Abstract
Computational models suggest the hippocampus plays an important role in the retrieval of sequences. However, empirical evidence supporting hippocampal involvement during sequence retrieval is lacking. The current study used functional magnetic resonance imaging (fMRI) to examine the role of the human hippocampus during the learning and retrieval of sequences. Participants were asked to learn four sequences comprised of six faces each. An overlapping condition, where sequences shared common elements, was comprised of two sequences in which two identical faces were shown as the middle images of both sequences. A non-overlapping condition contained two sequences that did not share any faces between them. A third random condition contained two sets of six faces that were always presented in a random order. The fMRI data were split into a learning phase and an experienced phase based upon each individuals behavioral performance. Patterns of hippocampal activity during presentation, delay, and choice periods were assessed both during learning (learning phase), and after subjects learned the sequences to criteria (experienced phase). The results revealed hippocampal activation during sequence learning, consistent with previous findings in rats and humans. Critically, the current results revealed hippocampal activation during the retrieval of learned sequences. No difference in hippocampal activation was seen between the overlapping and non-overlapping sequences during either sequence learning or the retrieval of sequences. The results extend our current knowledge by providing evidence that the hippocampus is active during the retrieval of learned sequences, consistent with current computational models of sequence learning and retrieval.
Keywords: Disambiguation, memory, medial temporal lobe, fMRI, sequence
Introduction
It is well documented that the hippocampus plays an important role in the formation of conjunctive or relational representations (Cohen and Eichenbaum, 1993; Rudy and Sutherland, 1995; Kirchhoff et al., 2000; O’Reilly and Rudy, 2001; Davachi and Wagner., 2002; Davachi et al., 2003; Squire et al., 2004; Hannula and Ranganath, 2008). Recent studies have suggested that in addition to bridging relational representations between items, the hippocampus may bridge representations across time (Hasselmo and Eichenbaum, 2005; Howard et al., 2005; Manns et al., 2007).
While prefrontal and striatal regions have traditionally been associated with the temporal ordering of information (Shimamura et al., 1990; Milner et al., 1991; Kesner et al., 1994; Cabeza et al., 1997; Suzuki et al., 2002; Tinaz et al., 2006), computational models suggest the hippocampus also uses the temporal organization of information during episodic memory (Lisman, 1999; Jensen and Lisman, 2005; Hasselmo and Eichenbaum, 2005; Howard et al., 2005). Specifically, the hippocampus may use temporal information in order to selectively retrieve specific prior experiences, particularly when a representation shares common elements with a similar prior experience, such as remembering where you parked your car today vs. yesterday, or remembering who came for dinner last Thanksgiving vs. Thanksgiving two years ago (Hasselmo and Eichenbaum, 2005). In support of this hypothesis, damaging the hippocampus after learning causes impairment in the ability of rats to correctly retrieve a sequence of odors which shares some common elements with another sequence of odors (Agster et al., 2002). In humans, the hippocampus has been shown to be active during sequence learning tasks (Schendan et al., 2003). However, while both the modeling and animal experiments predict that the hippocampus should also be involved during the retrieval of sequences, this has not been demonstrated in humans.
The current study was designed to examine hippocampal activation during both the learning and retrieval of overlapping and non-overlapping sequences. Participants were asked to learn and remember two overlapping and two non-overlapping sequences comprised of six faces while undergoing fMRI scanning. Sequences of faces were used to ensure the task was independent of spatial information. The behavioral data was used to split the fMRI data into a learning phase, where participants were learning each sequence, and an experienced phase, where participants were successfully retrieving the sequences. This analysis allowed us to assess hippocampal activation during both the learning and retrieval of sequences in overlapping and non-overlapping conditions.
Materials and Methods
Participants
Twenty-one participants between the ages of 18-35 (mean age 20.7 ± 0.69 years; eight male) with normal or corrected-to-normal vision were recruited from the community at Boston University. Two participants were eliminated from the study due to excess motion during fMRI scanning. Four other participants were eliminated because of poor behavioral performance leaving fifteen subjects for analysis. Informed consent was obtained from each participant before participation in accordance with the experimental protocol approved by both the Massachusetts General Hospital Internal Review Board and the Institutional Review Board of Boston University.
Experimental Protocol
Behavioral paradigm
Pictures of 36 different faces (18 male, 18 female) served as stimuli. Each stimulus was pseudo-randomly assigned to create six groups of six faces each with the stipulation that each group contain three male and three female faces with each face appearing in only one group. Each set of six faces was randomly paired and each pair was then randomly assigned to one of three conditions; overlapping sequence, non-overlapping sequence, and random.
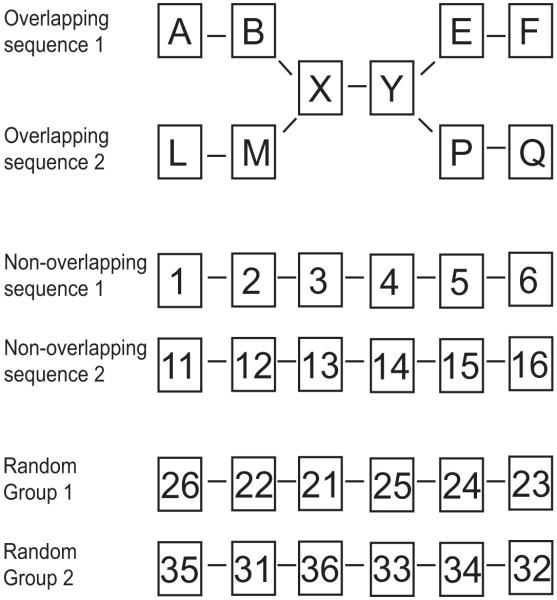
In the overlapping sequence condition, six faces were always shown in the same order and the third and fourth faces of each overlapping sequence were identical. The two sequences in the non-overlapping condition contained six faces that were always shown in the same order but did not share any faces between them. The random condition contained two sets of six faces that stayed the same across trials but were always presented in a random order (Fig. 1).
Figure 1.
Graphical representation of the overlapping, non-overlapping and random conditions. Each condition consisted of six faces represented by letters and numbers. The middle two faces of the two overlapping sequences were identical (X and Y). No faces were shared between the two non-overlapping sequences or between the two groups of faces in the random condition. Faces in the overlapping and non-overlapping sequences were always presented in the same order. The six faces in the random condition were presented in a new random order for each trial.
The task was designed to parallel the task used in rats by Agster and colleagues (2002). A trial consisted of Presentation, Delay, and Choice phases followed by an inter-trial interval (ITI) (Fig. 2). The Presentation phase consisted of the serial presentation of four faces (overlapping, non-overlapping, random) paired with a blurred face. The pairing with a blurred face was used to “force” participants to correctly choose the faces belonging in the sequence. Each stimulus was shown for four seconds. During the Delay period, a fixation cross was shown for eight seconds. In the choice phase, participants were shown two faces and asked to indicate which face was the one belonging in the sequence. The choice for the fifth face in the sequence was termed the “critical choice”, while the choice for the sixth face in the sequence was termed the “final choice”. Immediate feedback indicated to the participants whether or not their choice was correct.
Figure 2.
Behavioral paradigm. During the Presentation phase, the first four faces of a sequence were shown paired with a blurred face for 4 sec each. The Presentation phase was followed by an 8 sec Delay period where participants focused on a fixation dot. During the Choice period, participants selected between two faces in order to complete the fifth and sixth elements of the sequence. The choice for the fifth element was termed the “critical choice” and the choice for the sixth element was termed the “final choice”. Face pairs during the choice phase were presented for 4 sec and response feedback indicated to participants whether or not they were correct. The choice phase was followed by a 6 sec inter-trial-interval (ITI). A 2 sec cue was shown after the ITI to indicate to participants if the upcoming trial was going to be a sequence trial (either overlapping or non-overlapping) or a random trial. Finally, a 2 sec prompt for the participant to “Get Ready” for the next trial was shown after the cue.
Participants were explicitly instructed to learn the order of the faces presented in each sequence and were told they would be tested on their knowledge of the sequences after scanning. During the random trials, participants were instructed that the same six faces would appear in a random order each time. They were instructed to indicate via button press which of the two faces presented at the critical choice and final choice points belonged with the four faces shown during the presentation phase. Participants viewed all four sequences (two overlapping and two non-overlapping sequences) as well as the two random sets of faces in each run. The two sequences comprising the overlapping and non-overlapping conditions were always shown consecutively, as were both sets of six faces in the random condition. The order of presentation of the two sequences within a condition was split evenly across trials. The sequences used in the three conditions were counterbalanced so that each pair of six faces served as the stimuli in the overlapping, non-overlapping, and random conditions across participants.
There were 12 runs total. Each sequence was shown to the participants 12 times (for a total of 24 overlapping, 24 non-overlapping, and 24 random trials). The order of presentation of the three conditions within a run was counterbalanced across all 12 runs.
Post-scan test
After scanning, participants were administered a post-scan test to assess their knowledge of the sequences. Participants were shown all six faces of each sequence condition and were asked to order the faces by labeling them 1 through 6. Any participant that made more than two sequence errors on this test was excluded from the fMRI analysis. The participants were also asked to indicate on a 1-5 scale how difficult it was to learn each sequence with 1 being easy and 5 being difficult. A difficulty score was derived for the OL and NOL conditions by averaging the difficulty scores of the two sequences in each condition.
Imaging Acquisition
Imaging was conducted using a 3 T Siemens MAGNETOM TrioTim scanner (Siemens AG, Medical Solutions, Erlangen, Germany) with a 12-channel Tim® Matrix head coil. Two high-resolution T1-weighted multiplanar rapidly acquired gradient echo (MP-RAGE) structural scans were acquired using generalized autocalibrating partially parallel acquisitions (GRAPPA) (TR = 2530 ms; TE = 3.44 ms; flip angle = 7°;slices = 176, Field of view = 256; resolution = 1 mm × 1 mm × 1 mm). Functional T2*-weighted BOLD images were acquired using an echo planar imaging (EPI) sequence (TR = 2 s; TE = 30 ms; flip angle = 90 °; acquisition matrix = 64 × 64, Field of view = 256; slices = 32, resolution = 4.0 mm isotropic). Slices were aligned along the anterior commissure/posterior commissure line.
fMRI Pre-Processing
Imaging analysis was conducted using SPM5 (Wellcome Department of Cognitive Neurology, London, UK). The first step of preprocessing was to reorient all the BOLD images so the origin (i.e., coordinate xyz = [0 0 0]) was at the anterior commissure. Then the images were slice-time corrected to the first slice acquired in time. Motion correction was conducted next and included realigning and unwarping the BOLD images in order to correct for variance due to susceptibility by movement interactions (Andersson et al., 2001). The high-resolution structural images were then co-registered to the mean BOLD image created during the motion correction step and segmented into white and gray matter images. The bias-corrected structural images and the co-registered BOLD images were then spatially normalized into standard MNI space using the parameters derived during segmentation with resampling of the BOLD images to 2 mm3 isotropic voxels. The normalized structural images of all fifteen participants were averaged after normalization. Finally, all BOLD images were spatially smoothed using a 6 mm full-width at half-maximum Gaussian kernel.
Data Analysis
Behavioral analysis
A repeated measures ANOVA was used to assess whether differences existed between the overlapping, non-overlapping and random conditions in percent accuracy and reaction time at the critical and final choice points. Paired sample t tests were used to assess differences between conditions. Participants were excluded from the study if they scored less than 75% correct on the critical choice for either the overlapping or non-overlapping condition.
Learning phase vs. Experience phase behavioral analysis
Importantly, the behavioral data was used to split the fMRI data into a learning phase and experienced phase based on each individual participant’s behavioral performance at the critical choice point. All trials beginning with and following the trial in which participants correctly identified the critical choice stimulus six times in a row comprised the experienced phase while all trials before the experienced phase constituted the learning phase. For example, if a participant correctly identified the critical choice stimulus six times in a row beginning with the fifth run, then all trials in the fifth through twelfth run were included as part of the experienced phase whereas all trials in the first four runs were included as part of the learning phase. Six correct critical choices in a row required the participants to correctly identify the critical choice of both sets of faces in a condition three times in a row.
fMRI analysis
Twenty-four separate regressors were created for each participant as a function of condition (overlapping, non-overlapping, random), trial phase (presentation, delay, critical choice, final choice), and whether the trial was in the learning phase or experienced phase of the experiment. A single regressor was also created for the inter-trial-interval (ITI) as well as a regressor of no interest which included the two feedback periods, the trial cue, and the “Get Ready” cue. The six motion parameters obtained during the motion correction step were also added to the model as additional covariates of no interest. All regressors, except for the delay period regressors, were constructed as a series of square waves (or “box-cars”) using the onset of each event. The length of the square wave was set to equal the duration of stimulus presentation and then convolved with the hemodynamic response function in SPM5. In the case of the critical choice and final choice regressors, the length of the box car was determined by the reaction time of the participants for that particular trial. In order to minimize collinearity between regressors, delay period activity was modeled similarly to previous studies which examined delay related activity (Courtney et al., 1998, Schon et al., 2004). The design matrix was then analyzed using the General Linear Model approach in SPM5.
T-contrasts contrasting the four trial phases (presentation, delay, critical choice and final choice) of the overlapping sequence condition with the corresponding trial phase in the random condition were constructed. This analysis allowed us to assess activation in the hippocampus during both the learning and experienced phase of the overlapping sequence condition (e.g. learning phase overlapping presentation > learning phase random presentation; experienced phase overlapping presentation > experienced phase random presentation). Activation during the processing of non-overlapping sequences was also examined by constructing T-contrasts and contrasting all four trial phases of the non-overlapping condition with the corresponding trial phase of the random condition during the learning and experienced phase of the experiment. In order to assess whether hippocampal activation was greater during sequence disambiguation, activation during the presentation, delay, and critical choice period of the overlapping sequence was contrasted with activation during the corresponding non-overlapping sequence while participants were learning the sequences and after participants knew the sequences (experienced phase). T-contrasts were also constructed to assess the presentation, delay, and critical choice phase of each condition in the learning phase compared to the ITI (e.g. learning overlapping critical choice > ITI; learning non-overlapping delay > ITI). Group averaged Statistical Parametric Maps (SPMs) were created by entering the resulting contrast image into a one-sample t test using participant as a random factor. T-contrasts were constructed to examine whether there were hippocampal regions more active during the experienced phase compared to the learning phase (and vice versa). The group SPMs were corrected for multiple comparisons across the whole brain using a False Discovery Rate (FDR) of p < 0.05 with 5 as the voxel extent. Parameter estimates (beta-weights) were extracted directly from SPM5 and averaged across participants. Since the primary focus of this experiment was to examine hippocampal involvement in the processing of both overlapping and non-overlapping sequences, the discussion of results is limited to the hippocampal formation.
Results
Behavioral results
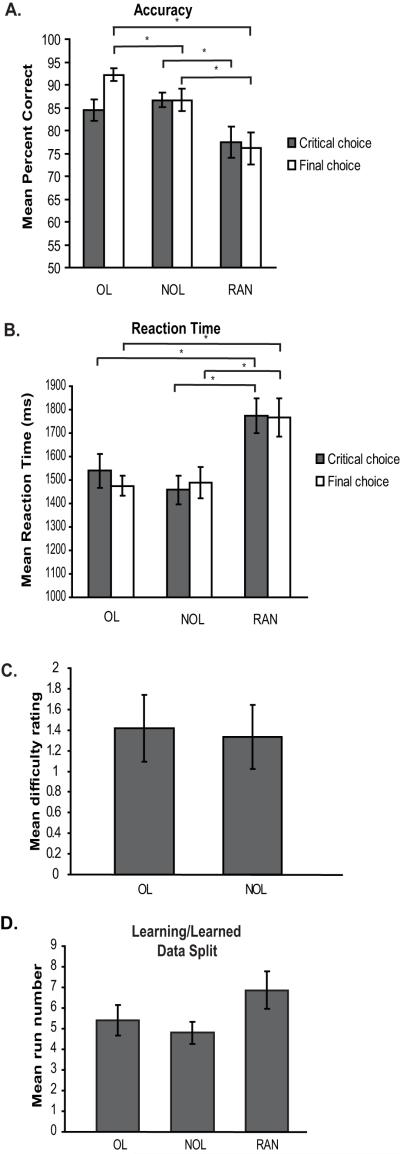
A one-way repeated measures ANOVA revealed a significant difference in percent accuracy across the overlapping (OL), non-overlapping (NOL), and random (RAN) conditions at both the critical choice (F(2,28) = 3.489, p = 0.044) and final choice (F(2,28) = 11.229, p < 0.001). The mean ± SEM percent accuracy for the OL, NOL, and RAN conditions at the critical choice were 84.44 ± 2.35%, 86.67 ± 1.64%, and 77.50 ± 3.42% respectively (Fig. 3a). Paired-sample t tests revealed a significant difference between the NOL and RAN conditions (t(14) = 2.310, p = 0.037) at the critical choice. No difference was seen between the OL and RAN conditions at the critical choice (t(14) = 1.1710, p = 0.109). Importantly, no significant difference was found between the OL and NOL conditions at the critical choice (t(14) = 0.835, p = 0.418). At the final choice, the mean ± SEM was 92.22 ± 1.46% for the OL condition, 86.67 ± 2.41% for the NOL condition and 76.11 ± 3.46% for the RAN condition (Fig. 3a). Paired-sample t tests revealed a significant difference in percent accuracy at the final choice between the OL and NOL conditions (t(14) = 2.354, p = 0.034), the OL and RAN conditions (t(14) = 4.405, p = 0.001), and the NOL and RAN conditions (t(14) = 2.572, p = 0.022).
Figure 3.
Behavioral results. (A) Mean percent accuracy at the critical choice point (grey bars) and final choice point (white bars) for the overlapping sequences (OL), non-overlapping sequences (NOL) and random (RAN) conditions. (B) Mean reaction time in milliseconds at the critical choice point (grey bars) and final choice point (white bars). (C) Mean difficulty rating. The mean difficulty rating provided by participants during post-scan testing for both the overlapping (OL) and non-overlapping (NOL) conditions. (D) Mean run number at which participants reached the behavioral criteria and the data split into the learning and experienced phase for each condition. Asterisks denote a significant difference between groups (* p < 0.05).
A one-way repeated measures ANOVA also revealed a significant difference in reaction times across all three conditions at both the critical choice (F(2,28) = 9.352, p = 0.001) and final choice (F(2,28) = 10.908, p < 0.001). The mean ± SEM reaction time in ms for the OL, NOL, and RAN conditions at the critical choice were 1539.78 ± 72.65 ms, 1458.18 ± 60.81 ms, and 1774.49 ± 74.28 ms respectively (Fig. 3b). Paired-sample t tests revealed significant differences in reaction time at the critical choice between the OL and RAN conditions (t(14) = 4.026, p = 0.001) and NOL and RAN conditions (t(14) = 3.784, p = 0.002). There was no difference found in reaction time between the OL and NOL conditions. The reaction times at the final choice were 1475.62 ± 43.21 ms for the OL condition, 1487.69 ± 66.68 ms for the NOL condition and 1766.55 ± 81.35 ms for the RAN condition (Fig. 3b). At the final choice, paired-sample t tests revealed significant differences in reaction time between the OL and RAN conditions (t(14) = 4.792, p < 0.001) and NOL and RAN conditions (t(14) = 3.263, p = 0.006). As a whole, the behavioral results suggest that the random condition was more difficult than both the overlapping sequence and non-overlapping sequence conditions. The behavioral finding that the sequence conditions were easier than the random condition suggests that participants benefit from learning the order of the faces in the sequence conditions. Critically, no difference in either percent accuracy or reaction time was found at the critical choice between the OL and NOL sequence conditions, suggesting the OL and NOL sequence conditions were of equal difficulty.
Post-scan test results
The results of the post-scan tests provide further evidence of a lack of difference in difficulty between the OL and NOL conditions. Participants subjective ratings of how difficult it was to learn each sequence were not significantly different (t(11) = 1.00, p = 0.339; mean OL difficulty, 1.417 ± 0.325, mean NOL difficulty, 1.333 ± 0.310, Fig. 3c).
Importantly, there was no significant difference in the number of errors made on the post-scan reordering task. Participants scored 100% correct when reordering the OL faces and 98.61% correct when reordering the NOL sequences. This finding demonstrates successful sequence learning.
Behavioral results for learning phase and experienced phase
The data was split for subsequent fMRI analysis into a learning phase and experienced phase separately for each condition at the point where each individual participant’s performance reached criteria. The mean run where participants reached the behavioral criteria of six correct critical choices in a row was 5.4 ± 0.75 in the OL condition, 4.80 ± 1.44 in the NOL condition and 6.8667 ± 1.87 in the RAN condition (Fig. 3d). A one-way repeated measures ANOVA revealed no significant difference between conditions at the run where participants reached behavioral criteria (F(2,28) = 2.319, p = 0.117).
fMRI Results
Results from the fMRI analysis consistently revealed hippocampal activation during retrieval of both overlapping and non-overlapping sequences compared to the random condition. Hippocampal activation was also seen during the learning of both overlapping and non-overlapping sequences. The results also revealed that the hippocampus was more active during the retrieval of sequences than during sequence learning, suggesting the hippocampus is involved in both sequence learning and the retrieval of learned sequences.
Presentation phase after participants learned the sequences
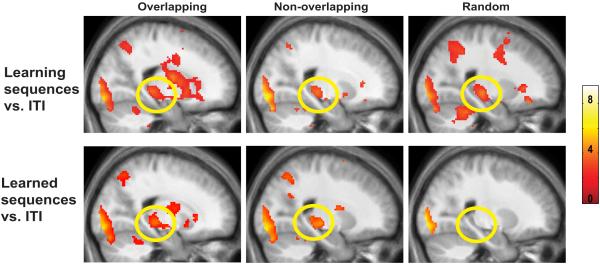
Hippocampal activation during the presentation phase was assessed after the participants learned the sequences to criteria by comparing activation during the presentation phase of the overlapping (OL) and non-overlapping (NOL) sequences with the random (RAN) condition during the experienced phase of the experiment (OL presentation experienced phase > RAN presentation experienced phase; NOL presentation experienced phase > RAN presentation experienced phase). Greater hippocampal activation was seen during the presentation phase of the OL trials compared to the presentation phase of the RAN trials (Fig. 4 top left panel), as well as during the presentation phase of the NOL trials compared to the RAN trials (Fig. 4 bottom left panel) after participants had acquired the correct sequence order (experienced phase). The finding of greater hippocampal activation during the viewing of the first four faces of a learned sequence compared to the viewing of four faces presented in random order during the experienced phase suggests the hippocampus is important for the retrieval of known sequences.
Figure 4.
Hippocampal activation after participants learned the sequences to criteria. The top panel shows hippocampal activation during the presentation phase (left) and critical choice phase (right) of the overlapping compared to the random condition (OL > RAN) while the bottom panel shows hippocampal activation during the presentation phase (left) and critical choice phase (right) of the non-overlapping condition compared to the random condition (NOL > RAN). Yellow arrows point towards hippocampal activation. Activations shown are FDR corrected to p < 0.05 voxel extent of 5. (OL = overlapping, NOL = non-overlapping, RAN = random, L = left hemisphere, R = right hemisphere).
Presentation phase during sequence learning
In contrast to the experienced phase, there were no significant differences in hippocampal activity between the presentation phase of the OL and RAN or the NOL and RAN conditions during the learning phase. However, we wanted to further explore whether there was hippocampal activation during the presentation portion of the learning phase since previous animal and fMRI research (Fortin et al., 2002; Schendan et al., 2003) has shown that the hippocampus is involved in sequence learning. Therefore, the presentation phase of the OL, NOL, and RAN conditions were each compared to the inter-trial-interval (ITI). Greater hippocampal activation was seen in all three experimental conditions during the learning phase when compared to the ITI (Fig. 5 top panel), replicating previous findings which have demonstrated that the hippocampus is involved in sequence learning (Fortin et al., 2002; Schendan et al., 2003).
Figure 5.
Sagittal sections through the right hippocampus (y = −32) for the presentation phase of the overlapping, non-overlapping, and random conditions. The top panel shows activations during the learning phase compared to the ITI for all three conditions while the bottom panel shows activations after participants had learned the sequences (experienced phase). The hippocampus was active during the presentation phase of all three conditions in the learning phase, but only the sequence conditions show hippocampal activation after the sequences were learned. Activations shown are FDR corrected to p < 0.05 voxel extent of 5. All hippocampal activations depicted were bilateral.
For comparison with the learning phase, we also contrasted the presentation phase of the OL, NOL, and RAN conditions during the experienced phase to the ITI. As demonstrated by the results presented in Figure 5, increases in hippocampal fMRI activity were found after participants had learned the correct sequence order (Fig. 5 bottom panel) during the presentation phase of the OL and NOL condition compared to the ITI, but not during the RAN presentation phase compared to the ITI. Hippocampal activation during the presentation phase of the OL and NOL sequences in the experienced phase provides evidence of hippocampal involvement during sequence retrieval. Together, hippocampal activation during sequence learning and the retrieval of sequences suggests the hippocampus plays a role in both learning and retrieval of temporal order.
Critical Choice after participants learned the sequences
We were particularly interested in examining hippocampal activity at the critical choice point since a correct answer at the critical choice point after participants had learned the OL and NOL sequences to criteria suggests successful retrieval of the sequences. The fMRI analysis revealed greater hippocampal activation during the critical choice phase of the OL trials compared to RAN trials (Fig. 4 top right panel) as well as during the critical choice of NOL trials compared to RAN trials (Fig. 4 bottom right panel) after participants had learned the sequences to criteria. The greater hippocampal activation seen during the critical choice of the OL and NOL sequences compared to the RAN condition after participants learned the sequences to criteria is most likely due to the successful retrieval of the sequence order.
Experienced vs. Learning phase at the Critical Choice
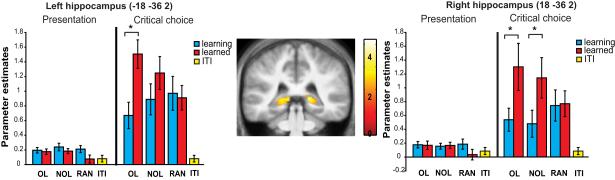
Activation during the critical choice of the experienced phase was directly contrasted with the activity during the critical choice of the learning phase in order to determine whether there was greater recruitment of the hippocampus during learning or after participants had learned the sequences to criteria. A region in the posterior hippocampus showed greater hippocampal activation during the critical choice of the OL and NOL sequences during the experienced phase compared to the learning phase (Fig. 6). Parameter estimates were extracted from the activated region of the left hippocampus (MNI coordinates −18, −36, 2) and right hippocampus (18, −36, 2) and revealed that all hippocampal signal changes, including hippocampal signal change during the presentation phase, were in the positive direction.
Figure 6.
Parameter estimates during sequence learning (blue bars) and after participants learned the sequences (red bars) of the overlapping condition (OL), non-overlapping condition (NOL), random condition (RAN), and ITI (yellow bars) for the left and right hippocampus at MNI coordinates ±18 −36 2. The left side of each bar graph shows parameter estimates for the presentation phase with the right side showing the parameter estimates for the critical choice. The center shows greater hippocampal activation during the critical choice of the experienced phase compared to the critical choice of the learning phase in the OL condition. Data shown are masked.
The parameter estimates were also used to examine hippocampal activation during the critical choice of the learning phase. A one-way repeated measures ANOVA revealed a difference between the three experimental conditions (OL, NOL, and RAN) and the ITI during the critical choice of the learning phase in the left hippocampus (F(3,42) = 10.639, p < 0.001) and right hippocampus (F(3,42) = 7.445, p < 0.001). Paired sample t tests revealed that the OL (left hippocampus t(14) = 3.623, p = 0.003; right hippocampus t(14) = 3.895, p = 0.002), NOL (left hippocampus t(14) = 4.358, p = 0.001; right hippocampus t(14) = 3.858, p = 0.002, and RAN (left hippocampus t(14) = 4.381, p = 0.001; right hippocampus t(14) = 4.095, p = 0.001, Fig. 6) conditions all had higher parameter estimates than the ITI. Greater hippocampal activation during the critical choice period of the learning phase is in line with the hypothesis that the hippocampus plays a role in sequence learning. Taken together, these results demonstrate that the hippocampus is active during both sequence learning and sequence retrieval and that the hippocampus is more strongly engaged during the retrieval of sequences.
Disambiguation of overlapping sequences
In order to determine whether there was an increased demand on the hippocampus during the learning and retrieval of sequences which have common elements, the presentation, delay, and critical choice portions of the OL condition were contrasted with the NOL condition in both the learning phase and experienced phase of the experiment (e.g. learning phase overlapping presentation > learning phase non-overlapping presentation; experienced phase overlapping critical choice > experienced phase non-overlapping critical choice). There were no differences found in any part of the OL trial compared to the NOL trial during the learning phase or during the experienced phase. A lack of difference between the OL and NOL sequences suggests the hippocampus was equally active during both the learning and retrieval of the OL and NOL sequences.
Delay period and Final choice
No difference was found in the hippocampus between the delay period of the OL or NOL sequence compared to the RAN condition in either the learning or experienced phase of the experiment. There was also no difference found between the delay period of the OL, NOL, and RAN condition compared to the ITI. Finally, no difference was found between the OL and NOL conditions compared to the RAN condition at the final choice in either the learning or experienced phase of the experiment.
Discussion
A defining characteristic of episodic memory is that the events comprising an episode are linked together in time (Tulving, 1984). One hypothesis proposes that it is the role of the hippocampus to link the events comprising an episodic memory together in a sequential manner across time (Levy, 1996, Howard et al., 2005), particularly if there is interference from similar prior experiences (Hasselmo and Eichenbaum, 2005; Zilli and Hasselmo, 2008). In this study, we set out to examine hippocampal involvement during the learning and retrieval of overlapping and non-overlapping sequences in a non-spatial sequence task. Our task was specifically designed to parallel a previous study conducted in rats (Agster et al., 2002). Our results demonstrate that the hippocampus is active during the learning of both overlapping and non-overlapping sequences (Fig. 5). Importantly, the hippocampus is also active during the retrieval of overlapping and non-overlapping sequences (Fig. 4), extending the role of the hippocampus in sequence learning to also include the retrieval of learned sequences.
Hippocampal activation during sequence retrieval
The current study demonstrates that the hippocampus is strongly engaged when participants view the first four faces of sequences that have been learned, regardless of overlap (Fig. 4). The hippocampus is also strongly activated when choosing the fifth face in a learned sequence (Fig. 4). Together, these findings provide evidence in humans that the hippocampus is involved in the retrieval of both overlapping and non-overlapping sequences.
Computational models suggest the hippocampus works with the entorhinal cortex in order to read out the sequence when an item from the sequence is presented (Jensen and Lisman, 1996; Lisman, 1999; Hasselmo and Eichenbaum, 2005; Hasselmo, 2007). It may be that the greater hippocampal activation seen during the presentation phase after participants had learned the sequence was a result of the hippocampus reading out the sequence. In 1985, Tulving proposed that a critical aspect of episodic memory is the ability to use previous experiences to predict future events (Tulving, 1985). During the post-scan interview, participants initially unable to identify the correct critical choice face reported being able to predict, or “see into the future”, upcoming stimuli. These participants knew which face was going to be the correct choice at the critical choice point after viewing only the first or second face in the presentation phase. This ability to predict upcoming stimuli typically occurred sometime in the middle of the experiment and closely coincided with the beginning of the experienced phase.
At the critical choice point, participants were asked to indicate if they knew which sequence they were presently viewing by choosing the correct fifth face in the sequence. When participants were consistently identifying the fifth face of the sequences correctly (i.e. in the experienced phase), there was a greater recruitment of the hippocampus than while participants were learning the sequences. The finding of greater hippocampal activation at the critical choice of the experienced phase compared to the learning phase (Fig. 6) is consistent with the hypothesis that the hippocampus plays a role in the retrieval of sequences.
Hippocampal activation during sequence learning
Our results demonstrate that the hippocampus is active during the learning of both overlapping and non-overlapping sequences. This result is consistent with previous work showing that the hippocampus is important for learning the order of events (Downes et al., 2002; Fortin et al., 2002; Kesner et al., 2002; Schendan et al., 2003; Hopkins et al., 2004). Prior research has shown that hippocampal activation correlates more strongly with a participant’s specific learning rate during the learning of overlapping compared to non-overlapping sequences (Kumaran and Maguire, 2006). In contrast to these findings, our results revealed no difference in the amount of hippocampal activation during the learning of overlapping and non-overlapping sequences. One possible explanation may be that the degree of overlap between the two overlapping sequences was not significant enough to warrant an increase in hippocampal activation in our task. The overlapping sequences in the present study were comprised of six elements with two consecutive common faces. In the previous study examining sequence disambiguation in humans, the sequences were comprised of 12 items with 4 elements in common (Kumaran and Maguire, 2006). The increase in both the number of items and the amount of overlap between the sequences may account for why they found greater hippocampal activation during the learning of overlapping sequences Alternatively, it may be that that the sequences need to be over-learned to detect differential hippocampal activity between the overlapping and non-overlapping sequences. In the Agster et al. (2002) study, rats were given more than 250 trials to learn the sequences. On average, participants in the current experiment reached behavioral criteria after about 10 trials. If the hippocampus is needed to disambiguate sequences, one might predict hippocampal activation during the retrieval of an overlapping sequence no matter how well the sequence was learned whereas hippocampal involvement during the retrieval of well learned non-overlapping sequences would decrease as other brain regions such as the striatum or prefrontal cortex become involved (Shimamura et al., 1990; Milner et al., 1991; Kesner et al., 1994; Cabeza et al., 1997; Suzuki et al., 2002; Willingham et al., 2002; Schendan et al. 2003). Our results do not show differential hippocampal activity between the overlapping and non-overlapping sequences after using FDR correction for multiple comparisons. However, at a reduced threshold of p < 0.01, a difference in hippocampal activation can be seen between the overlapping and non-overlapping sequences at the critical choice point after participants learned the sequences to criteria. Future studies could examine hippocampal activation during the retrieval of overlapping sequences after extensive pre-training.
Alternate computational models have attempted to determine what role the hippocampus plays in sequence retrieval. An initial model obtained accurate memory guided behavior using sequence retrieval at all points of a task, regardless of whether the sequences were overlapping or non-overlapping (Hasselmo and Eichenbaum, 2005). In contrast, a more recent model performed memory guided behavior after selectively learning to perform sequence retrieval only during points of overlap when it was necessary to disambiguate a choice (Zilli and Hasselmo, 2008). The more recent model requires less activity for performing a task, but requires a longer learning period. The fMRI data obtained here supports the initial model in which sequence retrieval within the hippocampus occurs at all points of a task, regardless of the amount of overlap.
In conclusion, the present findings are consistent with previous work in animals and humans suggesting that the hippocampus plays an important role in sequence learning (Fortin et al., 2002; Kesner et al., 2002; Schendan et al., 2003; Kumaran and Maguire, 2006). Importantly, the results of the current study also provide evidence that the hippocampus is active during the retrieval of learned sequences, consistent with computational models of sequence retrieval (Levy, 1996; Jensen and Lisman, 1996; Hasselmo and Eichenbaum, 2005).
Acknowledgements
This work was conducted with the support of the Cognitive Neuroimaging Laboratory, Silvio O. Conte Center for Memory and Brain (NIH P50 MH071702), Boston University (Boston, MA) and the Athinoula A. Martinos Center for Biomedical Imaging (NCRR P41RR14075), MGH/MIT/HMS (Charlestown, MA). We would like to thank Karin Schon and Michael Hasselmo for helpful discussion about the design of the study and helpful comments on this manuscript.
Grant sponsor: National Institutes of Health; National Center for Research Resources
Grant number: P50 MH071702; P41RR14075
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J Neurosci. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, Tulving E. Brain regions differentially involved in remembering what and when: A PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Mayes AR, MacDonald C, Hunkin NM. Temporal order memory in patients with Korsakoff’s syndrome and medial temporal amnesia. Neuropsychologia. 2002;40:853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum H. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Arc length coding by interference of theta frequency oscillations may underlie context-dependent hippocampal unit data and episodic memory function. Learn Mem. 2007;14:782–794. doi: 10.1101/lm.686607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Waldram K, Kesner RP. Sequences assessed by declarative and procedural tests of memory in amnesic patients with hippocampal damage. Neuropsychologia. 2004;42:1877–1886. doi: 10.1016/j.neuropsychologia.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: Toward a common explanation of medial temporal lobe function across domains. Psychol Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: Accounting for the phase precession of place cells. Learn Mem. 1996;3:279–287. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32:881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Marin A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The dynamics of hippocampal activation during encoding of overlapping sequences. Neuron. 2006;49:617–629. doi: 10.1016/j.neuron.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: Recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgments. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: An appraisal and reconfiguration. Hippocampus. 1995;5:375–389. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: A functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Fujii T, Tsukiura T, Okuda J, Umetsu A, Nagasaka T, Mugikura S, Yanagawa I, Takahashi S, Yamadori A. Neural basis of temporal context memory: A functional MRI study. Neuroimage. 2002;17:1790–1796. doi: 10.1006/nimg.2002.1303. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Schon K, Stern CE. Evidence for the importance of basal ganglia output nuclei in semantic event sequencing: An fMRI study. Brain Res. 2006:239–249. doi: 10.1016/j.brainres.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Tulving E. Précis of elements of episodic memory. Behav Brain Sci. 1984;7:223–268. [Google Scholar]
- Tulving E. Memory and consciousness. Can. Psychol. 1985;26:1–12. [Google Scholar]
- Willingham DB, Salidis J, Gabrieli DE. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Zilli EA, Hasselmo ME. Modeling the role of working memory and episodic memory in behavioral tasks. Hippocampus. 2008;18:193–209. doi: 10.1002/hipo.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]