Abstract
Serotonin (5-HT) appears to play a major role in controlling adult hippocampal neurogenesis and thereby it is relevant for theories linking failing adult neurogenesis to the pathogenesis of major depression and the mechanisms of action of antidepressants. Serotonergic drugs lacked acute effects on adult neurogenesis in many studies, which suggested a surprisingly long latency phase. Here we report that the selective serotonin reuptake inhibitor fluoxetine, which has no acute effect on precursor cell proliferation, causes the well-described increase in net neurogenesis upon prolonged treatment partly by promoting the survival and maturation of new postmitotic neurons. We hypothesized that this result is the cumulative effect of several 5-HT-dependent events in the course of adult neurogenesis. Thus, we used specific agonists and antagonists to 5-HT1a, 2, and 2c receptor subtypes to analyze their impact on different developmental stages. We found that 5-HT exerts acute and opposing effects on proliferation and survival or differentiation of precursor cells by activating the diverse receptor subtypes on different stages within the neuronal lineage in vivo. This was confirmed in vitro by demonstrating that 5-HT1a receptors are involved in self-renewal of precursor cells, whereas 5-HT2 receptors effect both proliferation and promote neuronal differentiation. We propose that under acute conditions 5-HT2 effects counteract the positive proliferative effect of 5-HT1a receptor activation. However, prolonged 5-HT2c receptor activation fosters an increase in late-stage progenitor cells and early postmitotic neurons, leading to a net increase in adult neurogenesis. Our data indicate that serotonin does not show effect latency in the adult dentate gyrus. Rather, the delayed response to serotonergic drugs with respect to endpoints downstream of the immediate receptor activity is largely due to the initially antagonistic and un-balanced action of different 5-HT receptors.
Keywords: fluoxetine, serotonin receptors, adult neurogenesis, dentate gyrus, stem cell
Introduction
Serotonin is long known as a potent regulator of adult hippocampal neurogenesis (Brezun and Daszuta, 1999, 2000; Radley and Jacobs, 2002; Santarelli et al., 2003; Banasr et al., 2004), but data on how this stimulation is actually achieved are limited and partially contradictory. Given the controversies over the ideas that adult neurogenesis might underlie the pathogenesis of depression and that adult neurogenesis might be necessary for antidepressant action (Jacobs et al., 2000; D'Sa and Duman, 2002; Sahay and Hen, 2007; Kempermann et al., 2008), the potential role of serotonin-based antidepressants in the regulation of adult neurogenesis have became a matter of broader interest. Malberg et al. (2000) were the first to show that cell proliferation and the number of newborn neurons derived from the adult subgranular zone (SGZ), the neurogenic niche in the adult hippocampal dentate gyrus, is increased after prolonged treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine (Flx). The neurogenic response had a latency of a few weeks. This delay was interpreted as possibly explaining the often reported latency in the clinical effect of antidepressants. It was postulated that newly generated neurons stimulated by Flx would require a few weeks to mature before they might contribute to the antidepressant action. However, this conclusion has always been debatable since an increase in precursor cell proliferation, not in neuronal maturation showed the characteristic delay. From a clinical perspective the putative effect latency is now also questionable because newer studies directly focusing on this issue failed to confirm any delayed effectiveness (Posternak and Zimmerman, 2005; Katz et al., 2006a,b; Mitchell, 2006).
Although, the effect of Flx on the number of proliferating cells in the dentate gyrus was first detectable at 14 days in the Malberg study, there were already suggestive mean differences at earlier time points. We therefore reasoned that there might indeed be acute effects of serotonin on adult neurogenesis that were somehow masked by the experimental design. A detailed study by Banasr et al. (2004), who had detected effects on cell proliferation, pointed into the same direction. Originating from our work on differential acute and chronic effects of voluntary wheel running on different stages of adult neurogenesis (Kronenberg et al., 2003; Steiner et al., 2008), we hypothesized that such balanced effect might also exist for serotonin-dependent regulation of adult neurogenesis. Our idea was that various serotonin receptors might have diverse, possibly opposing effects on different stages of neuronal development in the adult dentate gyrus leading to different net outcomes dependent on the time-point of investigation.
In a very detailed analysis, Encinas et al. have been the first to identify the cell types in the course of adult neurogenesis, on which serotonin (or the SSRI) acted. Two weeks of treatment with Flx increased the number of nestin-GFP-positive, non-radial precursor cells in the SGZ (Encinas et al., 2006), type-2a cells in our nomenclature (Kempermann et al., 2004). Encinas et al. suggested that the increase in type-2a cells was due to increased symmetric divisions as opposed to increased asymmetric divisions of the type-1 cells. Here, too, chronic treatment with Flx resulted in a net increase in adult neurogenesis. The authors suggested a sole effect of serotonin on precursor cell proliferation. This interpretation, however partially contradicts the diverse effects serotonin has on neuronal development in the early embryonic period (Gaspar et al., 2003).
We thus designed the present experiment in order to identify a potential mechanism that might help to explain the discrepancies between different experimental studies. We used a combination of in vivo and cell culture models to study in detail the acute and more chronic effects of serotonergic stimulation on the regulation of adult neurogenesis.
Materials and Methods
Animals and housing conditions
Six to eight weeks old adult female C57Bl/6 mice were purchased from Charles River. The second strain we used is a transgenic mouse expressing GFP driven by regulation of the nestin gene (Yamaguchi et al., 2000). We have previously used this approach to characterize nestin-expressing progenitor cells in the dentate gyrus (Kronenberg et al., 2003). A third generation of mice expressing enhanced (E) GFP under the DCX promoter (Gong et al., 2003) was used to investigate the expression of 5-HT receptors on newly born cells within their developmental steps (type-2b, type-3 and early immature postmitotic neurons) in adult hippocampal neurogenesis. The BAC transgenic mouse line has been developed within the Gene Expression Nervous System Atlas (GENSAT) BAC Transgenic Project. Breeding with FVBN mice at the animal facility of the Max Delbrück Center, Berlin, generated the transgenic offspring.
Mice were held five per cage under standard laboratory housing conditions with a light/dark cycle of 12 h each and free access to food and water. All experiments were performed according to national and institutional guidelines and were approved by an official committee (LaGeSo, Berlin, Germany). Fourteen animals per experiment were randomly distributed into two groups with the same size. One group received the drug, whereas saline was injected to the control group.
Drug treatments and experimental design
For all experiments, animals received a single i.p. injection of bromodeoxyuridine (BrdU, 50 mg/kg body weight; Sigma, Deisenhofen, Germany) and were killed according to the experimental design in Figure 1. A single BrdU injection is adequate and in accordance to our earlier studies (Kronenberg et al., 2003; Steiner et al., 2008).
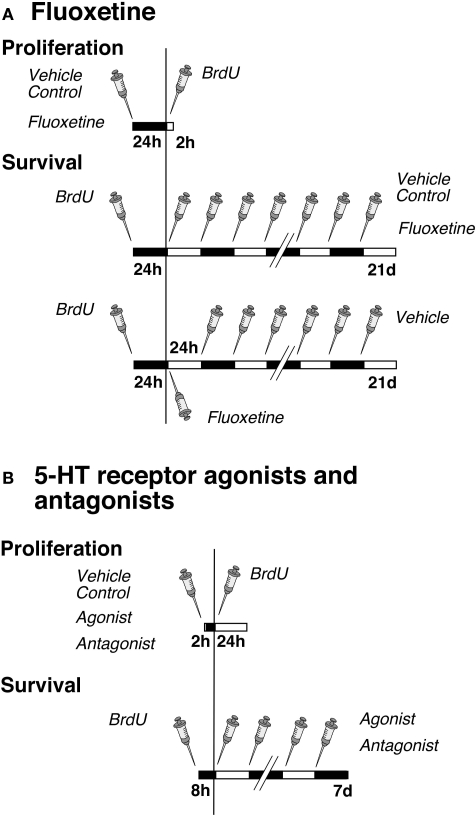
Figure 1.
Experimental design. (A) Time course of fluoxetine treatment for proliferation and survival experiments. Mice were injected either with NaCl (Vehicle Control) or fluoxetine once followed by one single injection of BrdU 24 h later. The survival paradigm is divided into two parts: Mice were injected first with BrdU followed by a daily administration of either NaCl or fluoxetine over 21 days; a third group received one single injection of fluoxetine followed by NaCl (Vehicle) once daily over 20 days. (B) Time course of serotonin receptor agonist and antagonist treatments for proliferation and survival experiments. Mice were injected once with NaCl (Vehicle Control), the antagonist or agonist followed by one single injection of BrdU after 2 h, and they were killed 24 h later. For cell survival animals were injected first with BrdU followed 8 h later by a daily administration of NaCl, the antagonist or agonist over 7 days.
The antidepressant Flx and pharmacological specific agonists and antagonists were used to dissect the interplay of various serotonin receptor subtypes in regulation of adult hippocampal neurogenesis. All drugs used for pharmacological manipulation of receptors were purchased from TOCRIS (Tocris Bioscience, Biozol, Germany) and were administered intraperitoneally (i.p.).
The experimental design for the Flx experiment was split into three parts (Figure 1). In the first setting we analyzed the number of dividing precursor cells at 1 day after one BrdU injection to investigate an acute effect of the drugs on cell proliferation. The mice were first injected with Flx (10 mg/kg body weight in 0.9% saline) or 0.9% saline vehicle and 24 h later with BrdU. The animals were then killed after 2 h. The second design focused on the potential effects of daily drug administration over 21 days on cell differentiation and survival of newly born neurons. Here BrdU was given once before the injection of Flx. The third part investigated a lasting effect of precursor cell survival after one single injection of Flx followed by 20 days of 0.9% saline vehicle.
The investigation of the role of serotonin receptor subtypes in adult neurogenesis contained two parts per experiment (Figure 1). In the first part, the effect on cell proliferation was examined. One group of mice was first injected with selective receptor agonists or antagonists or 0.9% saline vehicle (see following paragraphs for details). One single injection of BrdU followed 2 h later (it takes 2 h to let the drugs take effect). Mice were killed after 24 h. In the second group of animals BrdU was given 8 h (to let the marker be incorporated by as many as proliferating cells) before initiation of receptor stimulation or blocking. Cell differentiation and survival was discovered by a daily agonist or antagonist injection over 7 days.
5-HT1a receptor agonist and antagonist
For the in vivo experiment, the standard selective 5-HT1a agonist 8-OH DPAT (8-hydroxy-2-dipropylaminotetralin hydrobromide) and WAY100135 (S-N-tert-Butyl-3-(4-(2-methoxyphenyl))-piperazin-1-yl)-2-phenylpropanamide dihydrochloride), a potent selective 5-HT1a receptor antagonist with partially agonistic affinity, were dissolved in 0.9% saline. Dose calculations were based on (Rodgers and Cole, 1994; Yoshitake and Kehr, 2004). One group of mice received either 8-OH DPAT (1 mg/kg dissolved in saline, 0.1 ml/20 g) or WAY100135 (10 mg/kg dissolved in saline, 0.1 ml/20 g), and the control group received an equivalent volume of saline. For the in vitro part, another 5-HT1a receptor antagonist was used, Nan-190 (3 μM), which did not worked successfully in vivo. In addition, a second agonist was tested in its role on regulation, Ipsapirone (0.1 μM).
5-HT2 and 5-HT2c receptor agonists and antagonists
Cinanserin hydrochloride (N-(2-((3-(Dimethylamino)propyl)thio)phenyl)-3-phenyl-2-propenamide hydrochloride) is a widely used drug, which blocks the entire 5-HT2 receptor family. One group of mice received 2 mg/kg body weight of Cinanserin dissolved in saline. Another group of animals was treated with α-methyl-5-hydroxytryptamine-maleate, which is used as an entire 5-HT2 receptor family agonist with high affinity in the following concentration: 0.5 mg/kg body weight.
The potent and selective 5-HT2c receptor agonist WAY161503 (8.9-dicloro-2.3.4.4a-tetrahydro-1H-pyrazino(1.2-a)quinoxalin-5(6H)-one hydrochloride) has partially 5-HT2a agonistic activity and is used as an antidepressant following systemic administration in vivo. Dose calculations were based on (Welmaker et al., 2000; Cryan et al., 2004). One group of mice received 10 mg/kg per body weight of WAY161503 dissolved saline; the control group received an equivalent volume of saline.
Cell culture
Precursor cells were cultured from 8- to 12-weeks-old female C57Bl/6 animals. The mice were sacrificed and the dentate gyrus dissected out. The tissue from five to six animals was pooled. The tissue chunk was digested with papain, Dispase, and DNAse for 30–40 min. After washing with PBS, the single cell suspension was suspended in a 22 % percoll. This suspension was then centrifuged at 1500 rpm for 15 min. The supernatant was discarded and the pellet after washing in several rounds of PBS was resuspended in media and plated. The surface was coated with Poly-D-lysine 10 μg/ml and laminin 5 μg/ml.
For self-renewal experiments cells were plated at a very low density (“clonal density”) of 104 cells/cm2 (Babu et al., 2007). The cells were plated in conditioned medium together with fresh medium 1:1 ratio with 20 ng/ml of both epidermal growth factor (EGF) and Fibroblast Growth Factor-2 (FGF-2). The conditioned medium was prepared from medium incubated for 48 h in proliferating precursor cells.
For differentiating cells were plated at a density of 2 × 104 cells/cm2. The growth factors were withdrawn the following day. Wherever necessary the pharmacological agents were added while the growth factors were withdrawn. The drugs were added to the culture medium in following concentration: 8-OH DPAT (2 μM), Ipsapirone (0.1 μM), α-methyl-5-HT-maleate (20 μM), WAY161503 (0.5 μM), NAN-190 (3 μM), WAY100135 (2 μM) and Cinanserin (30 μM).
For clonal analysis 1000 cells were plated per well of a 96 well plate. After 8–10 days spheres were counted and represented as percentages. For immunocytochemical analysis, the cells were fixed with 4% PFA for 30 min at room temperature. The cells were then washed in PBS. The primary and secondary antibodies were incubated in blocking buffers consisting of 5% Donkey Serum. Permeabilization was carried out with 0.25% of TritonX-100, which was only done while blocking and omitted for primary antibody incubations.
RT-PCR
Neural precursor cells cultures were lysed in RLT buffer and RNA extracted using RNeasy mini kit according to the manufacturers (Invitrogen) suggestions. The RNA extracted was reverse transcribed in a 50 μl final reaction mix using the Superscript First-Strand RT reaction kit (Invitrogen) to obtain the cDNA. 0.5 μl of the cDNA was used to amplify the gene of interest with specific primers. The following primers were used: 5-HT1aR-Forward- 5′-ATCTCGCTCACTTGGCTCAT -3′, 5-HT1aR-Reverse- 5′- CCACTACCTGGCTGACCATT -3′, 5-HT2aRa-Forward- 5′- ATAGCCGCTTCAACTCCAGA -3′, 5-HT2aR-Reverse- 5′- ACGGCCATGATATTGGTGAT-3′, 5-HT2cR-Forward- 5′- TGATTGGACTGAGGGACGAAAGCA -3′ and 5-HT2cR-Reverse- 5′- TTCCCACAAAGCACCGACAGGATA -3′. The PCR products were analyzed by agarose gel electrophoresis.
Immunohistochemistry
Mice were deeply anesthetized with ketamine and perfused transcardially with 0.9% sodium chloride followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. Brains were dissected from the skulls, postfixed in 4% PFA at 4°C over night, and transferred into 30% sucrose. Brains were cut on a sliding microtome (Leica, Bensheim, Germany) in the coronal plane in 40-μm thick sections and cryoprotected. Sections were stained free floating with all antibodies diluted in Tris-buffered saline (TBS) containing 3% donkey serum and 0.1% Triton X-100. For BrdU staining, DNA was denatured in 2N HCl for 30 min at 37°C. Sections were then rinsed in 0.1 M borate buffer and washed in TBS.
Primary antibodies were applied in the following concentrations: anti-BrdU (rat, 1:500; Harlan Seralab, Indianapolis, IN, USA), anti-GFP (rabbit, 1: 400, Acris Antibodies, DPC Biermann, Germany), anti-GFP (goat, 1:1000, Acris Antibodies, DPC Biermann, Germany), anti-GFP (mouse, 1:400, Acris Antibodies, DPC Biermann, Germany), anti-Ki67 (rabbit, 1:500, Novocastra, UK), anti-S100β (rabbit, 1:2500; Swant, Belinzona, Switzerland), anti-S100β (mouse, 1:1000, SIGMA), anti-NeuN (mouse, 1:100; Chemicon, Temecula, CA, USA), anti-DCX (goat, 1:200; Santa Cruz Biotechnologies, Santa Cruz, CA, USA) and anti-Calretinin (goat, 1:250; Swant, Switzerland) anti-GFAP (guinea pig, 1:1000 Advanced ImmunoChemistry), 5-HT1a Receptor (guinea pig, 1:500, Chemicon), 5-HT2a Receptor (rabbit, 1:250, Sigma), 5-HT2c Receptor (rabbit, 1:250, Chemicon).
Immunohistochemistry followed the peroxidase method with biotinylated secondary antibodies (all: 1:500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA; Distributor Dianova, Hamburg, Germany), ABC Elite reagent (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine (DAB; Sigma) as chromogen. For immunofluorescence FITC-, RhodX- or Cy5-conjugated secondary antibodies were all used at a concentration of 1:250 (Jackson ImmunoResearch). Fluorescent sections were coverslipped in polyvinyl alcohol with diazabicyclooctane (DABCO, Sigma) as anti-fading agent.
In situ hybridization
For in situ hybridization, brains were embedded in Tissue-Tec OCT compound (Sakura) and cut in 20-μm thick sections on a cryostat. In situ hybridization was performed as follows. DIG-labeled riboprobe (nucleotide region 1251–1688 of the transcript for mouse Htr1a; NCBI reference NM_008308.4) was synthesized in vitro with Promega Riboprobe System – SP6/T7, DIG RNA labeling mix (Roche), and purified with RNAeasy mini kit (Qiagen). Pre-hybridized cryosections were incubated overnight at 70°C in hybridization buffer containing 500 ng/ml of DIG-labeled riboprobe. Incubation with alkaline phosphatase-conjugated anti-DIG antibody (Roche) followed at 4°C overnight, and staining with BCIP/NBT (Sigma-Aldrich) at RT for 1 week. The detection of the 5-HT1a receptor transcript was combined with immunofluorescence for BrdU, and DAPI staining to visualize the nuclei. BrdU staining was done immediately after in situ hybridization and as described above.
Quantification and imaging
One-in-six series of sections of each brain were DAB stained, and immunoreactive cells were counted throughout the rostrocaudal extent of the dentate gyrus. Results were multiplied by six to obtain the total number of BrdU-positive cells per dentate gyrus. One-in-twelve series of sections were tripled-labeled as described. For phenotypic analysis of BrdU-labeled cells, 100 randomly selected cells per animal were analyzed for co-staining with glial or neuronal markers, using a Leica TCS SP2 confocal microscope. Confocal microscopy was performed using a spectral confocal microscope (TCS SP2; Leica, Bensheim, Germany). Appropriate gain and black level settings were determined on control tissues stained with secondary antibodies alone. All images were taken in sequential scanning mode and further processed in Adobe Photoshop 7.0 for Macintosh. Only general contrast adaptations were made and figures were not otherwise manipulated.
To obtain the absolute number of BrdU-labeled cells of a certain phenotype we multiplied the absolute number of BrdU-labeled cells with the ratio of the phenotype of interest.
Statistical Analysis
All numerical analyses were performed using Statview 5.0.1 for Macintosh. ANOVA was followed by Fisher's post hoc test, where appropriate. All values are expressed as mean ± SD. p-Values of <0.05 were considered statistically significant.
Results
Fluoxetine exerts a survival-promoting effect on newborn cells in the dentate gyrus
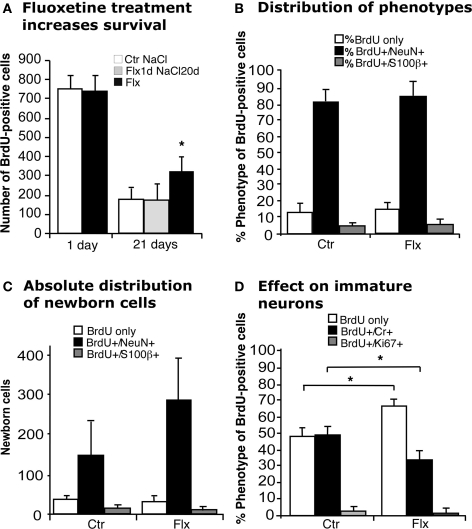
Injecting BrdU 24 h before the beginning of 21 days of treatment with fluoxetine (Flx, Figure 1) exposed newborn cells to the effect of the drug after the completion of their cell cycle. In this paradigm we found a survival-promoting effect by Flx (Figure 2A). We next asked, whether this survival effect might act only on the cells that had just undergone division. We thus gave BrdU, injected Flx 1 day later and continued with daily vehicle saline injections for the remainder of the 21-day period. Here no changes were found (Figure 2A). This finding points out that acute stimulation by Flx does not exert a lasting survival effect.
Figure 2.
Effects of acute and chronic fluoxetine treatment. (A) Compared to control (Ctr NaCl), chronic but not acute fluoxetine treatment significantly increased the number of BrdU-positive cells in the dentate gyrus. Injection of BrdU 24 h before the beginning of 21 days chronic fluoxetine administration exerted a survival-promoting effect on newborn cells, whereas one single injection of the drug followed by 20 days of saline had no effect. Chronic fluoxetine treatment resulted in a net increase in newborn cells (C) without affecting the proportion of new neurons (BrdU+/NeuN+) (B), but significantly decreased the ratio of BrdU-positive cells expressing the transient postmitotic marker Calretinin (Cr) (D). Here, the increased proportion of BrdU-labeled cells of undetermined phenotype presumably reflects the increase in NeuN-positive cells.
When analyzing the phenotype of BrdU-positive cells after 21 days of prolonged Flx treatment we found that the proportion of new neurons (BrdU+/NeuN+) was not influenced by Flx treatment (Figure 2B), resulting in a net increase of newborn neurons (Ctr 147 ± 97 vs. Flx 279 ± 110; p = 0.0536; Figure 2C). Interestingly, when we looked at the proportion of cells expressing transient postmitotic marker Calretinin we found a significant decrease in the treatment group (Ctr 49 ± 8.3% vs. Flx 32.6 ± 4.8%; p = 0.0019, Figure 2D). No change was found in the number of cells with co-labeling for BrdU and proliferation marker Ki67 indicating that the number of label-retaining proliferating precursor cells was not affected.
Since acute treatment with Flx neither affected cell proliferation in the adult dentate gyrus (Malberg et al., 2000; Encinas et al., 2006) nor cell survival (as shown here), but prolonged treatment with Flx showed an effect on neurogenesis, we next studied acute and chronic effects driven by different 5-HT receptors.
Expression of 5-HT1 and 2 receptors in the adult dentate gyrus
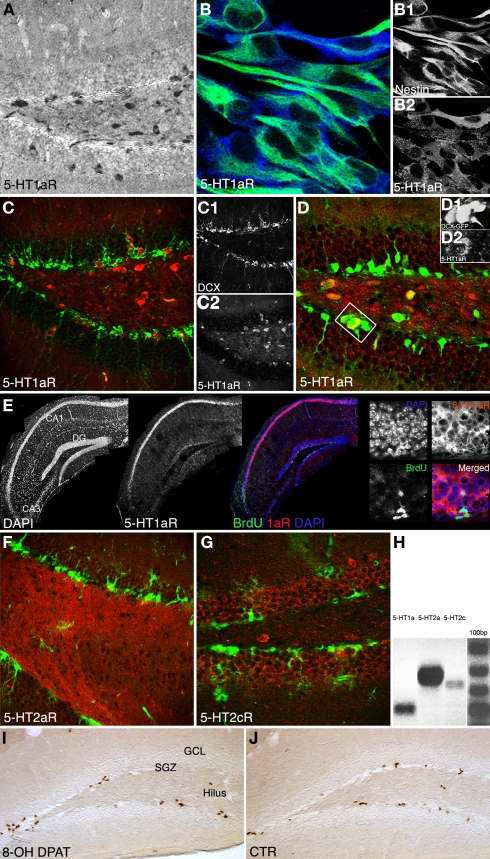
We first investigated, whether 5-HT receptors are expressed in the adult dentate gyrus and if so, by which cell types. Coronal brain sections of adult female mice showed 5-HT1a receptor expression in neurons both in the hilus and the granule cell layer including the SGZ (Figures 3A,C,D). Our data show that the majority of cells in the SGZ do not or only weakly express 5-HT1a, instead it seems to be a sub-population of cells that is positive for the receptor. We hypothesized that among these the precursor cells of the SGZ would be found. In vitro, hippocampal precursor cells displayed intense 5-HT1a receptor expressions (Figure 3B). Confocal images of in situ hybridization revealed the typical strong 5-HT1a receptor expression in the CA1 region and fainter in the dentate gyrus (Figure 3E). Combination of 5-HT1a receptor in situ hybridization with BrdU immunohistochemistry further indicated that the receptor is found on proliferating (precursor) cells in the dentate gyrus. Strong expression was also found on local interneurons, suggesting that some of the observed effects might also be indirect. For further in vivo investigations we used DCX-EGFP reporter mice to analyze receptor expression on doublecortin (Dcx)-expressing precursor cells in their full morphology. We found staining with the 5-HT1a receptor antibody in a few Dcx-positive, horizontal type-2 cells in the SGZ (Figure 3D). Immunohistochemistry with a selective 5-HT2a receptor antibody revealed dense staining in the hilus (Figure 3F) whereas staining for 5-HT2c receptors marked the granule cell layer of the dentate gyrus (Figure 3G).
Figure 3.
Qualitative results. (A–G) 5-HT1a, 2a and 2c receptor expression pattern in vivo, and in vitro. (A) Anti-5-HT1aR staining (DAB reaction) shows receptor expression in neurons both in the hilus and granule cell layer including the subgranular zone, Scale bar 80 μm. (B) Proliferating precursor cells [Nestin, green (B1)] in cell culture show an intense 5-HT1aR expression [blue, (B2)], Scale bar 10 μm. (C) 5-HT1a receptor expression in the dentate gyrus [Doublecortin, green (C1); 5-HT1aR, red (C2)]. (D) The receptor is detectable in horizontal type-2 cells revealed as an overlap with DCX-EGFP [green (D1), 5-HT1aR in red (D2). (E) Single focal plane of the hippocampus after in situ hybridization with riboprobe for the 5-HT1a receptor, immunofluorescence for BrdU, and DAPI; the confocal image reveals strong receptor expression in CA1, fainter in the dentate gyrus (DG), and no staining in the CA3 region. (F) 5-HT2a receptor expression pattern (red) in the dentate gyrus reveals a dense staining in the hilus whereas anti-5-HT2c receptor staining [(G), red] mostly marks the granule cell layer (Nestin in green). (H) Expression analysis of 5-HT receptors: PCR analysis was done from reverse transcribed RNA obtained from neural precursor cells under proliferative conditions. There is abundant expression of 5-HT1aR (Lane 1) and 5-HT2aR (Lane 2). Also low levels of 5-HT2c receptors expressed in the precursor cells can be seen (Lane 3). (I,J) Anti-BrdU DAB reaction. A single i.p., injection of the 5-HT1aR agonist 8-OH DPAT 2h pre-BrdU increases the number of BrdU-positive cells in the subgranular zone (SGZ) 24 h later compare to control (CTR in J; GCL, granule cell layer; Scale bar 120 μm).
In addition RT-PCR of neural precursor cell RNA revealed 5-HT1a and 5-HT2 receptor expression (Figure 3H).
Stimulation of the 5-HT1a receptor increases cell proliferation in vivo
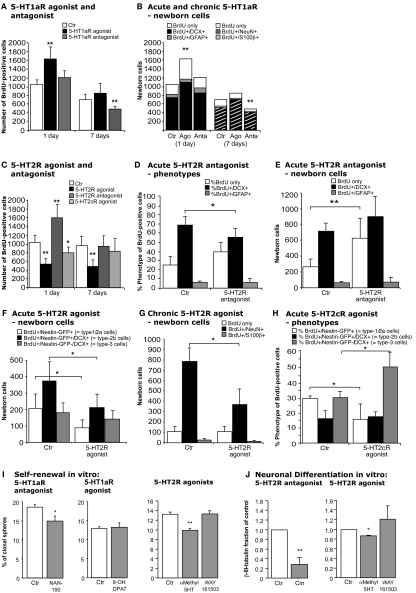
In vivo, upon treatment with the selective 5-HT1a receptor agonist 8-OH DPAT BrdU-positive cells formed characteristic clusters in the SGZ of the dentate gyrus (Figures 3I,J) as described earlier (Banasr et al., 2004). Acute treatment with 8-OH DPAT 2 h pre-BrdU (Figure 1B) significantly increased precursor cell proliferation measured 1 day after BrdU injection (Ctr 1052 ± 106 vs. 1627 ± 279; p = 0.0062; Figure 4A), whereas 1 week of treatment had no significant effect. Analogous treatment with 5-HT1aR antagonist WAY100135 showed the opposite pattern with no effect on precursor cell proliferation but a significant decrease in the number of BrdU-positive cells in animals that received the antagonist for 7 days (Ctr 670 ± 122 vs. 480 ± 55; p = 0.0019; Figure 4A).
Figure 4.
Effects of acute and chronic 5-HT1a, 2, and 2c receptor agonists and antagonists treatment. (A,B) 5-HT1aR effects. (A) Acute treatment with the 5-HT1a receptor agonist 8-OH DPAT produces a significant increase in cell proliferation 1 day after BrdU injection, whereas chronic stimulation for 1 week has no effect. In contrast, acute 5-HT1a receptor blocking with WAY100135 causes no change in cell proliferation but decreases the number of BrdU-positive cells 1 week later. (B) Confocal analysis of the differentiation profile reveals a net increase in the proportion of BrdU/DCX-positive cells after acute stimulation, and a decrease of newborn neurons (BrdU+/NeuN+) upon chronic treatment with the antagonist. (C–H) Effects of acute and chronic 5-HT2 receptor agonist and antagonist treatment, and 5-HT2c receptor stimulation on precursor cell proliferation and differentiation in the adult dentate gyrus. (C) As compared to control, acute treatment with the 5-HT2 receptor antagonist Cinanserin leads to a large increase in the number of proliferating cells 1 day after BrdU injection, but shows no differences 1 week later. In contrast, acute stimulation with the agonist α-methyl-5-HT-maleate as well as chronic treatment over 7 days significantly decreases the number of BrdU-positive cells. Acute 5-HT2c receptor agonist treatment also decreases cell proliferation, but has no effect on survival. Phenotypic analysis reveals a significant decrease in BrdU+/Dcx+ cells after acute 5-HT2R antagonist treatment (D) that result in a largely net increase in the number of BrdU+ cells of undetermined phenotype (E). Phenotypic analysis of acute and chronic effects of the 5-HT2R agonist reveal a net decrease in type-1/2a and type-2b cells after acute treatment (F), and a significantly reduced number of newborn neurons after 7 days (G). Acute 5-HT2c receptor agonist treatment exerts a shift from type-1/2a (which decreases) to type-3 and newly postmitotic cells (which increases), with the type-2b stage being unaffected (H). Data present the absolute number of BrdU-positive cells per dentate gyrus as well as their phenotype by percentage and absolute number, mean ± SD. (I) In vitro, self-renewal potential of neural precursor cells indicated by the capacity to form spheres when plated at clonal densities. Upon 5-HT1aR antagonist (NAN-190) addition as well as upon 5-HT2R stimulation with α-methyl-5-HT the self-renewal potential was significantly decreased, whereas the 5-HT1aR agonist 8-OH DPAT had no effect when added to the culture. (J) Neuronal differentiation of precursor cells was detected by β-III-tubulin antibody staining, and the number of neurons was counted. 5-HT2R antagonist Cinanserin (Cin) potently decreased neuronal differentiation from adult neural precursor cells suggesting an essential role for 5-HT2R in neuronal differentiation. 5-HT2R agonist addition but not 5-HT2cR agonist (WAY161503) also inhibited neuronal differentiation.
For phenotypic analysis sections were stained with the transient neuronal marker Dcx or the neuronal marker NeuN. Calculating the net effects on adult neurogenesis thus led to an identical pattern than the BrdU data (Figure 4B): 5-HT1aR agonist (8-OH DPAT) treatment increased cell proliferation of Dcx-positive cells (Ctr 763 ± 116 vs. 1105 ± 172; p = 0.0119) with the number of BrdU-only cells remaining unchanged suggesting a net increase in proliferating type-2b and type-3 cells (see Figure 5) after acute 5-HT1a receptor stimulation. In contrast, 1 week of treatment made this difference disappear and no effect on the total number of BrdU/NeuN-positive cells was found. Conversely, the number of new neurons significantly decreased after treatment with the 5-HT1aR antagonist WAY100135 for 7 days (Ctr 572 ± 125 vs. 404 ± 51; p = 0.0083, Figure 4B).
Figure 5.
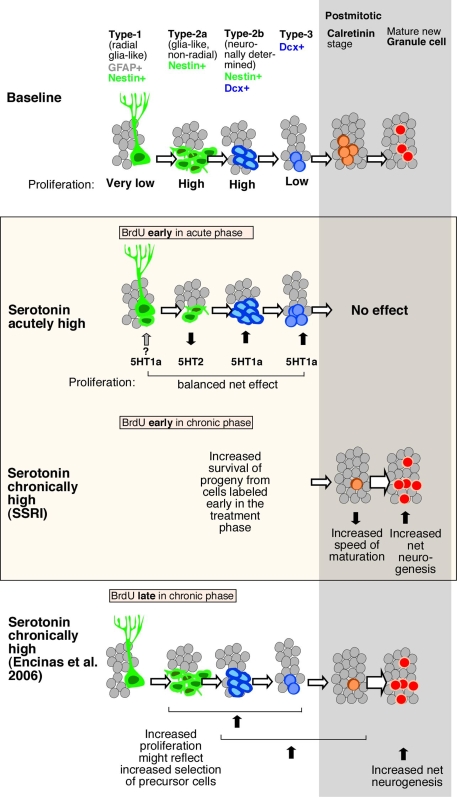
Schematic drawing of effects of Flx treatment and 5-HT receptors stimulation on adult hippocampal neurogenesis. Under baseline levels, from left to right, low proliferative radial glia-like cells (type-1 with GFAP-expression) give rise to three stages of transient amplifying lineage-determined progenitor cells (type-2a, -2b, and 3 with Dcx-expression) to postmitotic immature granule cells (transient Calretinin-expression) and mature neurons. The middle panel highlighted in color, shows the effect of acute and chronically manipulated serotonin levels on proliferation and differentiation. While 5-HT receptors reveal a balanced net effect on earlier stages, the SSRI Flx effect more advanced stages and increases net neurogenesis. The bottom panel in comparison summarizes the data by Encinas et al. (2006), who proposed a selective effect on early precursor cell proliferation and increased net neurogenesis.
5-HT2R and 5-HT1aR signaling have oppositional effects on adult neurogenesis
Interestingly, in vivo treatment with 5-HT2 receptor antagonist Cinanserin resembled the effects of 5-HT1aR agonist. There was an acute increase in cell proliferation but no effect after 7 days (Figure 4C). Most of the net increase in BrdU-positive cells was explained by an undetermined phenotype (Ctr 267 ± 115 vs. 626 ± 245; p = 0.0063) whereas the number of BrdU+/Dcx+ cells even decreased (68.5 ± 8.9% vs. 56.1 ± 9.4%; p = 0.0299; Figures 4D,E). Staining for GFAP or the endothelial marker Glut1 revealed no change in GFAP-positive or endothelial cells (data not shown). Consequently, the most parsimonious explanation is an increase in type-2a progenitor cells, which are Dcx-negative.
For the complementary experiment the 5-HT2 receptor agonist α-methyl-5-HT was used. In contrast to the results for the 5-HT1aR agonist, we here found a decrease in the number of BrdU-positive cells after both 1 and 7 days of treatment (Figure 4C). To further differentiate the 5-HT2 receptor driven results the selective 5-HT2cR agonist WAY161503 was used. Again, we found a moderate decrease in cell proliferation (Ctr 1129 ± 169 vs. 864 ± 161; p = 0.0481; Figure 4C), but no effect after 7 days of treatment. Nestin-GFP reporter gene mice were used for direct characterization of the different types of precursor cells (Yamaguchi et al., 2000; Filippov et al., 2003). Phenotypic analysis for acute 5-HT2 receptor agonist treatment revealed a net decrease of BrdU/Nestin-GFP-positive type-1/2a and BrdU/Nestin-GFP/Dcx-positive type-2b cells with type-3 cells and newly postmitotic neurons remaining unchanged (Figure 4F). However, 7 days of treatment significantly reduced the number of newborn neurons (787 ± 126 vs. 371 ± 145; p = 0.0025; Figure 4G). In concert with the data from the antagonist treatment these data confirm that 5-HT2 receptor effects mainly act on type-2 precursor cells. Phenotypic analysis for acute 5-HT2cR agonist showed a shift from type-1/2a (which decreased) to type-3 and newly postmitotic cells (which increased), with the type-2b stage being unaffected (Figure 4H). As with the pan-HT5R2 antagonist, the HT5R2c agonist showed no difference after 7 days of treatment.
Stimulation of 5-HT1a receptors in vitro primarily promotes self-renewal whereas 5-HT2R signaling is also involved in neuronal differentiation
To further investigate whether the in vivo effects might occur directly on precursor cells, or – as the data on receptor expression might suggest – rather indirect by surrounding cells we turned to adherent monolayer cultures (Babu et al., 2007) and three-dimensional neurospheres of hippocampal precursor cells.
First, the capacity for self-renewal in the presence of a 5-HT1a receptor antagonist was studied in a three-dimensional neurospheres culture of adult neural precursor cells. Cells were grown at low density in the presence of the mitogens EGF and FGF-2 to allow self-renewal when the 5-HT1a receptor antagonist NAN-190 was added. NAN-190 significantly decreased the percentage of secondary spheres generated by the individualized cells (Ctr 18.56 ± 0.75, NAN 14.92 ± 1.22; p = 0.0349, Figure 4I). Treatment with the selective 5-HT1a receptor agonist 8-OH DPAT did not significantly affect the sphere forming capacity of neural precursor cells (Figure 4I).
Interestingly, when neural precursor cells were incubated with Cinanserin, they did not generate any spheres in the presents of the 5-HT2 receptor antagonist. To test whether agonists of 5-HT2 receptors would play a role in regulating the self-renewal potential of hippocampal precursor cells, the 5-HT2R agonist α-methyl-5-HT was added. Here too, the number of spheres generated was significantly decreased as it was for Nan-190 (Ctr 13.18 ± 1.42%, α-methyl-5-HT 9.85 ± 0.41%; p = 0.002; Figure 4I). We did not, however, observe any difference with the selective 5-HT2c receptor agonist WAY 161503 (Ctr 13.18 ± 1.42%, WAY 161503 13.23 ± 0.64%; p = 0.476; Figure 4I). Those in vitro data mirror the antagonistic effect of 5-HT1a and 5-HT2 receptors as seen in vivo.
Next, the effect on neuronal differentiation of precursor cells in vitro was investigated. In the adherent monolayer culture model system, differentiation is induced by withdrawal of the growth factors. Here, the addition of the selective 5-HT1aR agonist 8-OH DPAT failed to regulate neuronal differentiation measured by β-III-tubulin expression. Ipsapirone, another 5-HT1a receptor agonist revealed the same (not shown). Next, non-stimulant action of serotonin via 5-HT1a receptors on neural precursor cells was investigated by incubation the cells with 5-HT1a receptor antagonists. In this setting the antagonists WAY100135 and NAN-190 appear to decreased neuronal differentiation but failed to reach statistical significance at p = 0.05. These results are in contrast to the in vivo data suggesting a rather indirect effect, which will be discussed later.
Pharmacological inactivation of 5-HT2 receptors by Cinanserin under differentiation conditions instead showed a significant reduction in the number of β-III-tubulin-positive neurons (Ctr value set to 1, Cinanserin 0.28 ± 0.08; p = 0.0037; Figure 4J). The cell density in cultures incubated with Cinanserin reflected the effect of the antagonist. Interestingly, we observed a similar but less pronounced result upon α-methyl-5-HT incubation, which also decreased the fraction of newly generated neurons in presence of the 5-HT2 receptor agonist (0.87 ± 0.008; p = 0.0043; Figure 4J). We did not detect any statistically significant change in the fraction of neurons differentiated upon WAY161503 exposure, the specific 5HT2c receptor agonist (1.21 ± 0.27; p = 0.5254; Figure 4J).
Discussion
The present study shows that in adult mice serotonin influences both cell proliferation and the survival of newly generated neurons in the dentate gyrus via different serotonin receptor activities (Table 1). We have demonstrated that acute activation of 5-HT1a and 5-HT2 receptor subtypes had counteracting effects on cell proliferation in vivo and self-renewal in vitro. These observations provide insight into why an acute increase in serotonin levels due to treatment with Flx does not produce a net increase in cell proliferation in vivo. The two receptors act antagonistically. Flx indirectly activates 5-HT1aR via increasing serotonin levels while a negative regulation of neurogenesis through 5-HT2R occurs.
Table 1.
Acute vs. chronic effects of fluoxetine and various serotonin receptor agonist and antagonist on adult hippocampal neurogenesis in vivo.
| Proliferation | Survival | |
|---|---|---|
| 21 days after BrdU | ||
| SSRI fluoxetine | – | ↑ |
| 7 days after BrdU | ||
| 5-HT1aR | ||
| 8-OH DPAT (selective agonist) | ↑ | – |
| WAY100135 (antagonist) | – | ↓ |
| 5-HT2R (entire family) | ||
| α-methyl-5-HT (agonist) | ↓ | ↓ |
| Cinanserin (antagonist) | ↑ | – |
| 5-HT2cR | ||
| WAY161503 (selective agonist) | ↓ | – |
Whereas acute activation increases proliferation, prolonged inhibition of 5-HT1a receptors results in a decrease in net neuronal survival and differentiation. Interestingly, chronic activation of the 5-HT2 receptor family also produced this effect indicating a lack of antagonistic action. Vice versa, blocking the 5-HT2 receptors did not cause a decrease in net neurogenesis that would mirror the down-regulatory effect of the 5-HT1a receptor antagonists. This might suggest that 5-HT1a activation is more required for baseline regulation of net neurogenesis, whereas 5-HT2 receptor activation is not. Consequently, adult neurogenesis might be functionally more sensitive to 5-HT1a receptor-mediated effects than to 5-HT2. This interpretation is supported by reports showing that 5-HT1a receptors are required for Flx-induced hippocampal neurogenesis (Santarelli et al., 2003) but does not explain the latency as observed by Malberg et al. (2000).
Yet, others proposed that 5-HT1a agonists also stimulate presynaptic receptors in the raphe nuclei, which inhibit the firing rate of serotonergic neurons and consequently reduce the signal at target postsynaptic receptors in the hippocampus. The time serotonergic raphe neurons need to regain their normal firing rate (increased activation) would explain the delay of therapeutic action (Blier, 2003). The apparent clinical effect latency of SSRIs has been questioned by several studies (Posternak and Zimmerman, 2005; Katz et al., 2006a,b, Mitchell, 2006). With respect to the link between this delay and the dynamics of adult neurogenesis we here add another argument.
Prolonged 5-HT2c receptor activation fostered an increase in late-stage progenitor cells and early postmitotic neurons, leading to a net increase in adult neurogenesis, similar to the effect of chronic Flx treatment. We propose that the balance between the two receptors that is seen under acute stimulation in vivo is lost after prolonged stimulation, because the 5-HT1a receptor-mediated effects will generate more cells that are responsive to the 5-HT2 receptor-mediated effects, thereby increasing both the number of proliferating late-stage precursor cells and net adult neurogenesis (Figure 5). A recently published study by Soumier et al. (2009a,b) also reported a 5-HT2c receptor-mediated effect in adult neurogenesis and antidepressant treatment (Soumier et al., 2009a). The authors have shown that the selective 5-HT2cR antagonist SB243213 increased cell proliferation but not survival in the ventral hippocampus and thus partially mimicked the effect of the novel antidepressant agomelatine (Soumier et al., 2009b). The antagonist effect is in accordance with our results (Cinanserin in Figure 4C). Both studies achieve the impact of serotonin receptor subtype 2c in antidepressant action.
Our results of 5-HT2 receptor stimulation in vivo differ from an earlier study (Banasr et al., 2004), which can be explained by differences in the experimental design and the drugs used (Table 2). Banasr et al. (2004) have shown that acute treatment with either DOI, a 5-HT2 receptor family agonist, or the selective 5-HT2c receptor agonist RO 600175 have no effect on cell proliferation in the SGZ of the dorsal hippocampus (2 h after BrdU). Our paradigm (24 h after BrdU) catches a larger number of proliferating cells (and throughout the SGZ of the entire dorsal and ventral hippocampus) and reflects the pool of proliferating and label-retaining progenitor cells. As depicted in Figures 4C,H, our protocol with 5-HT2c agonist treatment decreases the number of BrdU+ cells after 24 h but increases the relative number of “late” type-3 progenitor cells over 7 days. Taken together these results suggest that 5-HT2c receptor activity promotes proliferation and differentiation of neuronally determined progenitor cells.
Table 2.
Comparison of different 5-HT receptor studies.
| Banasr et al. (2004) | Our study | |
|---|---|---|
| Animals | Rats | Mice |
| BrdU i.p. | 75 mg/kg body weight | 50 mg/kg body weight |
| Treatment paradigm | 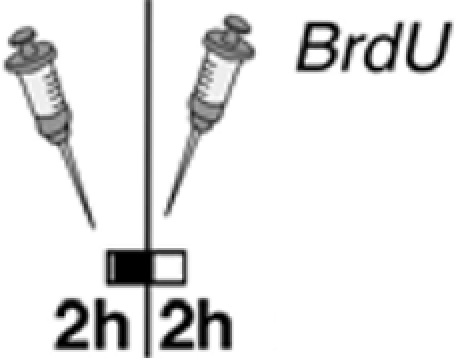 |
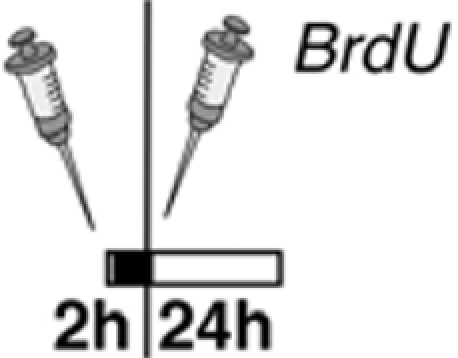 |
| 5-HT2 receptor family | No effect with DOI (agonist) | ↓ α-methyl-5-HT (agonist) |
| 5-HT2c receptor | No effect with RO 600175 (selective agonist) | ↓ WAY161503 (selective agonist) |
Furthermore, the present study demonstrates that serotonin receptor modulation is seen on sequential steps in the course of adult neurogenesis (Figure 5). To distinguish precursor cell stages, Nestin-GFP/GFAP-co-expression was used to mark type-1, whereas Nestin alone as type-2a and Nestin/Dcx-expression constituting type-2b. Type-3 cells characterize immature neurons with transient Dcx-expression, whereas early postmitotic immature granule cells also express Calretinin and NeuN (Kempermann et al., 2004). The acute stimulating effect through 5-HT1a receptors affected Dcx-positive cells, i.e., from type-2b onward, whereas activation of 5-HT2 also strongly affected Dcx-negative cells, most likely type-2a cells. As development proceeds from type-2a to type-2b and -3, the decrease in proliferation in response to 5-HT2 receptor activation is offset by the increase in proliferation at a later stage due to 5-HT1a receptor activation. The present data in vivo and vitro show that the phase of neuronal survival is influenced by 5-HT1a receptors while the phase of neuronal differentiation during transient Dcx-expression is regulated by 5-HT2 receptors. One problem with this hypothesis is that the key cell population of interest, the type-3 cells, is comparatively small, so that a large number of Nestin-GFP reporter gene mice would be needed to obtain the necessary power. In a study on the effect of kainic acid-induced seizures on adult neurogenesis we were able to detect a type-3-cell specific effect, but the seizure stimulus is much stronger than the pro-neurogenic effect of serotonin (Jessberger et al., 2005).
In line with other studies our data specify that chronic Flx treatment increased survival and differentiation of newborn cells (Encinas et al., 2006; Wang et al., 2008). In contrast to the Malberg study, however, Flx treatment in our hands had no effect on cell proliferation (Malberg et al., 2000). Table 3 shows a summary of the different treatment paradigms. It remains to be shown whether and how chronic Flx treatment might change the relative composition of the precursor cell pool in the course of adult neurogenesis. We have seen that 21 days of prolonged Flx treatment decreases the number of cells at the Calretinin stage despite an increase in net neurogenesis (NeuN co-expression). We interpret this as increased maturation of the newborn neurons, presumably shortening the Calretinin-positive stage and thereby lowering the number of cells at this stage at a given time. Chronic Flx treatment might promote and accelerate neuronal differentiation. These data are in line with a recent report suggesting that chronic Flx administration led to an increase in neuronal maturation (Wang et al., 2008) which would here be reflected in a decrease of cells at the Calretinin-positive immature neuronal stage.
Table 3.
Comparison of studies on Fluoxetine and adult neurogenesis.
| Malberg et al. (2000) | Encinas et al. (2006) | Our study | |
|---|---|---|---|
| Species | Rat | Mouse | Mouse |
| Fluoxetine 10 mg/kg | 1× per day for 1, 5, 14, or 28 days; 5 mg/kg body weight |
1× per day for 15 days; 10 mg/kg body weight |
1× per day for 1 or 21 days; body weight |
| BrdU | Day 4 after treatment | Day 14 of treatment | Day 1 before treatment |
| End of experiment | 2 h, 24 h, or 28 days after BrdU | 24 h or 30 days after BrdU | 22 days after BrdU |
To complete the picture of serotonin action on regulation of adult hippocampal neurogenesis and thus to get insights of antidepressant action, it is important to know to which degree the observed effects are direct or indirect. As depicted in Figure 3, our data on receptor expressions suggest a rather indirect effect by surrounding cells. There are 5-HT receptors on precursor cells of the SGZ but expression on local interneurons is actually more prominent. For a detailed analysis, we have tested the effect of the 5-HT2c receptor subtype on adult neurogenesis due to its earlier described impact in hippocampal LTP and BDNF mRNA expression (Vaidya et al., 1997; Tecott et al., 1998). Here, we have shown that acute 5-HT2cR activation with WAY161503 decreases proliferation. Recent publications indicate that WAY161503 inhibits the firing rate of serotonergic dorsal raphe neurons by a mechanism that involves 2c receptor-mediated activation of GABAergic neurons (Boothman et al., 2006). Given the existence of synaptic contacts on hilar interneurons via raphe afferents an increased GABA release may lead to an inhibitory control of cell proliferation and differentiation in the SGZ, suggesting an indirect effect. Another study showed that type-2a cells are the first to receive GABAergic synaptic input (Wang et al., 2005), which is excitatory at this early stage of neuronal development and promotes neuronal differentiation and maturation (Tozuka et al., 2005; Ge et al., 2006). This raises the possibility that 5-HT-induced effects on adult neurogenesis are at least partially mediated by GABA-related mechanisms.
Our data support an overall pro-neurogenic role of serotonin, and indicate that serotonin does not show a delayed neurogenic effectiveness in the adult dentate gyrus. We propose that the apparent delay is largely due to the antagonistic action of different 5-HT receptor systems and the time required for these actions to reach a balance. Details of this antagonism and how it changes with prolonged exposure to serotonin will have to be unraveled.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by Thyssenstiftung, Volkswagenstiftung and Deutsche Forschungsgemeinschaft (DFG). The authors would like to thank Signe Knespel, Daniela Lasse, and Ruth Zarmstorff for technical assistance.
References
- Babu H., Cheung G., Kettenmann H., Palmer T. D., Kempermann G. (2007). Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE 2, e388. 10.1371/journal.pone.0000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M., Hery M., Printemps R., Daszuta A. (2004). Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29, 450–460 10.1038/sj.npp.1300320 [DOI] [PubMed] [Google Scholar]
- Blier P. (2003). The pharmacology of putative early-onset antidepressant strategies. Eur. Neuropsychopharmacol. 13, 57–66 10.1016/S0924-977X(02)00173-6 [DOI] [PubMed] [Google Scholar]
- Boothman L., Raley J., Denk F., Hirani E., Sharp T. (2006). In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br. J. Pharmacol. 149, 861–869 10.1038/sj.bjp.0706935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun J. M., Daszuta A. (1999). Serotonin depletion in the adult rat produces differential changes in highly polysialylated form of neural cell adhesion molecule and tenascin-C immunoreactivity. J. Neurosci. Res. 55, 54–70 [DOI] [PubMed] [Google Scholar]
- Brezun J. M., Daszuta A. (2000). Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus 10, 37–46 [DOI] [PubMed] [Google Scholar]
- Cryan J. F., O'Leary O. F., Jin S. H., Friedland J. C., Ouyang M., Hirsch B. R., Page M. E., Dalvi A., Thomas S. A., Lucki I. (2004). Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc. Natl. Acad. Sci. U.S.A. 101, 8186–8191 10.1073/pnas.0401080101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Sa C., Duman R. S. (2002). Antidepressants and neuroplasticity. Bipolar Disord. 4, 183–194 10.1034/j.1399-5618.2002.01203.x [DOI] [PubMed] [Google Scholar]
- Encinas J. M., Vaahtokari A., Enikolopov G. (2006). Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. U.S.A. 103, 8233–8238 10.1073/pnas.0601992103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V., Kronenberg G., Pivneva T., Reuter K., Steiner B., Wang L. P., Yamaguchi M., Kettenmann H., Kempermann G. (2003). Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell. Neurosci. 23, 373–382 10.1016/S1044-7431(03)00060-5 [DOI] [PubMed] [Google Scholar]
- Gaspar P., Cases O., Maroteaux L. (2003). The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012 10.1038/nrn1256 [DOI] [PubMed] [Google Scholar]
- Ge S., Goh E. L., Sailor K. A., Kitabatake Y., Ming G. L., Song H. (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 10.1038/nature04404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Zheng C., Doughty M. L., Losos K., Didkovsky N., Schambra U. B., Nowak N. J., Joyner A., Leblanc G., Hatten M. E., Heintz N. (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 10.1038/nature02033 [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Praag H., Gage F. H. (2000). Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatry 5, 262–269 10.1038/sj.mp.4000712 [DOI] [PubMed] [Google Scholar]
- Jessberger S., Romer B., Babu H., Kempermann G. (2005). Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 196, 342–351 10.1016/j.expneurol.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Katz M. M., Bowden C. L., Berman N., Frazer A. (2006a). Resolving the onset of antidepressants’ clinical actions: critical for clinical practice and new drug development. J. Clin. Psychopharmacol. 26, 549–553 10.1097/01.jcp.0000246220.04422.de [DOI] [PubMed] [Google Scholar]
- Katz M. M., Frazer A., Bowden C. L. (2006b). “Delay” hypothesis of onset of antidepressant action. Br. J. Psychiatry 188, 586; author reply 587. 10.1192/bjp.188.6.586-a [DOI] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. (2004). Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452 10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Krebs J., Fabel K. (2008). The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry 21, 290–295 10.1097/YCO.0b013e3282fad375 [DOI] [PubMed] [Google Scholar]
- Kronenberg G., Reuter K., Steiner B., Brandt M. D., Jessberger S., Yamaguchi M., Kempermann G. (2003). Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467, 455–463 10.1002/cne.10945 [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Eisch A. J., Nestler E. J., Duman R. S. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. J. (2006). Two-week delay in onset of action of antidepressants: new evidence. Br. J. Psychiatry 188, 105–106 10.1192/bjp.bp.105.011692 [DOI] [PubMed] [Google Scholar]
- Posternak M. A., Zimmerman M. (2005). Is there a delay in the antidepressant effect? A meta-analysis. J. Clin. Psychiatry 66, 148–158 10.4088/JCP.v66n0201 [DOI] [PubMed] [Google Scholar]
- Radley J. J., Jacobs B. L. (2002). 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 955, 264–267 10.1016/S0006-8993(02)03477-7 [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Cole J. C. (1994). Anxiolytic-like effect of (S)-WAY 100135, a 5-HT1A receptor antagonist, in the murine elevated plus-maze test. Eur. J. Pharmacol. 261, 321–325 10.1016/0014-2999(94)90124-4 [DOI] [PubMed] [Google Scholar]
- Sahay A., Hen R. (2007). Adult hippocampal neurogenesis in depression. Nat. Neurosci. 10, 1110–1115 10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Soumier A., Banasr M., Goff L. K., Daszuta A. (2009a). Region- and phase-dependent effects of 5-HT(1A) and 5-HT(2C) receptor activation on adult neurogenesis. Eur. Neuropsychopharmacol. 20, 336–345 10.1016/j.euroneuro.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Soumier A., Banasr M., Lortet S., Masmejean F., Bernard N., Kerkerian-Le-Goff L., Gabriel C., Millan M. J., Mocaer E., Daszuta A. (2009b). Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology 34, 2390–2403 10.1038/npp.2009.72 [DOI] [PubMed] [Google Scholar]
- Steiner B., Zurborg S., Horster H., Fabel K., Kempermann G. (2008). Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience 154, 521–529 10.1016/j.neuroscience.2008.04.023 [DOI] [PubMed] [Google Scholar]
- Tecott L. H., Logue S. F., Wehner J. M., Kauer J. A. (1998). Perturbed dentate gyrus function in serotonin 5-HT2C receptor mutant mice. Proc. Natl. Acad. Sci. U.S.A. 95, 15026–15031 10.1073/pnas.95.25.15026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. (2005). GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47, 803–815 10.1016/j.neuron.2005.08.023 [DOI] [PubMed] [Google Scholar]
- Vaidya V. A., Marek G. J., Aghajanian G. K., Duman R. S. (1997). 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 17, 2785–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. W., David D. J., Monckton J. E., Battaglia F., Hen R. (2008). Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 28, 1374–1384 10.1523/JNEUROSCI.3632-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. P., Kempermann G., Kettenmann H. (2005). A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol. Cell. Neurosci. 29, 181–189 10.1016/j.mcn.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Welmaker G. S., Nelson J. A., Sabalski J. E., Sabb A. L., Potoski J. R., Graziano D., Kagan M., Coupet J., Dunlop J., Mazandarani H., Rosenzweig-Lipson S., Sukoff S., Zhang Y. (2000). Synthesis and 5-hydroxytryptamine (5-HT) activity of 2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5-(6H)ones and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinoxalines. Bioorg. Med. Chem. Lett. 10, 1991–1994 10.1016/S0960-894X(00)00400-5 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Saito H., Suzuki M., Mori K. (2000). Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 11, 1991–1996 10.1097/00001756-200006260-00037 [DOI] [PubMed] [Google Scholar]
- Yoshitake T., Kehr J. (2004). Differential effects of (R)-, (R, S)- and (S)-8-hydroxy-2-(di-n-propylamino)tetralin on hippocampal serotonin release and induction of hypothermia in awake rats. Life. Sci. 74, 2865–2875 10.1016/j.lfs.2003.10.024 [DOI] [PubMed] [Google Scholar]