 | 460 ± 10 |
| 4i | 30 ± 2 | 45 ± 2 | 18 ± 0 | 19 ± 1 | 18 ± 2 |
| 4j | 25 ± 4 | 35 ± 4 | 28 ± 2 | 18 ± 4 | 21 ± 1 |
| 4k | 1100 ± 60 | 980 ± 53 | 440 ± 14 | 350 ± 15 | 220 ± 16 |
| 4l | 100 ± 0 | 130 ± 10 | 56 ± 2 | 56 ± 3 | 140 ± 24 |
| 4m | 130 ± 20 | 140 ± 9 | 94 ± 8 | 110 ± 10 | 110 ± 13 |
| CA4 | 2.8 ± 1.1 | 42 ± 6.0 | 16 ± 1.4 | 1.9 ± 1.6 | 1.9 ± 1.6 |
n.d. = not determined.
IC50= compound concentration required to inhibit tumor cell proliferation by 50%. Data are expressed as the mean ± SE from the dose–response curves of at least three independent experiments.
Comparing the 3-unsubstituted derivatives 4a–e with their 3-methyl congeners 4f–j, the introduction of a methyl at the C-3 position of the benzo[b]thiophene system generally increased anti-proliferative activity against all five cell lines. The methoxy substitution and location on the benzene part of benzo[b]thiophene moiety plays an important role in affecting antiproliferative activity. In both these series, the methoxy group located at the C-4 or C-7 position enhanced activity, while the C-5 and C-6 substituents were detrimental.
In the series of 3-unsubstituted derivatives 4b–e, there was an evident difference in activity between the C-4/7 and the C-5/6 methoxy derivatives. Our findings showed that the methoxy group located at the C-4 or at the C-7 position (4b and 4e, respectively) resulted, on average, in antiproliferative activity, 19-fold greater than with the methoxy group at the C-5 or C-6 position (4b and 4e vs 4c and 4d). The C-5/6 dimethoxy derivative 4k had activity on average about twice that of either 4c or 4d, and 4k was particularly more active than either monosubstituted compound in the CEM/0 and HeLa cells.
A different effect was observed in the series of 3-methyl substituted derivatives 4g–j, in which the greatest activity occurred when the methoxy group was located at the C-4, C-6 or C-7 position, the least when it was located at the C-5 position. The C-3 methyl, C-6 methoxy derivative (4i) was selected as a scaffold for further modifications, by replacing the methoxy group with either an ethoxy group (4l) or an electron-withdrawing fluorine atom (4m). Both these changes resulted in a decrease in antiproliferative activity against all cell lines relative to 4i, but overall compounds 4l and 4m had very similar activity despite the significant difference in the chemical properties of the two C-6 substituents.
The marked improvement in activity of 4g relative to 4-methoxy-3-amino-2-(3′,4′,5′-trimethoxybenzoyl)benzo[b]thiophene derivative 3a derives from the substitution of an amino with a methyl group at the C-3 position of the 2-(3′,4′,5′-trimethoxy)benzo[b]thiophene core.
Comparing the 4-methoxy derivatives 3a, 4b and 4g, the order of activity for the substituent at the 3-position of the benzo[b]thiophene moiety was methyl (4g) > hydrogen (4b) ≫ amino (3a). With these three compounds, IC50 values were 16–23 nM for 4g and 75–99 nM for 4b, while for 3a all IC50’s were greater than 10 µM. Comparing the C-6 and C-7 methoxy derivatives 3cd and 4ij, replacement of the 3-amino group with a methyl resulted in minimal loss of activity. Thus, with a C-4 methoxy group, the C-3 substituent had a much greater impact on antiproliferative activity than was the case with either a C-6 or C-7 methoxy substituent. With the latter compounds, in which the benzo[b]thiophene methoxy substituent is more distant from the trimethoxybenzene ring, the ortho relationship between the 3′,4′,5′-trimethoxybenzoyl moiety and the C-3 amino group does not play an important role in activity. Thus the 3-amino function can be removed and replaced with a hydrogen or a methyl without significant change in activity.
Because 3c and 3d inhibited tubulin assembly,8 we next investigated whether the antiproliferative activities of the new compounds were related to an interaction with the microtubule system. Compounds 4b, 4e–g, 4i–j and 4lm were evaluated for their in vitro inhibition of the polymerization of 10 µM tubulin and for their inhibitory effects on the binding of [3H]colchicine to tubulin (in the latter assay, tubulin was examined at a concentration of 1 µM, while colchicine was at 5 µM).11,12 For comparison, derivatives 3cd and CA4 were examined in contemporaneous experiments as a reference compounds (Table 2). All tested compounds strongly inhibited tubulin assembly and derivatives 4e, 4f, 4g and 4m, with IC50 values of 0.58–0.71 µM, exhibited antitubulin activity about 1.5–2 times greater than that of CA4 (IC50, 1.0 µM), while 4b, 4i, 4j and 4l had IC50 values of 0.92–1.2 µM, essentially equivalent to that of CA4 and 3c. Comparing the 7-methoxy benzo[b]thiophene derivatives 3d, 4e and 4j, the 3-amino compound 3d was less effective than 3-unsubstituted and 3-methyl analogues 4e and 4j, respectively, as an inhibitor of tubulin polymerization.
Table 2.
Inhibition of tubulin polymerization and colchicine binding by compounds 3c–d, 4b, 4e–g, 4i–j, 4l–m and CA4
| Compound | Tubulin assemblya IC50 ± SD (µM) |
Colchicine bindingb % ± SD |
|
|---|---|---|---|
| 1 µM drug |
5 µM drug |
||
| 3c | 1.3 ±0.1 | 60 ± 0.1 | n.d. |
| 3d | 2.1 ± 0.3 | 39 ± 1 | n.d. |
| 4b | 1.2 ± 0.07 | 49 ± 1 | 81 ±4 |
| 4e | 0.58 ± 0.02 | 60 ± 0.3 | 88 ± 0.5 |
| 4f | 0.63 ± 0.1 | 51 ± 2 | 88 ± 4 |
| 4g | 0.67 ± 0.1 | 85 ± 7 | 93 ± 5 |
| 4i | 0.92 ± 0.05 | 66 ± 4 | 92 ± 0.3 |
| 4j | 0.95 ± 0.06 | 54 ± 3 | 87 ± 1 |
| 4l | 0.93 ± 0.01 | n.d. | 75 ± 3 |
| 4m | 0.71 ± 0.1 | 48 ± 2 | 80 ± 1 |
| CA4 (1) | 1.0 ± 0.09 | 88 ± 1 | 99 ± 2 |
n.d.: not determined.
Inhibition of tubulin polymerization. Tubulin was at 10 µM.
Inhibition of [3H]colchicine binding. Tubulin was at 1 µM, colchicine was at 5 µM.
Compounds 4f and 4m were almost 1.5-fold more active than CA4 as inhibitors of tubulin assembly, although both compounds were less active in their effects on cell growth.
In the colchicine studies, all compounds were active inhibitors of the binding reaction. Compound 4g, the agent with the greatest antiproliferative activity, was the most active inhibitor of the binding of [3H]colchicine to tubulin, since 85% and 93% inhibition occurred with this agent at 1 and 5 µM, respectively. Compound 4g was as active as CA4, which in these experiments inhibited colchicine binding by 88% and 99%. Although 4e was almost twice as active as 4i as an inhibitor of tubulin polymerization, these two compounds showed similar activity as inhibitors of colchicine binding.
While this group of compounds were all highly potent in the biological assays (inhibition of cell growth, tubulin assembly and colchicine binding), correlations between the three assay types were imperfect. Thus, while compound 4e was the best inhibitor of tubulin assembly, its effect on colchicine binding was matched by compounds 4i and 4j, both of which were almost half as active as assembly inhibitors. Compound 4e was, in general, somewhat less effective as an inhibitor of cell growth than the other two compounds 4i and 4j.
Because molecules exhibiting effects on tubulin assembly should cause alteration of cell cycle parameters, with preferential G2–M blockade, flow cytometry analysis was performed to determine the effect of compounds 4b, 4e–g, 4i–j and 4l–m on K562 (human chronic myelogenous leukemia) cells, which are usually employed by our research group to determine the alteration of cell cycle parameters following exposure to antitumour compounds.13 Cells were cultured for 24 h in the presence of each compound at the concentration able to inhibit cell growth 100% after 24 h. Analysis of sub-G0–G1 (apoptotic peak, A), G0–G1, S, and G2–M peaks revealed that the compounds caused somewhat different effects on cell cycle distribution (Table 3). After a 24 h treatment, all tested compounds caused a massive accumulation of cells in the G2–M phase (51–82%) relative to the untreated control (15.1%), with a simultaneous decrease of cells in the S and G0–G1. With compound 4e, the accumulation of cells in G2–M phase was accompanied by the appearance of a significant sub-G0–G1 peak (A = 17.7%) due to apoptosis.
Table 3.
Effects of compounds 4b, 4e–g, 4i–j and 4l–m on K562 cell cycle progression
| Compound | IC50a (nM) | IC100b (nM) | Cell cycle distribution (%) | |||
|---|---|---|---|---|---|---|
| Sub-G1c | G0G1 | S | G2/M | |||
| Control | 3.76 | 34.6 | 50.4 | 15.1 | ||
| 4b | 220 ± 50 | 800 | 8.28 | 2.13 | 18.9 | 78.9 |
| 4e | 95 ± 2 | 150 | 17.7 | 14.8 | 26.8 | 58.3 |
| 4f | 180 ± 20 | 500 | 6.00 | 3.72 | 13.8 | 82.5 |
| 4g | 38 ± 7 | 85 | 7.92 | 3.74 | 16.9 | 79.4 |
| 4i | 6 ± 0.1 | 25 | 7.80 | 11.7 | 22.1 | 66.2 |
| 4j | 42 ± 5 | 75 | 9.46 | 4.19 | 15.2 | 80.6 |
| 4l | 47 ± 8 | 230 | 11.2 | 10.8 | 38.1 | 51.2 |
| 4m | 220 ± 40 | 400 | 10.5 | 6.16 | 17.1 | 76.7 |
Compound concentration required to inhibit tumor cell proliferation by 50%. Data are expressed as the mean ± SE from the dose–response curves of at least three independent experiments.
Compound concentration required to inhibit tumor cell proliferation by 100%.
Percentage of the cell population with hypodiploid DNA content peak (apoptotic cells).
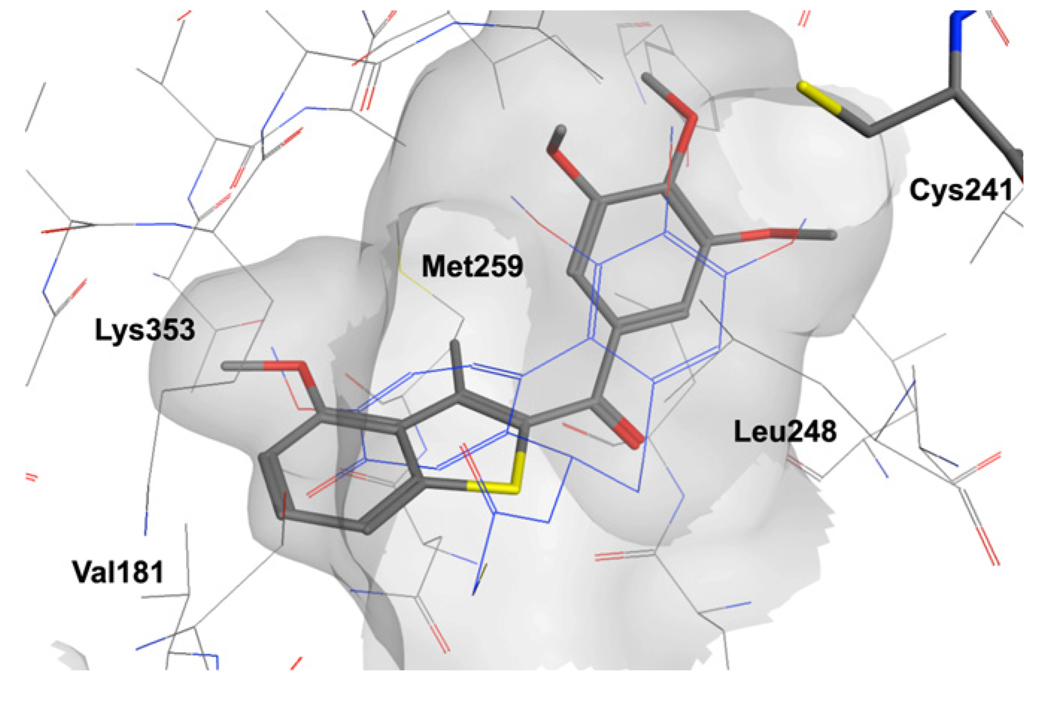
A set of molecular docking calculations was also performed to investigate the possible binding mode of this series of compounds. All the compounds reported in Table 1 were docked in the colchicine binding site of tubulin. The majority of the compounds showed a binding pose similar to the one observed with the co-crystallized DAMA-colchicine, with the trimethoxyphenyl ring placed in proximity of Cys241 (residue numbering derived from the crystal structure used). In addition, the benzo[b]thiophene ring is placed deep in the pocket, interacting with tubulin through a series of hydrophobic contacts (Fig. 1). In addition, the benzo[b]thiophene ring is placed deep in the pocket, interacting with tubulin through a series of hydrophobic contacts (Fig. 1). Compounds 3a–b, 4c–d, 4h and 4l either did not dock with the trimethoxyphenyl in contact with Cys241 or they docked outside the binding pocket, hence their results were not further analyzed. For compounds 3c–d, the benzo[b]thiophene was placed with the amino group oriented on the same side of the carbonyl linker, allowing a possible intramolecular hydrogen bond (see Fig. 1 in Supplementary data). In the case of methyl substituted analogues (4f–j), it is often the sulfur atom that is oriented on the same side of the carbonyl group, allowing, in the case of compound 4g, the positioning of the methoxy substituent in position 4 on the heterocyclic ring to overlap well with the corresponding moiety on ring C of the colchicine analogue (Fig. 1). In particular, the methoxy group establishes a nonpolar interaction deep in the biding pocket, in contact with Val181, which appear to be important for biological activity. Compound 4j showed a similar orientation of the heterocyclic ring to the one observed for 3c–d and also in this case, the methoxy substituent is in contact with Val181. It should also be noted that a similar binding pose was observed for the series of benzo[b]furan derivatives we have recently reported.14
Figure 1.
Presumptive binding mode of 4g. DAMA-colchicine in blue.
4. Conclusions
In continuing experiments to optimize the 2-(3′,4′,5′-trimethoxybenzoyl)-3-aminobenzo[b]thiophene core structure, we developed a new series of compounds with general structure 4, in which the 3-amino moiety was replaced with a hydrogen or methyl group. The results suggest that the hydrophobic methyl group is a good surrogate for the 3-amino moiety and resulted in an improvement in antiproliferative activity. Maximal activity was dependent on the location of the methoxy substituent on the benzene part of benzo[b]thiophene moiety. The SAR study showed that a single methoxy group located at the C-4 or C-7 position resulted in the best activity, while activity was decreased when the methoxy group was located at the C-5 position.
In the series of derivatives without a substituent at C-3, the C-5 and C-6 methoxy derivatives were significantly less active than C-4 and C-7 positional isomers. In the 3-methyl series, compounds with the methoxy group at the C-4, C-6 and C-7 position exhibited about 20-fold greater activity than the C-5 methoxy isomer.
The most active compound in this series was the 2-(3′,4′,5′-trimethoxybenzoyl)-3-methyl-4-methoxybenzo[b]thiophene derivative 4g, with IC50 antiproliferative values of 16–23 nM. Compound 4g also showed at least 100-fold greater activity than its 4-methoxy-3-amino-2-(3′,4′,5′-trimethoxy)benzo[b]thiophene counterpart 3a. Compound 4g was a potent inhibitor of tubulin polymerization, with an IC50 of 0.67 µM, and it arrested K562 cancer cells in the G2–M phase of the cell cycle. Several additional compounds (4e, 4f, 4g and 4m) were more potent than CA4 as inhibitors of tubulin assembly. Nevertheless, except for 4g, all members of the series were less active than CA4 as inhibitors of the binding of [3H]colchicine to tubulin. As a consequence of their interactions with tubulin, all compounds examined caused cell cycle arrest in the G2–M phase, with an increase from the control value of 15% to 51–82% of cells in G2–M. This is well supported by the molecular modeling studies, where it was observed that the methoxy group in these positions could occupy an hydrophobic region deep in the binding pocket.
5. Experimental
5.1. Chemistry
5.1.1. Materials and methods
2-Nitrobenzaldehyde (5a), 2-hydroxy-6-methoxybenzaldehyde (5b), 2-hydroxy-5-methoxybenzaldehyde (5c), 2-hydroxy-4-methoxybenzaldehyde (5d), 2-hydroxy-3z-methoxybenzaldehyde (5e), 1-(2-hydroxyphenyl)ethanone (5f), 2-hydroxy-6-methoxyacetophenone (5g), 2-hydroxy-5-methoxyacetophenone (5h), 2-hydroxy-4-methoxyacetophenone (5i), 2-hydroxy-3-methoxyacetophenone (5j), 2-hydroxy-4-ethoxyacetophenone (5l), 2-hydroxy-4-fluoroacetophenone (5m) are commercially available and were used as received. For the preparation of 2-hydroxy-4,5-dimethoxybenzaldehyde (5k) see: Foyer, R.; Rene, L.; Cavier, R.; Lemoine, J. Eur. J. Med. Chem. 1977, 12, 455.
1H NMR spectra were recorded on a Bruker AC 200 spectrometer. Chemical shifts (δ) are given in ppm upfield from tetramethylsilane as internal standard, and the spectra were recorded in appropriate deuterated solvents, as indicated. Melting points (mp) were determined on a Buchi-Tottoli apparatus and are uncorrected. All products reported showed 1H NMR spectra in agreement with the assigned structures. Elemental analyses were conducted by the Microanalytical Laboratory of the Chemistry Department of the University of Ferrara. Mass spectra were obtained by electrospray ionization (ESI) in positive mode using an ESI Micromass ZMD 2000 mass spectrometer. All reactions were carried out under an inert atmosphere of dry nitrogen, unless otherwise described. Standard syringe techniques were applied for transferring dry solvents. Reaction courses and product mixtures were routinely monitored by TLC on silica gel (precoated F254 Merck plates) and visualized with aqueous KMnO4. Flash chromatography was performed using 230–400 mesh silica gel and the indicated solvent system. Organic solutions were dried over anhydrous Na2SO4. Calcium chloride was used in the distillation of DMF, and the distilled solvent was stored over molecular sieves (3 Å). The MW reaction was performed in a focused MW oven (Discover, CEM, 2450 MHz).
5.2. General procedure (A) for the synthesis of compounds 6b–m
To a solution of phenol 5b–m (5 mmol) in 8 mL of DMF containing DABCO (1.68 g, 15 mmol) was added N,N-dimethylthiocarbamyl chloride (1.85 g, 15 mmol) in one portion. The temperature rose rapidly to 50 °C, and the mixture was held at this temperature for 5 h. After this time, the mixture was poured into water (30 mL), and the product was extracted with dichloromethane (3 × 30 mL). The combined organic phases were washed with 5% HCl (20 mL), 0.1 M NaOH (20 mL) and brine (20 mL), dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel.
5.2.1. O-2-Formyl-3-methoxyphenyl N,N-dimethylcarbamothioate (6b)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 6b as a white solid, yield: 79%, mp 119–121 °C. 1H NMR (CDCl3) δ: 3.43 (s, 3H), 3.48 (s, 3H), 3.87 (s, 3H), 7.19 (d, J = 8.0 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 10.1 (s, 1H).
5.2.2. O-2-Formyl-4-methoxyphenyl N,N-dimethylcarbamothioate (6c)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 6c as a white solid, yield: 92%, mp 87–89 °C. 1H NMR (CDCl3) δ: 3.41 (s, 3H), 3.47 (s, 3H), 3.86 (s, 3H), 7.03 (d, J = 8.8 Hz, 1H), 7.15 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 10.0 (s, 1H).
5.2.3. O-2-Formyl-5-methoxyphenyl N,N-dimethylcarbamothioate (6d)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 6d as a yellow solid, yield: 72%, mp 91–93 °C. 1H NMR (CDCl3) δ: 3.41 (s, 3H), 3.47 (s, 3H), 3.88 (s, 3H), 6.63 (s, 1H), 6.91 (d, J = 8.8 Hz, 1H), 7.83 (d, J = 8.8 Hz, 1H), 9.91 (s, 1H).
5.2.4. O-2-Formyl-6-methoxyphenyl N,N-dimethylcarbamothioate (6e)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 6e as a white solid, yield: 72%, mp 142–144 °C. 1H NMR (CDCl3) δ: 3.40 (s, 3H), 3.46 (s, 3H), 3.94 (s, 3H), 6.70 (d, J = 8.0 Hz, 1H), 6.89 (d, J = 8.0 Hz, 1H), 7.56 (t, J = 8.2 Hz, 1H), 10.3 (s, 1H).
5.2.5. O-2-Acetylphenyl N,N-dimethylcarbamothioate (6f)
The residue was chromatographed with EtOAc–petroleum ether 1:9 as eluent to give 6f as a white solid, yield: 95%, mp 77–79 °C 1H NMR (CDCl3) δ: 2.66 (s, 3H), 3.36 (s, 3H), 3.45 (s, 3H), 7.07 (d, J = 8.2 Hz, 1H), 7.32 (t, J = 7.2 Hz, 1H), 7.57 (t, J = 7.2 Hz, 1H), 7.80 (d, J = 8.2 Hz, 1H).
5.2.6. O-2-Acetyl-3-methoxyphenyl N,N-dimethylcarbamothioate (6g)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 6g as a white solid, yield: 79%, mp 89–92 °C 1H NMR (CDCl3) δ: 2.53 (s, 3H), 3.29 (s, 3H), 3.39 (s, 3H), 3.86 (s, 3H), 6.68 (d, J = 8.4 Hz, 1H), 6.81 (d, J = 8.4 Hz, 1H), 7.35 (d, J = 8.4 Hz, 1H).
5.2.7. O-2-Acetyl-4-methoxyphenyl N,N-dimethylcarbamothioate (6h)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 6h as a white solid, yield: 94%, mp 83–85 °C 1H NMR (CDCl3) δ: 2.52 (s, 3H), 3.38 (s, 3H), 3.45 (s, 3H), 3.84 (s, 3H), 7.01 (s, 1H), 7.04 (m, 1H), 7.29 (m, 1H).
5.2.8. O-2-Acetyl-5-methoxyphenyl N,N-dimethylcarbamothioate (6i)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 6i as colourless oil, yield: 95%. 1H NMR (CDCl3) δ: 1.42 (t, J = 7.0 Hz, 3H), 2.49 (s, 3H), 3.39 (s, 3H), 3.46 (s, 3H), 4.04 (q, J = 7.0 Hz, 2H), 6.56 (d, J = 2.6 Hz, 1H), 6.84 (dd, J = 2.6 and 8.8 Hz, 1H), 7.80 (d, J = 8.8 Hz, 1H).
5.2.9. O-2-Acetyl-6-methoxyphenyl N,N-dimethylcarbamothioate (6j)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 6j as a white solid, yield: 78%, mp 144–146 °C 1H NMR (CDCl3) δ: 2.55 (s, 3H), 3.40 (s, 3H), 3.46 (s, 3H), 3.84 (s, 3H), 7.12 (d, J = 8.0 Hz, 1H), 7.27 (t, J = 8.4 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H).
5.2.10. O-2-Formyl-4,5-dimethoxyphenyl N,N-dimethylcarbamothioate (6k)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 6k as a white solid, yield: 69%, mp 172–174 °C. 1H NMR (CDCl3) δ: 3.42 (s, 3H), 3.48 (s, 3H), 3.94 (s, 6H), 6.61 (s, 1H), 7.36 (s, 1H), 9.94 (s, 1H).
5.2.11. O-2-Acetyl-5-ethoxyphenyl N,N-dimethylcarbamothioate (6l)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 6l as a white solid, yield: 81%, mp 84–86 °C 1H NMR (CDCl3) δ: 2.48 (s, 3H), 3.38 (s, 3H), 3.45 (s, 3H), 3.84 (s, 3H), 6.58 (d, J = 2.8 Hz, 1H), 6.84 (dd, J = 2.4 and 8.8 Hz, 1H), 7.80 (d, J = 8.8 Hz, 1H).
5.2.12. O-2-Acetyl-5-fluorophenyl N,N-dimethylcarbamothioate (6m)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 6m as a colourless oil, yield: 90%. 1H NMR (CDCl3) δ: 2.52 (s, 3H), 3.39 (s, 3H), 3.45 (s, 3H), 6.85 (dd, J = 2.8 and 7.0 Hz, 1H), 7.03 (m, 1H), 7.82 (dd, J = 6.4 and 8.8 Hz, 1H).
5.3. General procedure (B) for the synthesis of compounds 7b–m
The N,N-dimethylcarbamothioate 6b–m (0.5 mmol) was placed in a 10 mL closed vial and irradiated in a focused MW oven at the conditions described for each compound. The progress of the reaction was followed by thin-layer chromatography. The reaction mixture was then cooled and purified by flash column chromatography on silica gel.
5.3.1. S-2-Formyl-3-methoxyphenyl N,N-dimethylcarbamothioate (7b)
MW irradiation conditions: power (P): 200 W, temperature (T): 210 °C, ramp time (RT): 2 min; time: 2 min. The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 7b as a yellow oil, yield: 60 %. 1H NMR (CDCl3) δ: 3.01 (s, 3H), 3.13 (s, 3H), 3.90 (s, 3H), 7.02 (d, J = 8.4 Hz, 1H), 7.21 (d, J = 8.4 Hz, 1H), 7.47 (t, J = 8.4 Hz, 1H), 10.5 (s, 1H).
5.3.2. S-2-Formyl-4-methoxyphenyl N,N-dimethylcarbamothioate (7c)
MW conditions: P: 280 W, T: 240 °C, RT: 2 min; time: 1 min. The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 7c as a yellow oil, yield: 48%. 1H NMR (CDCl3) δ: 3.03 (s, 3H), 3.16 (s, 3H), 3.87 (s, 3H), 7.12 (d, J = 8.2 Hz, 1H), 7.46 (d, J = 8.2 Hz, 1H), 7.52 (s, 1H), 10.3 (s, 1H).
5.3.3. S-2-Formyl-5-methoxyphenyl N,N-dimethylcarbamothioate (7d)
MW conditions: P: 280 W, T: 170 °C, RT: 2 min; time: 10 min. The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 7d as a white solid, yield: 92%, mp 81–83 °C. 1H NMR (CDCl3) δ: 3.02 (s, 3H), 3.17 (s, 3H), 3.87 (s, 3H), 7.07 (d, J = 8.4 Hz, 1H), 7.08 (s, 1H), 8.01 (d, J = 8.4 Hz, 1H), 10.25 (s, 1H).
5.3.4. S-2-Formyl-6-methoxyphenyl N,N-dimethylcarbamothioate (7e)
MW conditions: P: 200 W, T: 210 °C, RT: 2 min; time: 2 min. The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 7e as a yellow oil, yield: 67%. 1H NMR (CDCl3) δ: 3.01 (s, 3H), 3.21 (s, 3H), 4.31 (s, 3H), 7.18 (d, J = 8.0 Hz, 1H), 7.52 (t, J = 8.4 Hz, 1H), 7.62 (d, J = 8.4 Hz, 1H), 10.4 (s, 1H).
5.3.5. S-2-Acetylphenyl N,N-dimethylcarbamothioate (7f)
MW conditions: P: 200 W, T: 210 °C, RT: 2 min; time: 3 min. The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 7f as a brown oil, yield: 61%. 1H NMR (CDCl3) δ: 2.57 (s, 3H), 3.04 (s, 3H), 3.07 (s, 3H), 7.45 (m, 2H), 7.59 (m, 2H).
5.3.6. S-2-Acetyl-3-methoxyphenyl N,N-dimethylcarbamothioate (7g)
MW conditions: P: 200 W, T: 210 °C, RT: 2 min; time: 5 min. The residue was chromatographed with EtOAc–petroleum ether 4:6 as eluent to give 7g as a brown oil, yield: 82%. 1H NMR (CDCl3) δ: 2.46 (s, 3H), 2.99 (s, 3H), 3.02 (s, 3H), 3.79 (s, 3H), 6.92 (d J = 8.4 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 7.27 (t, J = 8.4 Hz, 1H).
5.3.7. S-2-Acetyl-4-methoxyphenyl N,N-dimethylcarbamothioate (7h)
MW conditions: P: 280 W, T: 240 °C, RT: 2 min; time: 1 min. The residue was chromatographed with EtOAc–petroleum ether 4:6 as eluent to give 7h as a brown oil, yield: 53%. 1H NMR (CDCl3) δ: 2.56 (s, 3H), 3.04 (s, 3H), 3.10 (s, 3H), 3.83 (s, 3H), 6.95 (d, J = 8.6 Hz, 1H), 6.99 (s, 1H), 7.44 (d, J = 8.6 Hz, 1H).
5.3.8. S-2-Acetyl-5-methoxyphenyl N,N-dimethylcarbamothioate (7i)
MW conditions: P: 200 W, T: 210 °C, RT: 2 min; time: 4 min. The residue was chromatographed with EtOAc–petroleum ether 4:6 as eluent to give 7i as a brown oil, yield: 51%. 1H NMR (CDCl3) δ: 2.56 (s, 3H), 3.04 (s, 3H), 3.09 (s, 3H), 3.84 (s, 3H), 6.89 (d, J = 2.8 and 8.8 Hz, 1H), 7.14 (d, J = 2.6 Hz, 1H), 7.65 (d, J = 8.6 Hz, 1H).
5.3.9. S-2-Acetyl-6-methoxyphenyl N,N-dimethylcarbamothioate (7j)
MW conditions: P: 240 W, T: 210 °C, RT: 2 min; time: 2 min. The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 7j as a brown oil, yield: 69 %. 1H NMR (CDCl3) δ: 2.58 (s, 3H), 2.96 (s, 3H), 3.24 (s, 3H), 4.24 (s, 3H), 7.12 (d, J = 8.0 Hz, 1H), 7.48 (t, J = 8.4 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H).
5.3.10. S-2-Formyl-4,5-dimethoxyphenyl N,N-dimethylcarbamothioate (7k)
MW conditions: P: 300 W, T: 240 °C, RT: 2 min; time: 4 min. The residue was chromatographed with EtOAc–petroleum ether 1:1 as eluent to give 7k as a yellow oil, yield: 55%. 1H NMR (CDCl3) δ: 3.03 (s, 3H), 3.16 (s, 3H), 3.96 (s, 6H), 6.99 (s, 1H), 7.54 (s, 1H), 10.3 (s, 1H).
5.3.11. S-2-Acetyl-5-ethoxyphenyl N,N-dimethylcarbamothioate (7l)
MW conditions: P: 280 W, T: 220 °C, RT: 2 min; time: 2 min. The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 7l as a brown oil, yield: 53%. 1H NMR (CDCl3) δ: 1.41 (t, J = 7.0 Hz, 3H), 2.56 (s, 3H), 3.04 (s, 3H), 3.09 (s, 3H), 4.09 (q, J = 7.0 Hz, 2H), 6.87 (d, J = 2.8 and 8.6 Hz, 1H), 7.14 (d, J = 2.6 Hz, 1H), 7.65 (d, J = 8.6 Hz, 1H).
5.3.12. S-2-Acetyl-5-fluorophenyl N,N-dimethylcarbamothioate (7m)
MW conditions: P: 240 W, T: 210 °C, RT: 1 min; time: 4 min. The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 7m as a brown oil, yield: 78%. 1H NMR (CDCl3) δ: 2.58 (s, 3H), 3.04 (s, 3H), 3.08 (s, 3H), 7.12 (m, 1H), 7.36 (dd, J = 2.6 and 8.8 Hz, 1H), 7.64 (dd, J = 5.6 and 8.6 Hz, 1H).
5.4. Synthesis of 2-(benzylthio) benzaldehyde
To a cold solution (ice bath) containing 2-nitrobenzaldehyde (3.0 g, 20 mmol) and benzylmercaptan (2.48 g, 20 mmol) in 20 mL of DMF was added dropwise a solution of KOH (2 g, 36 mmol) in 5 mL of water. The mixture was stirred at 4 °C for 2 h and then poured into ice-water. The solid was collected, washed with cold water (50 mL), dried in vacuo over P2O5 and recrystallised from petroleum ether. Yield: 76%, brown oil. 1H NMR (CDCl3) δ: 4.13 (s, 2H), 7.28 (m, 6H), 7.33 (t, J = 8.0 Hz, 1H), 7.49 (m, 1H), 7.82 (d, J = 4.4 Hz, 1H), 10.2 (s, 1H).
5.5. Synthesis of 2-mercaptobenzaldehyde (8a)
A solution of 2-benzylthiobenzaldehyde (4.56 g, 20 mmol) in dry benzene (40 mL) was added dropwise over 30 min to a suspension of finely powdered anhydrous aluminium chloride (4.5 g, 33 mmol) in dry benzene (40 mL), and the mixture was stirred under nitrogen at room temperature for 48 h. The green-brown mixture was decomposed by the cautious addition of ice-water (100 mL), and the organic layer was separated and washed successively with water (50 mL) and 5% aqueous sodium hydroxide (2 × 50 mL). The alkaline extracts were acidified (pH 2) and extracted with dichloromethane (3 × 50 mL). The organic extracts were washed with brine, dried and evaporated to yield 2-mercaptobenzaldehyde as a brown oil, yield: 24%. 1H NMR (CDCl3) δ: 7.31 (m, 2H), 7.33 (t, J = 8.0 Hz, 1H), 7.82 (d, J = 4.4 Hz, 1H), 10.1 (s, 1H), 10.4 (s, 1H).
5.6. General procedure (C) for the synthesis of 2-thiophenols 8b–m
The S-aryl compound 7b–m (3 mmol) was dissolved in MeOH (10 mL). A 3 N aqueous solution of NaOH (12 mL) was added, and the reaction mixture was heated to reflux for 2 h. The reaction mixture was cooled to 25 °C and acidified to pH 5 by the addition of 10% aqueous HCl. The resulting mixture was extracted with EtOAc (3 × 15 mL), and the combined organic extracts were washed with brine, dried over Na2SO4 and concentrated in vacuo to furnish the corresponding 2-mercaptoarylaldehyde, which was used without further purification for the next reaction.
5.7. General procedure (D) for the synthesis of 2-(3′,4′,5′-trimethoxybenzoyl) benzo[b]thiophenes 4a–m
To a solution of the appropriate substituted 2-mercaptobenzadehyde 8b–e and 8k or 2-mercaptoacetophenone 8f–j and 8lm (1 mmol) in dry acetone (15 mL) was added 2-bromo-1-(3,4,5-trimethoxyphenyl)ethanone (289 mg, 1 mmol) and anhydrous potassium carbonate (276 mg, 2 mmol) while stirring, and the reaction mixture was refluxed for 18 h. After cooling, the reaction mixture was evaporated, and the residue was dissolved in a mixture of dichloromethane (15 mL) and water (5 mL). The organic layer was washed with brine, dried and concentrated under reduced pressure to obtain a residue, which was purified by flash column chromatography.
5.7.1. (Benzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl) methanone (4a)
The residue was chromatographed with EtOAc–petroleum ether 1.5:8.5 as eluent to give 4a as a white solid, yield: 96%, mp 113–115 °C. 1H NMR (CDCl3) δ: 3.93 (s, 6H), 3.96 (s, 3H), 7.19 (s, 2H), 7.42 (t, J = 8.0 Hz, 1H), 7.52 (t, J = 8.0 Hz, 1H), 7.92 (m, 3H). Anal. Calcd for C18H16O4S: C, 65.84; H, 4.91. Found: C, 65.72; H, 4.78.
5.7.2. (4-Methoxybenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4b)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 4b as a white solid, yield: 55%, mp 163–165 °C. 1H NMR (CDCl3) δ: 3.92 (s, 3H), 3.93 (s, 6H), 3.96 (s, 3H), 6.77 (dd, J = 7.6 and 1.0 Hz, 1H), 7.19 (s, 2H), 7.44 (m, 2H), 8.07 (s, 1H). Anal. Calcd for C19H18O5S: C, 63.76; H, 5.06. Found: C, 63.56; H, 4.98.
5.7.3. (5-Methoxybenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4c)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 4c as a yellow oil, yield: 78%. 1H NMR (CDCl3) δ: 3.88 (s, 3H), 3.92 (s, 6H), 3.96 (s, 3H), 7.12 (m, 2H), 7.33 (s, 2H), 7.49 (s, 1H), 7.51 (d, J = 8.4Hz, 1H). Anal. Calcd for C19H18O5S: C, 63.76; H, 5.06. Found: C, 63.61; H, 4.89.
5.7.4. (6-Methoxybenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4d)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 4d as a white solid, yield: 63%, mp 114–116 °C. 1H NMR (CDCl3) δ: 3.92 (s, 3H), 3.93 (s, 6H), 3.95 (s, 3H), 7.03 (dd, J = 8.8 and 2.4 Hz, 1H), 7.16 (s, 2H), 7.34 (d, J = 2.0 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.83 (d, J = 1.0 Hz, 1H). Anal. Calcd for C19H18O5S: C, 63.76; H, 5.06. Found: C, 63.62; H, 4.78.
5.7.5. (7-Methoxybenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4e)
The residue was chromatographed with EtOAc–petroleum ether 2:8 as eluent to give 4e as a yellow solid, yield: 68%, mp 105–107 °C. 1H NMR (CDCl3) δ: 3.92 (s, 3H), 3.96 (s, 6H), 4.03 (s, 3H), 6.89 (d, J = 7.2 Hz, 1H), 7.20 (s, 2H), 7.38 (t, J = 8.0 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.92 (s, 1H). Anal. Calcd for C19H18O5S: C, 63.76; H, 5.06. Found: C, 63.61; H, 4.92.
5.7.6. (3,4,5-Trimethoxyphenyl)(3-methylbenzo[b]thiophen-2-yl)methanone (4f)
The residue was chromatographed with EtOAc–petroleum ether 1:9 as eluent to give 4f as a white solid, yield: 56%, mp 124–126 °C 1H NMR (CDCl3) δ: 2.61 (s, 3H), 3.89 (s, 6H), 3.95 (s, 3H), 7.20 (s, 2H), 7.43 (m, 2H), 7.90 (m, 2H). Anal. Calcd for C19H18O4S: C, 66.65; H, 5.30. Found: C, 66.51; H, 5.18.
5.7.7. (4-Methoxy-4-methylbenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4g)
The residue was chromatographed with EtOAc–petroleum ether 1:9 as eluent to give 4g as a white solid, yield: 60%, mp 160–162 °C 1H NMR (CDCl3) δ: 2.73 (s, 3H), 3.89 (s, 6H), 3.94 (s, 6H), 6.73 (dd, J = 7.6 and 1.0 Hz, 1H), 7.18 (s, 2H), 7.38 (m, 2H). Anal. Calcd for C20H20O5S: C, 64.50; H, 5.41. Found: C, 64.38; H, 5.23.
5.7.8. (5-Methoxy-3-methylbenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4h)
The residue was chromatographed with EtOAc–petroleum ether 1:9 as eluent to give 4h as a white solid yield: 52%, mp 132–133 °C 1H NMR (CDCl3) δ: 2.58 (s, 3H), 3.89 (s, 3H), 3.92 (s, 3H), 3.94 (s, 3H), 3.96 (s, 3H), 7.11 (d, J = 8.8 Hz, 1H), 7.20 (s, 2H), 7.24 (s, 1H), 7.72 (d, J = 8.8 Hz, 1H). Anal. Calcd for C20H20O5S: C, 64.50; H, 5.41. Found: C, 64.32; H, 5.21.
5.7.9. (6-Methoxy-3-methylbenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4i)
The residue was chromatographed with EtOAc–petroleum ether 1.5:8.5 as eluent to give 4i as a white solid, yield: 60%, mp 130–132 °C. 1H NMR (CDCl3) δ: 2.59 (s, 3H), 3.89 (s, 3H), 3.91 (s, 3H), 3.92 (s, 3H), 3.94 (s, 3H), 7.11 (d, J = 2.8 and 8.8 Hz, 1H), 7.17 (s, 2H), 7.39 (s, 1H), 7.74 (d, J = 8.8 Hz, 1H). Anal. Calcd for C20H20O5S: C, 64.50; H, 5.41. Found: C, 64.33; H, 5.19.
5.7.10. (7-Methoxy-3-methylbenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4j)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 4j as a yellow solid, yield: 71%, mp 112–114 °C. 1H NMR (CDCl3) δ: 2.43 (s, 3H), 3.89 (s, 3H), 3.94 (s, 6H), 3.98 (s, 3H), 6.87 (d, J = 7.2 Hz, 1H), 7.12 (s, 2H), 7.42 (t, J = 8.0 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H). Anal. Calcd for C20H20O5S: C, 64.50; H, 5.41. Found: C, 64.32; H, 5.20.
5.7.11. (5,6-Dimethoxybenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4k)
The residue was chromatographed with EtOAc–petroleum ether 4:6 as eluent to give 4k as a yellow solid, yield: 62%, mp 153–155 °C. 1H NMR (CDCl3) δ: 3.93 (s, 6H), 3.95 (s, 6H), 4.00 (s, 3H), 7.17 (s, 2H), 7.26(s, 1H), 7.30 (s, 1H), 7.80 (s, 1H). Anal. Calcd for C20H20O6S: C, 61.84; H, 5.19. Found: C, 61.62; H, 5.02.
5.7.12. (6-Ethoxy-3-methylbenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4l)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 4l as a red solid, yield: 64%, mp 157–158 °C. 1H NMR (CDCl3) δ: 1.50 (t, J = 7.2 Hz, 3H), 2.58 (s, 3H), 3.89 (s, 6H), 3.94 (s, 3H), 4.14 (t, J = 6.8 Hz, 2H), 7.10 (d, J = 2.8 and 8.6 Hz, 1H), 7.16 (s, 2H), 7.29 (s, 1H), 7.75 (d, J = 8.8 Hz, 1H). Anal. Calcd for C21H22O5S: C, 65.27; H, 5.74. Found: C, 65.01; H, 5.62.
5.7.13. (6-Fluoro-3-methylbenzo[b]thiophen-2-yl)(3,4,5-trimethoxyphenyl)methanone (4m)
The residue was chromatographed with EtOAc–petroleum ether 3:7 as eluent to give 4m as a white solid, yield: 95%, mp 130–132 °C. 1H NMR (CDCl3) δ: 2.60 (s, 3H), 3.90 (s, 6H), 3.96 (s, 3H), 7.16 (s, 2H), 7.23 (m, 1H), 7.52 (dd, J = 2.6 and 8.8 Hz, 1H), 7.84 (dd, J = 5.6 and 8.6 Hz, 1H). Anal. Calcd for C19H17FO4S: C, 63.32; H, 4.75. Found: C, 63.11; H, 4.56.
5.8. Cell growth inhibitory activity
Murine leukemia L1210, murine mammary carcinoma FM3A and human T-lymphocyte Molt 4 and CEM and human cervix carcinoma (HeLa) cells were suspended at 300,000–500,000 cells/mL of culture medium, and 100 µL of a cell suspension was added to 100 µL of an appropriate dilution of the test compounds in wells of 96-well microtiter plates. After incubation at 37 °C for two days, cell number was determined using a Coulter counter. The IC50 value was defined as the compound concentration required to inhibit cell proliferation by 50%.
5.9. Effects on tubulin polymerization and on colchicine binding to tubulin
Bovine brain tubulin was purified as described previously.15 To evaluate the effect of the compounds on tubulin assembly in vitro,11 varying concentrations were preincubated with 10 µM tubulin in glutamate buffer at 30 °C and then cooled to 0 °C. After addition of GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C, and the assembly of tubulin was observed turbidimetrically. The IC50 value was defined as the compound concentration that inhibited the extent of assembly by 50% after a 20 min incubation. The ability of the test compounds to inhibit colchicine binding to tubulin was measured as described,15 except that the reaction mixtures contained 1 µM tubulin, 5 µM [3H]colchicine and 1 µM or 5 µM test compound.
5.10. Flow cytometric analysis of cell cycle distribution
For details, see Ref. 8a.
5.11. Molecular modeling
All molecular modeling studies were performed on a MacPro dual 2.66 GHz Xeon running Ubuntu 8. The tubulin structure was downloaded from the PDB data bank (http://www. rcsb.org/-PDB code: 1SA0).16 Hydrogen atoms were added to the protein, using Molecular Operating Environment (MOE) 2009.10,17 and minimized keeping all the heavy atoms fixed until a RMSD gradient of 0.05 kcal mol−1 Å−1 was reached. Ligand structures were built with MOE and minimized using the MMFF94x forcefield until a RMSD gradient of 0.05 kcal mol−1 Å−1 was reached. The docking simulations were performed using FlexX18 with the MOE interface.
Supplementary Material
Acknowledgments
Financial support was provided by GOA (Krediet no. 10/014) of the K. U. Leuven. The authors would like to thank Mrs. Lizette van Berckelaer, Dr. Alberto Casolari and Elisa Durini for technical assistance.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmc.2010.05.068.
References and notes
- 1.(a) Worderman L, Mitchison TJ. Dynamics of Microtubule Assembly In vivo. In: Hyams J, Lloyd C, editors. Microtubules. New York: Wiley_Liss, Inc; 1994. [Google Scholar]; (b) Hearn BR, Shaw SJ, Myles DC. Compr. Med. Chem. II. 2007;7:81. [Google Scholar]; (c) Pasquier E, Andrè N, Braguer D. Curr. Cancer Drug Targets. 2007;7:566. doi: 10.2174/156800907781662266. [DOI] [PubMed] [Google Scholar]
- 2.(a) Pellegrinelli F, Budman DR. Cancer Invest. 2005;23:264. doi: 10.1081/cnv-200055970. [DOI] [PubMed] [Google Scholar]; (b) Chaplin DJ, Horsman MR, Siemann DW. Curr. Opin. Invest. Drugs. 2006;7:522. [PubMed] [Google Scholar]; (c) Walczak CE. Curr. Opin. Cell Biol. 2000;12:52. doi: 10.1016/s0955-0674(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hadfield JA, Ducki S, Hirst N, McGown AT. Prog. Cell Cycle Res. 2003;5:309. [PubMed] [Google Scholar]; (b) Ducki S. Anticancer Agents Med. Chem. 2009;9:336. doi: 10.2174/1871520610909030336. [DOI] [PubMed] [Google Scholar]; (c) Kiselyov A, Bulakin KV. Anticancer Agents Med. Chem. 2007;7:189. doi: 10.2174/187152007780058650. [DOI] [PubMed] [Google Scholar]
- 4.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Experientia. 1989;45:209. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 5.Lin CM, Ho HH, Pettit GR, Hamel E. Biochemistry. 1989;28:6984. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 6.(a) Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. J. Med. Chem. 2006;49:3033. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]; (b) Mahindroo N, Liou JP, Chang JY, Hsieh HP. Expert Opin. Ther. Patents. 2006;16:647. [Google Scholar]; (c) Chaudhary A, Pandeya SN, Kumar P, Sharma PP, Gupta S, Soni N, Verma KK, Bhardwaj G. Mini-Rev. Med. Chem. 2007;7:1186. doi: 10.2174/138955707782795647. [DOI] [PubMed] [Google Scholar]; (d) Hsie HP, Liou JP, Mahindroo N. Curr. Pharm. Des. 2005;11:1655. doi: 10.2174/1381612053764751. [DOI] [PubMed] [Google Scholar]
- 7.(a) Pinney KG, Bounds AD, Dingerman KM, Mocharla VP, Pettit GR, Bai R, Hamel E. Bioorg. Med. Chem. Lett. 1999;9:1081. doi: 10.1016/s0960-894x(99)00143-2. [DOI] [PubMed] [Google Scholar]; (b) Flynn BL, Verdier-Pinard P, Hamel E. Org. Lett. 2001;3:651. doi: 10.1021/ol0067179. [DOI] [PubMed] [Google Scholar]
- 8.(a) Romagnoli R, Baraldi PG, Carrion MD, Lopez Cara C, Preti D, Fruttarolo F, Pavani MG, Tabrizi MA, Tolomeo M, Grimaudo S, Di Antonella C, Balzarini J, Hadfield JA, Brancale A, Hamel E. J. Med. Chem. 2007;50:2273. doi: 10.1021/jm070050f. [DOI] [PubMed] [Google Scholar]; (b) Romagnoli R, Baraldi PG, Jung MK, Iaconinoto MA, Carrion MD, Preti D, Tabrizi MA, Fruttarlo F, De Clercq E, Balzarini J, Hamel E. Bioorg. Med. Chem. Lett. 2005;15:4048. doi: 10.1016/j.bmcl.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 9.For a review on the mechanism and application of Newman–Kwart rearrangement see: Lloyd-Jones GC, Moseley JD, Renny JS. Synthesis. 2008;5:661. [Google Scholar]
- 10.Beck JR. J. Heterocycl. Chem. 1978;15:513. [Google Scholar]
- 11.Hamel E. Cell Biochem. Biophys. 2003;38:1. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 12.Verdier-Pinard P, Lai J-Y, Yoo H-D, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Mol. Pharmacol. 1998;53:62. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 13.(a) Lozzio CB, Lozzio BB. Blood. 1975;45:321. [PubMed] [Google Scholar]; (b) Viola G, Vedaldi D, Dall’acqua F, Fortunato E, Basso G, Bianchi N, Zuccato C, Borgatti M, Lampronti I, Gambari R. Biochem. Pharmacol. 2008;75:810. doi: 10.1016/j.bcp.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Romagnoli R, Baraldi PG, Carrion MD, Lopez-Cara C, Cruz-Lopez O, Tolomeo M, Grimaudo S, Di Cristina A, Pipitone MR, Balzarini J, Zonta N, Brancale A, Hamel E. Bioorg. Med. Chem. 2009;17:6862. doi: 10.1016/j.bmc.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamel E, Lin CM. Biochemistry. 1984;23:4173. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 16.Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Nature. 2004;428:198. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 17.Molecular Operating Environment (MOE 2007.09) Montreal, Quebec, Canada: Chemical Computing Group, Inc.; http://www.chemcomp.com. [Google Scholar]
- 18.FlexX 3.0. BioSolveIT GmbH. Germany: Sankt Augustin; http://www.biosolveit.de. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.