Abstract
Trichobilharzia is a genus of thread-like schistosomes with a cosmopolitan distribution in birds. Species of Trichobilharzia achieve notoriety as major etiological agents of cercarial dermatitis, or swimmer’s itch. There are 40 species described in the literature, for which the majority lacks molecular sequence information. To better understand the phylogenetic relationships, diversity, species boundaries, host use, and geographic distribution of this genus, we surveyed 378 birds and over 10,000 snails from North America. The phylogenetic analysis was based on nuclear 18S, 28S rDNA, internal transcribed spacer region and mitochondrial cytochrome oxidase I sequence data. Specimens were recovered that could be related to 6 of the 14 described species of Trichobilharzia from North America (T. physellae, T. querquedulae, T. szidati, T. stagnicolae, T. franki, and T. brantae). An additional 5 lineages were found that could not be related directly to previously described species. Trichobilharzia brantae, transmitted by Gyraulus parvus, grouped outside the clade containing the recognized species of Trichobilharzia. A subgroup of the Trichobilharzia clade designated Clade Q was comprised of closely related species whose adults and eggs are similar, yet the European species use lymnaeids whereas the North American species use physids as snail hosts. This molecular phylogeny provides a useful framework to: 1) facilitate identification of worms, including those involved in dermatitis outbreaks; 2) test hypotheses about the evolution, diversification, host-parasite interactions and character evolution of Trichobilharzia; and 3) guide future taxonomic revision of Trichobilharzia.
TrichobilharziaSkrjabin and Zakharov, 1920 is the most speciose genus within the Schistosomatidae Weinland 1858. The genus is comprised of 40 described species worldwide (Blair and Islam, 1983; Horák et al., 2002), of which 14 (Table I) have been described from North America. Members of this genus are reported to infect 5 orders of aquatic birds and 4 families of freshwater snails (Horák et al., 2002). Adult worms occur in the mesenteric or nasal veins of their definitive hosts, usually ducks, except in Africa where they also have been reported from other groups of aquatic birds such as grebes and ibises (Fain, 1956; Blair and Islam, 1983) and in Japan, from passerine birds (Oda, 1973). Blair and Islam (1983) and Horák et al. (2002) present the most recent reviews of this genus. The known snail intermediate hosts for most of the species are members of the basommatophoran families Lymnaeidae Rafinesque, 1815 and Physidae Fitzinger, 1833. Some species of Trichobilharzia also infect snails of Planorbidae Rafinesque, 1815, another basommatophoran family (Basch, 1966; Nassi, 1987; Rind, 1991), and the Pleuroceridae Fischer, 1885, a caenogastropod family (Ito, 1960a, b). The 14 described North American species of Trichobilharzia (Table I) are transmitted by physid snails (T. physellae, T. querquedulae, T. adamsi, T. cameroni, and T. oregonensis) or lymnaeid snails (T. stagnicolae, T. elvae, T. alaskensis, and T. ocellata) snails. The snail hosts of T. waubesensis, T. kegonsensis, T. burnetti, and T. horiconensis, and T. brantae (until this study) are unknown. All of the North American species occur in anatid ducks (Anatinae, Aythyinae, Merginae), except T. brantae, which infects geese (Anserinae).
Table I.
List of the North American species of Trichobilharzia and their definitive and/or intermediate hosts from the type descriptions.
| Species | Intermediate host | Definitive host | Type Locality | |
|---|---|---|---|---|
| Trichobilharzia adamsi | Edwards & Jansch, 1955 | Physa gyrina† | * Exp: peking duck | Canada |
| Trichobilharzia alaskensis | Harkema, 1960 | Lymnaea stagnalis† | Exp: peking duck | Alaska |
| Trichobilharzia brantae | Farr & Blankemeyer, 1956 | unknown | Branta canadensis† | Virginia |
| Trichobilharzia burnetti | Brackett, 1942 | unknown | Aythya collaris† | Wisconsin |
| Trichobilharzia cameroni | Wu, 1953 | Physa gyrina† | Exp: canary, pigeon, domestic duck | Canada |
| Trichobilharzia elvae | (Miller, 1923) Talbot, 1936 | Lymnaea stagnalis† | Exp: peking & black duck | Michigan |
| Trichobilharzia horiconensis | Brackett, 1942 | unknown | Anas americana† | Wisconsin |
| Trichobilharzia kegonsensis | Brackett, 1942 | unknown | Aythya valisinera† | Wisconsin |
| Trichobilharzia ocellata | (La Valette, 1855) Brumpt, 1931 | Lymnaea stagnalis† | Exp: domestic duck | Germany |
| Trichobilharzia oregonensis | MacFarlane & Macy 1946 | Physa gyrina† | Exp: peking duck | Oregon |
| Trichobilharzia physellae | (Talbot, 1936) McMullen & Beaver, 1945 | Physa parkeri† | Exp: domestic duck | Michigan |
| Trichobilharzia querquedulae | McLeod, 1937 | Anas discors† | Canada | |
| Trichobilharzia stagnicolae | (Talbot, 1936) McMullen & Beaver, 1945 | Stagnicola emarginata† | Exp: canary | Michigan |
| Trichobilharzia waubesensis | Brackett, 1942 | unknown | Anas americana† | Wisconsin |
Exp=experimental exposure;
Type host.
Cercariae of species of Trichobilharzia were the first to be implicated in causing cercarial dermatitis or swimmer’s itch (Cort, 1928), an underappreciated and underreported condition occurring worldwide except in Antarctica (Cort, 1950; Lindblade, 1998; Larsen et al., 2004). Contemporary swimmer’s itch cases are most frequently caused by Trichobilharzia cercariae (Loken et al., 1995; Kolářová et al., 1997; Farahnak and Essalat, 2003; Voronin and Beer, 2002; Bouree and Caumes, 2004; Sheng et al., 2004; Żbikowska, 2004; Coady et al., 2006), although several other genera of avian schistosomes can also cause swimmer’s itch (Buckley, 1938; Cort, 1950; Stunkard and Hinchliffe, 1952; Chu, 1958; Tang and Tang, 1976).
Avian schistosomes, including Trichobilharzia, are a challenging group to identify and fully characterize due to the difficulties in obtaining intact adult specimens, the paucity of informative adult characters, the short duration of infection in birds and the difficulty of experimentally completing life cycles thereby relating the adult worms in birds to their larval stages, including cercaria, from snails. Moreover, changes over the last several decades in land use and water management have altered habitats for birds and snails, leaving no guarantee that transmission dynamics and species composition reported in the original species descriptions remain the same. Compounding this problem, morphological and behavioral features of the worms may vary depending on their age, season, age and size of the host, and whether or not worms have been collected from a primary or minor host (McMullen and Beaver, 1945; Wu, 1953; Farr and Blankemeyer, 1956; Stunkard, 1959; Combes, 1967; Bayssade-Dufour et al., 2006). In an understandable effort to identify dermatitis-causing schistosomes, some species were named based on only cercariae or on variable or difficult to locate adult morphological features (e.g. length, testes arrangement, position of the cecal reunion). The lack of clearly distinguishable features to identify Trichobilharzia and other avian schistosomes, including their cercariae, has impeded our understanding of the etiology and epidemiology of swimmer’s itch.
The application of molecular systematics methods to this group of worms offers great promise as an initial step in resolving many of these difficulties. Molecular markers have expanded our understanding of schistosome parasites by permitting much less ambiguous identification of species or distinct genetic lineages (Morgan et al., 2003; Vilas et al., 2005; Brant and Loker, 2005; Brant et al., 2006; Štefka et al., 2009). The solid reference points provided by DNA sequence data permit differentiation of morphologically similar parasites and the linking of different life cycle stages that may have been collected decades apart (Vilas et al., 2005; Brant et al., 2006). These DNA sequences can be used to augment taxonomy and species delimitation, as corroborating evidence for existing hypotheses, or for falsifying systematic hypotheses (DeSalle et al., 2005).
Throughout this paper, we focus on a clade of schistosomes found in birds, herein designated as the BTGD clade (sensu Brant et al., 2006). Carmichael (1984) using morphological characters was the first to propose the phylogenetic relationships within Schistosomatidae and placed the genus Trichobilharzia within the BTGD clade. To date molecular phylogenetic analyses undertaken for Trichobilharzia include only 3 species (T. frankiMüller and Kimmig, 1994, T. szidati Neuhaus, 1952, and T. regenti Horák et al., 1998), all believed to be primarily European in distribution (Picard and Jousson 2001; Dvořák et al., 2002; Ferté et al., 2005; Rudolfová et al., 2005, 2007; Jouet et al., 2008; Aldhoun et al., 2009). This leaves the remaining 37 putative species virtually unknown with respect to molecular markers. To expand our knowledge of the systematics of Trichobilharzia, we surveyed North American birds and freshwater snails and incorporated these data into a molecular phylogenetic analysis. These results will be valuable for future taxonomic revisions of the genus and this framework will shed new light on understanding the origins, radiation, evolution and patterns of host usage of this diverse group of blood flukes. They will also assist investigators seeking more precise identification of the cercariae involved in dermatitis outbreaks, and will contribute to the eventual unraveling of this complex etiology of this common affliction.
MATERIALS AND METHODS
Specimen collection and examination
Birds were obtained from a variety of sources: our own hunting/collecting; hunters; or frozen carcasses provided by the State of New Mexico Department of Game and Fish or the Museum of Southwestern Biology, Division of Ornithology. The viscera and nasal tissues of freshly killed birds were examined in saline for schistosomes between 30 min to 12 hr postmortem. Frozen birds were thawed and examined immediately. The intestine was divided into thirds and scrapings were made to look for eggs. Worms were teased out and either relaxed and killed in hot water or put immediately into 95% ethanol for subsequent DNA analysis. Young of the year birds, targeted and collected by us before their flight feathers had developed, were collected in Churchill, Manitoba and Douglas Lake, Michigan as a way to guarantee that their parasites were acquired from their natal habitats.
Snails were collected by hand or wire mesh scoop and kept cool and moist until returned to the lab. Each snail was isolated individually in a 24-well tissue culture plate in artificial spring water and placed in natural light to induce cercarial shedding. If conditions allowed, snails that did not shed the first day were placed in aerated containers with lettuce and screened again 2 to 7 days later. In most cases, snails shed cercariae within 30 min after being placed in natural light. All schistosome cercariae were saved in 95% ethanol.
Adult worms were stained in Semichon’s acetocarmine and mounted in Canada balsam on slides for measurements and morphological observation (Pritchard and Kruse, 1982). Specimens collected from this study were identified both by morphology (when possible) and by DNA sequence. Morphological determinations were made by comparison with the original species descriptions, and if available, with voucher specimens from the U.S. National Parasite Collection: Trichobilharzia kegonsensis (USNPC 044865), Trichobilharzia horiconensis (USNPC 044866), Trichobilharzia burnetti (USNPC 044867), Trichobilharzia waubesensis (USNPC 044868), Trichobilharzia querquedulae (USNPC 079068), Trichobilharzia physellae (USNPC 079636, 083314), and Trichobilharzia brantae (USNPC 047609). Voucher specimens for adults and cercariae from this study were deposited in the Division of Parasitology, Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico (Accession numbers: T. brantae male and cercariae MSB Para176, 182, 184; T. physellae males and cercariae MSB Para177–178; T. stagnicolae cercariae MSB Para179; T. querquedulae males and cercariae MSB Para180, 181, 183).
Life cycle investigations
Host verification
In an attempt to verify host use, snails or domestic ducks were exposed experimentally to species of Trichobilharzia. Miracidia were hatched from eggs by rinsing and then diluting the feces of the avian host in artificial spring water in an Erlenmeyer flask. All but the neck of the flask was covered with aluminum foil, leaving just the top exposed to light, to concentrate miracidia (McMullen and Beaver, 1945). Flasks were placed in natural light and miracidia were collected within 30 min. Snails were isolated individually in a tissue culture well plate with artificial spring water and were exposed each to 3 miracidia. The wells were examined after 1 hr to ensure no miracidia remained. Snails were screened for cercarial shedding 3–6 wk post-infection (PI). To verify that adult worms and miracidia derived from the same bird were the same species (in cases where both were collected), cox1 and ITS were sequenced for both. Any worms collected from experimental infections were also sequenced for cox1 and ITS and compared to the initial life cycle stage used in the experiment to verify it was the same species. This was done both to confirm that adults and cercariae were the same species and that all worms collected from experimental infections were identical.
Trichobilharzia querquedulae
In April 2004, at Bitter Lake National Wildlife Refuge, lab-reared strains of Physa acuta Draparnaund, 1805 n=30, and Stagnicola elodes (Say, 1821) n=30 and pre-screened and trematode-negative wild-caught found not to be shedding cercariae over a 3-day period Gyraulus parvus (Say, 1817) n=10 were exposed to miracidia. Half the snails of each species were exposed to miracidia from cinnamon teal, Anas cyanoptera Vieillot, 1816, and the other half to miracidia from blue winged teal, Anas discors L.
Trichobilharzia physellae
In March 2005, at Bitter Lake National Wildlife Refuge, New Mexico, lab-reared Physa gyrina (Say, 1821) n=30, and S. elodes n=30 were exposed to miracidia from either the lesser scaup, Aythya affinis (Eyton, 1838), or the bufflehead, Bucephalus albeola L. At Douglas Lake, Michigan, in August 2005, wild-caught Stagnicola emarginata Say, 1821 (n=10) and Physa parkeri Currier, 1868 (n=10), determined not to be shedding cercariae over a 3-day period, were exposed to miracidia of T. physellae hatched from the feces of Mergus merganser L. collected from the lake.
Trichobilharzia stagnicolae
In August 2005, individuals of the type host species, S. emarginata, were collected from the type locality for T. stagnicolae at Douglas Lake, University of Michigan Biological Station (McMullen and Beaver, 1945). Morphology of the cercariae collected was consistent with the original descriptions by Talbot (1936) and McMullen and Beaver (1945). Six domestic mallards and 6 peking ducks, all 10-days-old, were exposed to about 100 cercariae each for up to 30 min. The birds’ feet were checked for cercarial dermatitis to determine if penetration had occurred. Feces were examined every other day from 1–4 wk PI. The birds were killed at either 2 and 4 wk PI and examined for worms. Ten specimens each of wild-caught S. emarginata (n=10) and P. parkeri (n=10) from Douglas Lake determined not to be shedding cercariae over a 3-day period were exposed to miracidia of T. stagnicolae from of the common merganser, M. merganser, collected from the same lake.
Sequencing data and phylogenetic analysis
DNA was extracted from fresh or alcohol preserved worms with the DNeasy Tissue Kit (Qiagen, Valencia, California) according to manufacturer’s guidelines or HotShot Lysis (Truett et al., 2000). In a few cases, multiple worms from a single host were extracted. DNA was amplified by polymerase chain reaction (Takara Ex Taq kit, Takara Biomedicals, Otsu, Japan) and sequenced using previously published primers. For 18S–28S, we used primers listed in Brant et al. (2006). For ITS we used its4, its5 (Dvořák et al., 2002), 3S (Bowles et al., 1995), and 4S (Bowles and McManus, 1993). We designed primers for cox1: CO1F15: 5′-TTT NTY TCT TTR GAT CAT AAG C-3′ and CO1R15: 5′-TGA GCW AYH ACA AAY CAH GTA TC-3′ and an internal sequencing primer CO1RH3R: 5′-TAA ACC TCA GGA TGC CCA AAA AA-3′. PCR products were purified with Montage Microcon columns (Millipore, Billerica, Maryland). Sequencing reactions were performed with Applied Biosystems BigDye direct sequencing kit, version 3.1 (Applied Biosystems, Foster City, California).
Phylogenetic analyses were performed on 6 different datasets. The first dataset was comprised of combined 18S–28S sequence data to place the samples collected for this study within the larger context of the family Schistosomatidae (Snyder, 2004; Brant et al., 2006). The second dataset comprised a combined matrix of 18S–28S-partial ITS (ITS1-5.8S-ITS2)-cox1 regions to reconstruct the relationships within the genus with existing isolates of Trichobilharzia from GenBank and included a greater sampling of individuals from more localities and hosts from our collections. The third and fourth datasets included separate analyses to look at congruence between nuclear DNA (ITS1-58S-ITS2) and mtDNA (cox1). The ITS1-5.8S-ITS2 region was used because it was the only region that was conserved enough to align unambiguously all available schistosomes. The fifth dataset was ITS1, and included T. franki samples as well as samples of Trichobilharzia that were designated as unidentified from Europe from the studies of Picard and Jousson (2001) and Rudolfová et al., (2007). The sixth dataset was ITS2 that included T. franki samples from Jouet et al., (2008). The last 2 analyses were to assess the positions of all available European taxa in GenBank relative to the North American taxa.
Phylogenetic analyses using maximum parsimony (MP), maximum likelihood (ML), and Minimum evolution (ME) were carried out using PAUP* ver 4.0b10 (Swofford, 2002) and Bayesian inference (BI) using MrBayes (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003). jModeltest (Posada, 2008) was used to determine the best nucleotide substitution model for ML and ME analyses. In cases where the Bayesian Information Criteria (B.I.C) or Akaike Information Criteria (A.I.C) criteria selected different models, both were used in analyses and in all cases, the tree topologies were the same. The combined 18S-28S-ITS-cox1 was rooted with members of SchistosomaWeinland, 1858 since ITS sequences were available only for Schistosoma.
The model, GTR+I+G, from jModeltest was used for both combined datasets, as well as for the ITS1 dataset. For cox1, ITS2, and ITS1-5.8S-ITS2, the model TVM+I+G was selected for ML and ME analyses. For BI, a mixed model approach was implemented to account for the potential differences in evolutionary model parameters between data partitions (both genes and codon positions). Parsimony trees were reconstructed using heuristic searches, random taxon-input order and tree-bisection and reconnection (TBR) branch swapping. Optimal ME and ML trees were determined from heuristic searches (50 replicates for ME, 5 replicates for ML), random taxon-input order, and TBR. Nodal support was estimated by bootstrap (100 replicates) and was determined for the MP and ME trees using heuristic searches, each with random taxon-input order. In all BI that included cox1, the dataset was partitioned by codon positions. For the BI of the 18S-28S-ITS-cox1 dataset, there were 4 partitions defined by first 18S, 28S (Nst=6 rates=gamma ngammacat=4), second cox1 codon1, third cox1 codon2, and fourth cox1 codon3 (Nst=6 rates-invgamma ngammacat=4). All parameters were unlinked between partitions. For all the analyses, 4 chains were run simultaneously for 5 × 105 generations, trees sampled every 100 cycles, the first 5,000 trees with preasymptotic likelihood scores were discarded as burnin, and the retained trees were used to generate 50% majority-rule consensus trees and posterior probabilities.
RESULTS
Specimen collections
Results of the survey for species of Trichobilharzia in North American birds and snails are reported in Table II and Figure 1. Ten lineages of Trichobilharzia were collected and the collection localities of specimens used in the phylogenetic analyses are listed in Table III. A total of 378 birds of 46 species were necropsied (Fig. 1, Table II), of which 92 birds were infected with Trichobilharzia (overall prevalence 24.5%). Nine of the 10 lineages reported were collected as adults and/or miracidia. Approximately 10,000 snails representing 4 families and 21 species were examined, of which 20 snails were infected with a species of Trichobilharzia, representing 7 of the 10 species (Table II). In most cases, prevalence of Trichobilharzia infections in snails was around 1%. However, at Glen Lake in Michigan, the prevalence of T. stagnicolae in Stagnicola emarginata was 30%. No nasal schistosomes were found in birds nor were any cercariae from snails genetically similar to the avian nasal schistosome, T. regenti. Three species of Trichobilharzia (T. physellae, T. querquedulae, and T. brantae) were identified whose adult morphology corresponded to the original species descriptions (Tables IV, V). One species collected was from cercariae with sequence data that matched GenBank sequences attributed to T. szidati (Table III). Adult worms in the lineage identified as T. querquedulae could be differentiated from T. physellae and T. franki by the position of the cecal reunion and the number of testes (Table IV). Also, adults of the lineage identified as T. physellae are consistently smaller and have a shorter gynaecophoric canal as compared to T. querquedulae (Table IV).
Table II.
List of the hosts examined harboring species of Trichobilharzia. Localities are labeled with the U.S. state abbreviation, except for Manitoba, Canada (MB). Schistosomes were found in hosts and localities in bold. See text for further description.
| North America Avian Host | Number examined | Viscera positive | Nasals examined | Species of Trichobilharzia | Locality |
|---|---|---|---|---|---|
| Larus delawarensis | 5 | 0 | 0 | - | LA, CA |
| Larus delawarensis | 10 | 0 | 0 | - | CA |
| Larus californicus | 1 | 0 | 0 | - | CA |
| Larus fuscus | 1 | 0 | 0 | - | LA |
| Sterna maxima | 1 | 0 | 0 | - | LA |
| Anhinga anhinga | 10 | 0 | 0 | - | FL, LA |
| Phalacrocorax auritus | 2 | 0 | 0 | - | LA |
| Pluvialis dominicus | 1 | 0 | 0 | - | LA |
| Egretta tricolor | 1 | 0 | 0/1 | - | LA |
| Egretta thula | 1 | 0 | 0/1 | - | LA |
| Plegadis chihi | 6 | 0 | 0 | - | LA |
| Eudocimus albus | 12 | 0 | 0/5 | - | LA |
| Aramus guarauna | 1 | 0 | 0 | - | FL |
| Podilymbus podiceps | 2 | 0 | 0 | - | NM |
| Gavia immer | 1 | 0 | 0 | - | NM |
| Xanthocephalus xanthocephalus | 1 | 0 | 0 | - | NM |
| Aythya affinis | 28 | 11 | 0/7 | T. physellae | AK, CA, LA, NM, PA |
| Aythya americana | 4 | 0 | 0 | - | CA, LA, NM |
| Aythya collaris | 5 | 1 | 0 | T. physellae | CA, LA, NM |
| Aythya marila | 9 | 0 | 0 | AK, PA, MB | |
| Aythya valisineria | 5 | 1 | 0/3 | T. physellae | NM, NV |
| Anas acuta | 23 | 1 | 0/1 | Trichobilharzia sp. E | AK, CA, LA, NV, NM, MB |
| Anas americana | 23 | 8 | 0/1 | Trichobilharzia spp. A and B | AK, CA, NM |
| Anas carolinensis | 41 | 4 | 0/7 | Trichobilharzia physellae | AK, CA, LA, NM, PA |
| Anas clypeata | 22 | 20 | 0/13 | T. querquedulae | AK, CA, LA, NE, NM, MB |
| Anas cyanoptera | 12 | 11 | 0/2 | T. querquedulae | CA, NM |
| Anas discors | 20 | 20 | 0/4 | T. querquedulae | CA, FL, LA, NM, PA |
| Anas fulvigula | 5 | 0 | 0/3 | - | LA |
| Anas platyrhynchos | 12 | 2 | 0/3 | T. physellae | AK, LA, MI, PA, |
| Anas rubripes | 3 | 0 | 0 | - | PA |
| Anas strepera | 27 | 1 | 0/5 | T. physellae | LA, NM, PA |
| Aix sponsa | 4 | 1 | 0/1 | T. physellae | LA, NM, PA |
| Bucephala albeola | 8 | 1 | 0 | T. physellae | NM, NV, PA |
| Bucephala clangula | 3 | 0 | 0 | - | CA, NM |
| Clangula hyemalis | 9 | 1 | 0 | T. physellae | AK |
| Histrionicus histrionicus | 1 | 0 | 0 | - | AK |
| Lophodytes cucullatus | 5 | 1 | 0 | Trichobilharzia sp. C | LA, PA |
| Mergus merganser | 6 | 3 | 0/1 | T. stagnicolae + T. physellae | MI |
| Mergus serrator | 3 | 0 | 0 | - | CA, NM |
| Oxyura jamaicensis | 6 | 0 | 0/2 | - | CA, NM, NV |
| Melanitta fusca | 5 | 0 | 0 | - | AK, MB |
| Melanitta perspicillata | 3 | 0 | 0 | - | AK |
| Somateria mollissima | 1 | 0 | 0/1 | - | MB |
| Cygnus columbianus | 13 | 0 | 0/5 | * | NV, NM |
| Chen caerulescens | 9 | 2 | 0/7 | Trichobilharzia brantae | LA, NM, MB |
| Branta canadensis | 7 | 3 | 0/5 | T. brantae | NM, NV, MB |
| Snail Hosts | |||||
| Physidae | |||||
| Physa gyrina | T. physellae | CA, MT, MN, NE, NM, NV, MB | |||
| Physa acuta | T. querquedulae | experimental in NM | |||
| Physa parkeri | T. physellae | MI, MN | |||
| Aplexa sp. | - | MB | |||
| Lymnaeidae | |||||
| Stagnicola emarginata | T. stagnicolae | MI, MN | |||
| Stagnicola elrodi | T. szidati | MT, MN, MB | |||
| Stagnicola elodes | - | MN, NM | |||
| Stagnicola sp. | Trichobilharzia sp. D | MB | |||
| Stagnicola sp. | Trichobilharzia sp. E | MB | |||
| Radix auricularia | - | NM | |||
| Fossaria sp. | - | NM | |||
| Lymnaea stagnalis | T. szidati | AK, MI, MT, MN, MB | |||
| Bulimnaea megasoma | - | MN | |||
| Planorbidae | |||||
| Gyraulus parvus | T. brantae | CO, MT, MN, MB | |||
| Planorbula armigera | - | MN | |||
| Promenetus exacuous | - | MN | |||
| Pecosorbis kansasensis | - | NM | |||
| Helisoma trivolvus | - | LA, NM, MI, MN | |||
| Helisoma anceps | - | MI, MN | |||
This host had Allobilharzia visceralis reported in Brant, 2007. The ‘+’ indicates co-infection in all three hosts examined.
FIGURE 1.
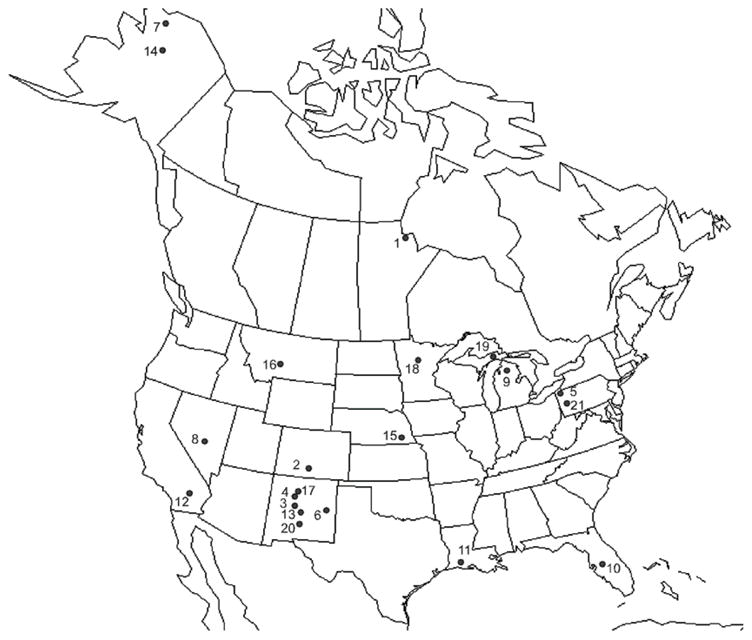
Collection localities. Refer to Table III for more details. Localities are as follows: 1 Canada: Manitoba, Churchill 58.7541 N; 93.8066 W, July/August 2007; 2 Colorado: El Paso Co. 38.827 N; 104.804 W, June 2007; 3 New Mexico: Bernalillo Co. 35.1305 N; 106.6822 W, July 2002; 4 New Mexico: Sandoval Co. 35.8485 N; 106.4907 W, July 2006; 5 Pennsylvania: Erie Co. 42.1703 N; 80.0868 W, November 2004; 6 New Mexico: Chavez Co. 33.45 N; 104.4 W, April 2005, March 2006; 7 Alaska: North Slope Borough 68.9820 N; 148.8318 W, June 2005; 8 Nevada: Churchill Co. 39.9 N; 118.817W, November 2005; 9 Michigan: Cheboygan Co. 45.581 N; 84.697 W, July, 1999, August 2005; 10 Florida; 11 Louisiana, Cameron Parish 26.661 N; 92.688 W, November 2003; 12 California: Imperial Co. 33.2988 N; 115.5875 W, November 2004; 13 New Mexico: Socorro Co. 33.7131 N 106.9579 W, April 2004; 14 Alaska: Yukon-Koyukuk Borough 65.665 N; 149.098 W, May 2005; 15 Nebraska: Nemaha Co. 40.467 N; 95.7 W, November 2004; 16 Montana: Big Fork Lake Co. 47.483 N; 114.217 W, 1999; 17 New Mexico: Taos Co. 36.8467 N; 105.3794 W, June 2004; 18 Minnesota: Itasca Co. 47.510 N; 94.185 W, July 2008; 19 Michigan: Luce Co. 46.667 N; 85.733 W, July 1999; 20 New Mexico: Sierra Co. 32.9071 N; 107.3116 W, February 2005; 21 Pennsylvania: Crawford Co. 41.575 N; 80.212 W, November 2004.
Table III.
The host and locality origin of the specimens used in this study. Numbered localities were collected for this study and relate to Fig. 1.
| Schistosome Taxa | Host | Life cycle stage | GenBank Accession numbers | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Locality | 18S | 28S | ITS | CO1 | ||||
| Trichobilharzia brantae | ||||||||
| W340 sngo MB | Chen caerulescens | A* | 1 Canada | FJ174451 | FJ174467 | FJ174533 | FJ174482 | This paper |
| W346 Gyraulus MB | Gyraulus parvus | C | 1 Canada | FJ174534 | This paper | |||
| W330 Gyraulus CO | Gyraulus parvus | C | 2 Colorado | FJ174450 | FJ174466 | FJ174532 | FJ174484 | This paper |
| W331 Gyraulus CO | Gyraulus parvus | C | 2 Colorado | FJ174531 | This paper | |||
| This paper | ||||||||
| Trichobilharzia franki | Radix sp. | C | Germany | FJ711767 | FJ711768 | FJ174530 | This paper | |
| T. franki Cz | Radix auricularia | C | Czech Republic | AF356845 | Dvořák et al., 2002 | |||
| T. franki Ra1 | Radix auricularia | C | Czech Republic | AY713969 | Rudolfová et al., 2005 | |||
| T. franki Ra2 | Radix auricularia | C | Poland | AY713964 | Rudolfová et al., 2005 | |||
| T. franki Ra3 | Radix auricularia | C | Poland | AY713966 | Rudolfová et al., 2005 | |||
| T. franki RSFO1 | Radix auricularia | C | France | AY795572 | Ferte et al., 2005 | |||
| T. franki Ls1 | Lymnaea stagnalis | C | Czech Republic | AY713973 | Rudolfová et al., 2007 | |||
| T. franki auri1 | Radix auricularia | C | Switzerland | AJ312041 | Picard & Jousson, 2001 | |||
| T. franki auri2 | Radix auricularia | C | Switzerland | AJ312042 | Picard & Jousson, 2001 | |||
| T. franki ov1 | Radix ovata | C | Switzerland | AJ312043 | Picard & Jousson, 2001 | |||
| T. franki ov2 | Radix ovata | C | Switzerland | AJ312044 | Picard & Jousson, 2001 | |||
| T. franki ov3 | Radix ovata | C | Switzerland | AJ312045 | Picard & Jousson, 2001 | |||
| T. franki ov4 | Radix ovata | C | Switzerland | AJ312046 | Picard & Jousson, 2001 | |||
| Trichobilharzia physellae | ||||||||
| W146 Physa NM | Physa gyrina | C | 3 New Mexico | FJ174568 | FJ174513 | This paper | ||
| W263PhysaNM | Physa gyrina | C | 4 New Mexico | FJ174562 | FJ174523 | This paper | ||
| W171 lesc PA | Aythya affinis | A | 5 Pennsylvania | FJ174564 | FJ174515 | This paper | ||
| W193 lesc NM | Aythya affinis | A | 6 New Mexico | FJ174457 | FJ174473 | FJ174518 | This paper | |
| W212 lesc AK | Aythya affinis | A | 7 Alaska | FJ174563 | FJ174512 | This paper | ||
| W256 lesc NM | Aythya affinis | A | 6 New Mexico | FJ174575 | FJ174522 | This paper | ||
| W194 ridu NM | Aythya collaris | A | 6 New Mexico | FJ174566 | FJ174517 | This paper | ||
| W249 caba NV | Aythya valisineria | A | 8 Nevada | FJ174565 | This paper | |||
| W255 buhe NM | Bucephala albeola | A | 6 New Mexico | FJ174458 | FJ174474 | FJ174561 | FJ174514 | This paper |
| W211 olsq AK | Clangula hyemalis | A | 7 Alaska | FJ174516 | This paper | |||
| W230.1 come MI | Mergus merganser | M | 9 Michigan | FJ174567 | FJ174521 | This paper | ||
| W234 come MI | Mergus merganser | M | 9 Michigan | FJ174569 | FJ174519 | This paper | ||
| W236 Physa MI | Physa parkeri | C | 9 Michigan | FJ174459 | FJ174475 | FJ174520 | This paper | |
| Trichobilharzia querquedulae | ||||||||
| E45 blte FL | Anas discors | A | 10 Florida | FJ174453 | FJ174469 | FJ174555 | FJ174510 | This paper |
| E64 blte FL | Anas discors | A | 10 Florida | FJ174511 | This paper | |||
| W137 blte LA | Anas discors | A | 11 Louisana | FJ174452 | FJ174468 | FJ174558 | FJ174498 | This paper |
| W156 blte NM | Anas discors | A | 6 New Mexico | FJ174554 | FJ174502 | This paper | ||
| W190 blte CA | Anas discors | A | 12 California | FJ174550 | FJ174507 | This paper | ||
| W148.1 cite NM‡ | Anas cyanoptera | A | 13 New Mexico | FJ174559 | FJ174499 | This paper | ||
| W148.2 cite NM | Anas cyanoptera | A | 13 New Mexico | FJ174500 | This paper | |||
| W155.3 cite NM | Anas cyanoptera | A | 13 New Mexico | FJ174553 | FJ174501 | This paper | ||
| W180 cite CA | Anas cyanoptera | A | 12 California | FJ174454 | FJ174470 | FJ174556 | FJ174505 | This paper |
| W135 nosh LA | Anas clypeata | A | 11 Louisana | FJ174557 | FJ174497 | This paper | ||
| W203 nosh AK | Anas clypeata | A | 14 Alaska | FJ174552 | FJ174508 | This paper | ||
| W158 nosh NM | Anas clypeata | A | 6 New Mexico | FJ174549 | FJ174503 | This paper | ||
| W162 nosh NM | Anas clypeata | A | 6 New Mexico | FJ174551 | FJ174504 | This paper | ||
| W183 nosh CA | Anas clypeata | A | 12 California | FJ174560 | FJ174506 | This paper | ||
| SDS1006 nosh NE | Anas clypeata | A | 15 Nebraska | FJ174548 | This paper | |||
| W345 nosh MB | Anas clypeata | A | 1 Canada | FJ174547 | FJ174509 | This paper | ||
| Trichobilharzia regenti | Radix peregra | C | Czech Republic | AY157219 | AY157245 | AY157190 | Lockyer et al., 2003 | |
| T. regenti Pl14 | Anas clypeata | M | Poland | EF094533 | Rudolfová et al., 2006 | |||
| T. regenti Pl17 | Aythya fuligula | M | Poland | EF094534 | Rudolfová et al., 2006 | |||
| T. regenti Pl20 | Anas platyrhynchus | M | Poland | EF094535 | Rudolfová et al., 2006 | |||
| T. regenti Pl27 | Anas platyrhynchus | M | Poland | EF094537 | Rudolfová et al., 2006 | |||
| T. regenti Cz31 | Anas platyrhynchus | M | Poland | EF094538 | Rudolfová et al., 2006 | |||
| T. regenti Cz79 | Anas clypeata | M | Czech Republic | EF094540 | Rudolfová et al., 2006 | |||
| T. regenti ad1 | Anas platyrhynchus | A | Switzerland | AJ312049 | Picard & Jousson, 2001 | |||
| T. regenti ov1 | Radix ovata | C | Switzerland | AJ312047 | Picard & Jousson, 2001 | |||
| T. regenti ov2 | Radix ovata | C | Switzerland | AJ312048 | Picard & Jousson, 2001 | |||
| Trichobilharzia stagnicolae | ||||||||
| W240 come MI | Mergus merganser | M | 9 Michigan | FJ174462 | FJ174478 | FJ174544 | FJ174490 | This paper |
| W230.2 come MI | Mergus merganser | M | 9 Michigan | FJ174545 | FJ174493 | This paper | ||
| Stagnicola MT | Stagnicola sp. | C | 16 Montana | FJ174541 | FJ174488 | This paper | ||
| DouglasLake Stagnicola MI | Stagnicola emarginata | C | 9 Michigan | FJ174463 | FJ174479 | FJ174546 | FJ174489 | This paper |
| W164 Stagnicola NM | Stagnicola sp. | C | 17 New Mexico | FJ174461 | FJ174477 | FJ174540 | FJ174492 | This paper |
| W224 Stagnicola MI | Stagnicola emarginata | C | 9 Michigan | FJ174542 | FJ174494 | This paper | ||
| W229 Stagnicola MI | Stagnicola emarginata | C | 9 Michigan | FJ174543 | FJ174491 | This paper | ||
| W400 Stagnicola MN | Stagnicola emarginata | C | 18 Minnesota | |||||
| Trichobilharzia szidati | Lymnaea stagnalis | C | Czech Republic | AF263828 | AY157191 | Dvořák et al., 2002 | ||
| AY157219 | AY157245 | Lockyer et al., 2003 | ||||||
| Blind Sucker Lymnaea MI | Lymnaea stagnalis | C | 19 Michigan | FJ174460 | FJ174476 | FJ174538 | FJ174496 | This paper |
| Flathead Stagnicola MT | Stagnicola elrodi | C | 16 Montana | FJ174539 | FJ174495 | This paper | ||
| T. szidati Ls5 | Lymnaea stagnalis | C | Poland | AY713967 | Rudolfová et al., 2006 | |||
| T. szidati ToA | Lymnaea stagnalis | C | Netherlands | AY713970 | Rudolfová et al., 2005 | |||
| T. szidati ToE | Lymnaea stagnalis | C | Germany | AY713971 | Rudolfová et al., 2005 | |||
| T. szidati Tsz | Lymnaea stagnalis | C | Czech Republic | AY713972 | Rudolfová et al., 2005 | |||
| T. szidati Pl21 | Anas platyrhynchos | M | Poland | EF094536 | Rudolfová et al., 2006 | |||
| T. szidati Cz11 | Anas platyrhynchos | A | Czech Republic | EF094541 | Rudolfová et al., 2006 | |||
| Unspecified species of Trichobilharzia | ||||||||
| Trichobilharzia sp. 3 Pl10 | Anas penelope | M | Poland | EF094531 | Aldhoun et al., 2009 | |||
| Trichobilharzia sp. 3 Pl7 | Anas penelope | M | Poland | EF094532 | Aldhoun et al., 2009 | |||
| Trichobilharzia sp. EAN17 | Radix peregra | C | France | EU413971 | Jouet et al., 2008 | |||
| Trichobilharzia sp. EAN35 | Radix peregra | C | France | EU413974 | Jouet et al., 2008 | |||
| Trichobilharzia sp. A | ||||||||
| W149 amwi NM | Anas americana | A | 13 New Mexico | FJ174456 | FJ174472 | FJ174574 | FJ174524 | This paper |
| W182 amwi CA | Anas americana | A | 12 California | FJ174573 | FJ174525 | This paper | ||
| W192 amwi NM | Anas americana | A | 20 New Mexico | FJ174455 | FJ174471 | FJ174572 | FJ174526 | This paper |
| W213 amwi AK | Anas americana | A | 7 Alaska | FJ174570 | FJ174527 | This paper | ||
| Trichobilharzia sp. B | ||||||||
| W205 amwi AK | Anas americana | A | 14 Alaska | FJ174571 | FJ174528 | This paper | ||
| Trichobilharzia sp. C | ||||||||
| W173 home PA | Lophodytes cucullatus | A | 21 Pennsylvania | FJ174576 | FJ174529 | This paper | ||
| Trichobilharzia sp. D | ||||||||
| W376 Stagnicola MB | Stagnicola sp. | C | 1 Canada | FJ174465 | FJ174481 | FJ174537 | FJ174485 | This paper |
| Trichobilharzia sp. E | ||||||||
| W332 Stagnicola MB | Stagnicola sp. | C | 1 Canada | FJ174464 | FJ174480 | FJ174483 | This paper | |
| W336 Stagnicola MB | Stagnicola sp. | C | 1 Canada | FJ174535 | FJ174486 | This paper | ||
| W344 pita MB | Anas acuta | A | 1 Canada | FJ174536 | FJ174487 | This paper | ||
| Other schistosomatids | ||||||||
| Ornithobilharzia canaliculata | Larus delawarensis | A | U.S.A. | AY157222 | AY157248 | Lockyer et al., 2003 | ||
| Austrobilharzia variglandis | Larus delawarensis | A | U.S.A. | AY157224 | AY157250 | AY157196 | Lockyer et al., 2003 | |
| Austrobilharzia terrigalensis | Batillaria australis | C | Australia | AY157223 | AY157249 | AY157195 | Lockyer et al., 2003 | |
| Macrobilharzia macrobilharzia | Anhinga anhinga | A | U.S.A. | AY829260 | AY858885 | Brant et al., 2006 | ||
| Bivitellobilharzia nairi | Elephas maximus | A | Sri Lanka | AY829261 | AY858888 | AY829249 | Brant et al., 2006 | |
| Schistosoma japonicum | †Mus musculus | A | Tanzania | AY157226 | AY157607 | Lockyer et al., 2003 | ||
| Orientobilharzia turkestanicum | Ovis aries | A | Iran | AF442499 | AY157254 | Lockyer et al., 2003 | ||
| Schistosoma hippopotami | Bulinus truncatus | C | Uganda | AY197343 | Morgan et al., 2003 | |||
| Schistosoma incognitum | Bandicota indica | A | Thailand | AY157229 | AY157255 | Lockyer et al., 2003 | ||
| Schistosome spindale | †Mus musculus | A | Sri Lanka | Z11979 | Johnston et al., 1993 | |||
| AY157257 | Lockyer et al., 2003 | |||||||
| Schistosoma margrebowiei | †Mus musculus | A | Zambia | AY157233 | AY157260 | Lockyer et al., 2003 | ||
| Schistosoma leiperi | †Mesocricetus auratus | A | South Africa | AY157234 | AY157261 | Lockyer et al., 2003 | ||
| Schistosoma haematobium | †Mesocricetus auratus | A | Mali | Z11976 | AY157263 | Lockyer et al., 2003 | ||
| Schistosoma intercalatum | †Mus musculus | A | Sao Tome | AY157235 | AY157262 | Lockyer et al., 2003 | ||
| Schistosomatium douthitii | †Mesocricetus auratus | A | U.S.A. | AY157221 | AY157247 | Lockyer et al., 2003 | ||
| Heterobilharzia americana | †Mesocricetus auratus | A | U.S.A. | AY157220 | AY157246 | Lockyer et al., 2003 | ||
| W1285 Biomphalaria KE | Biomphalaria sudanica | C | Kenya | AY829258 | AY858886 | Brant et al., 2006 | ||
| Bilharziella polonica | Anas platyrhynchus | A | Ukraine, Czech Republic | AY157214 | AY157240 | EF094539 | AY157186 |
Lockyer et al., 2003 Rudolfová et al., 2006 |
| W2081 Ceratophallus KE | Ceratophallus sp. | C | Kenya | AY829259 | AY858887 | Brant et al., 2006 | ||
| Dendritobilharzia pulverulenta | Gallus, Mergus | A | U.S.A. | AY157215 | AY157241 | AY157187 | Lockyer et al., 2003 | |
| EF071988 | Brant, 2007 | |||||||
| Gigantobilharzia huronensis | Agelaius phoeniceus | A | U.S.A. | AY157216 | AY157242 | AY157188 | Lockyer et al., 2003 | |
| Gigantobilharzia huronensis | Agelaius phoeniceus | A | U.S.A. | EF071987 | Brant, 2007 | |||
| Allobilharzia visceralis | Cygnus cygnus | A | Iceland | DQ067561 | Kolářová et al., 2006 | |||
| Allobilharzia visceralis | Cygnus columbianus | A | U.S.A. | EF114220 | EF114222 | EF071989 | EF114219 | Brant, 2007 |
| Allobilharzia visceralis | Cygnus columbianus | A | U.S.A. | EF114221 | EF114223 | EF071991 | EF114224 | Brant, 2007 |
| Outgroups | ||||||||
| Cardiocephaloides longicollis | Larus ridibundus | Ukraine | AY222089 | AY222171 | Olson et al., 2003 | |||
| Alaria alata | Nyctereutes procyonoides | Ukraine | AY222091 | AF184263 | Olson et al., 2003 | |||
| Brachylaima thompsoni | Blarina brevicauda | U.S.A. | AY222085 | Olson et al., 2003 | ||||
| AF184262 | Tkach et al., 2001 | |||||||
| Urogonimus macrostomus | Anas platyrhynchus | Ukraine | AY222086 | AY222168 | Olson et al., 2003 | |||
| Leucochloridium perturbatum | Turdus merula | Czech Republic | AY222087 | AY222169 | Olson et al., 2003 | |||
| Clinostomum sp. USA | Rana catesbeiana | U.S.A. | AY222095 | AY222095 | Olson et al., 2003 | |||
| Aporocotyle spinosicanalis | Merluccius merluccius | United Kingdom | AJ287477 | Cribb et al., 2001 | ||||
| AY222177 | Olson et al., 2003 | |||||||
| Plethorchis acanthus | Mugil cephalus | Australia | AY222096 | AY222178 | Olson et al., 2003 | |||
| Unicaecum sp. | Trachemys scripta | U.S.A. | AY604719 | AY604711 | Snyder, 2004 | |||
| Vasotrema robustum | Apalone spinifera | U.S.A. | AY829257 | AY858883 | Brant et al., 2006 | |||
| Spirorchis scripta | Chrysemys picta marginata | U.S.A. | AY829256 | AY858882 | Brant et al., 2006 | |||
| Hapalorhynchus gracilis | Chelydra serpentina | U.S.A. | AY604718 | AY604710 | Snyder, 2004 | |||
| Griphobilharzia amoena | Crocodylus johnstoni | Australia | AY899915 | AY899914 | Brant et al., 2006 | |||
| Carettacola hawaiiensis | Chelonia mydas | U.S.A. | AY604717 | AY604709 | Snyder, 2004 | |||
| Learedius learedi | Chelonia mydas | U.S.A. | AY604715 | AY604707 | Snyder, 2004 | |||
| Hapalotrema mehrai | Chelonia mydas | U.S.A. | AY604716 | AY604708 | Snyder, 2004 | |||
A=adults M=miracidia C=cercariae;
experimental;
W148.1 and W148.2 are worms from the same host individual
Table IV.
Morphological comparisons useful in diagnosing both adults and cercariae. Measurements in micrometers unless otherwise indicated.
| T. querquedulae | T. querquedulae | T. physellae | T. physellae | T. franki | |
|---|---|---|---|---|---|
| Reference | this paper (average) | McLeod, 1937 | this paper (average) | McMullen & Beaver 1945 | Müller & Kimmig 1994 |
| Adults | n=3 | n=3 | |||
| length of males | 4.8 mm | 3.7 mm | 2.6 mm | 1.3–7.5 mm | 3.2–4.0 mm |
| VS - OS* | 417 | 274–375 | 320 | 160–340 | 485–530 |
| VS - GC | 400 | NA† | 440 | NA | 495–550(522) |
| length SV | 325 | NA | 400 | NA | |
| length GC | 225 | 375 | 186 | 100–190 | 212–291(246) |
| cecal reunion | not seen | between SV & GC | not seen | between VS & SV | between SV & GC |
| testes size | 18–23 | NA | 25–30 | 28–32 | 95–106 |
| number of testes | >200 | 210–240 | >100 | 96–160 | 41–64 |
| egg shape | spindle with spine | spindle with spine | spindle with spine | spindle with spine | spindle with spine |
| eggs in utero | - | 140 × 30 | - | 170 × 65 | 206 × 69 |
| eggs in feces | 150 × 35 | - | 180 × 70 | - | - |
| Cercariae | |||||
| snail host | Physa gyrina exp. | P. gyrina | P. gyrina | P. parkeri, P. gyrina | Radix auricularia |
| n=5 | n=5 | ||||
| length body | 327 | - | 270 | 265 | 307 |
| length tail | 410 | - | 352 | 374 | 419 |
| length furcae | 221–224 | - | 188 | 196 | 234 |
| ratio: body:furcae | 0.68 | 0.7 | 0.74 | 0.76 | |
| ratio: body:tail | 1.25 | - | 1.3 | 1.41 | 1.36 |
VS=ventral sucker, OS=oral sucker, GC=gynaecophoric canal, SV=seminal vesicle, exp=experimental infection.
NA=not available.
Table V.
Morphological comparisons of the key differentiating features among closely related genera of avian schistosomes.
| Trichobliharzia brantae | Allobilharzia | Trichobilharzia | Jilinobilharzia | |
|---|---|---|---|---|
| References |
Farr & Blankenmeyer, 1956 This study |
Kolářová et al., 2006 Brant, 2007 |
Skrjabin & Zakharov, 1920 Blair & Islam, 1983 |
Lui & Bai, 1976 |
| Total length males | 33.5 mm | 65 mm | 2.2–12 mm | 3.6–4.6 mm |
| Cecal reunion | at or anterior to the seminal vesicle | posterior to gynaecophoric canal | variable but within range of the seminal vesicle | middle gynaecophoric canal, posterior to seminal vesicle |
| Position of the seminal vesicle | between ventral sucker and gynaecophoric canal | between ventral sucker and gynaecophoric canal | between ventral sucker and gynaecophoric canal | in gynaecophoric canal |
| Start of the gynaecophoric canal | posterior to seminal vesicle | posterior to seminal vesicle | posterior to seminal vesicle | posterior to ventral sucker |
| End of the gynaecophoric canal | well before start of testes | well before start of testes | at start of testes | at start of testes |
| Testes | 585 | >400 | 57–240 | 83–132 |
| Average egg shape | ovoid with spine | long nonsymmetrical with spine | spindle with spine | spindle with spine |
| Cercaria flame cells | 5+1 | unknown | 6+1 | unknown |
A fifth schistosome we identified provisionally as T. stagnicolae (Talbot, 1936; McMullen and Beaver, 1945) was collected as miracidia from mergansers. McMullen and Beaver (1945) described T. stagnicolae from an experimental infection in canaries; their study is the only description and record of T. stagnicolae adults. Subsequent reports of presumptive T. stagnicolae in the literature have been as cercariae from S. emarginata (Swales, 1936; McLeod, 1940; Elliott, 1942; Zischke and Zischke, 1968; Keas and Blankespoor, 1997; Leighton et al., 2000; Blankespoor et al., 2001; Coady et al., 2006). In our study, we collected miracidia from mergansers, Mergus merganser, from the type locality, Douglas Lake, but were unable to locate adult worms or eggs. Our assignment of the name T. stagnicolae to the samples we collected was justified based on the following: 1) our samples were collected from the type locality and the type snail; 2) we infected successfully the type snail with miracidia collected from the type locality; 3) our cercarial measurements were the same as those reported by Talbot (1936) and McMullen and Beaver (1945); 4) previous fecal examinations of M. merganser on Douglas Lake revealed eggs of T. stagnicolae (Blankespoor et al., 2001); and 5) our collections of cercariae from S. emarginata from several lakes in northern Michigan, in northern Minnesota, and New Mexico (Table III), all genetically matched what we collected at Douglas Lake, the type locality (0%–1.2% for cox1). The finding of a distinct genetic lineage with widespread representation strongly suggests our samples are T. stagnicolae.
The common morphological features used to differentiate the above mentioned 5 species from other avian schistosomes are provided in Tables IV and V. The infections we observed in mergansers with both T. physellae and T. stagnicolae may occur more frequently than commonly recognized. Not only can double infections be easily overlooked because of difficulties in finding both adult worms and eggs, but care is also required to ensure adults and eggs are both assigned to the correct species.
The 5 remaining lineages sequenced, 4 of which were represented by portions of adult worms recovered from birds, grouped with species of Trichobilharzia, but could not be matched definitively to any existing species description or to GenBank sequences associated with a formal species name. To facilitate our discussion of these species, we designated these specimens as Trichobilharzia spp. A–E (Table III). Trichobilharzia spp. A and B were found only in the American widgeon, Anas americana Gmelin, 1789, from 3 widespread U.S. localities along the Pacific flyway. Trichobilharzia sp. A was also found in a widgeon from New Mexico. Trichobilharzia sp. C was from the hooded merganser, Lophodytes cucullatus L., from the eastern U.S. For Trichobilharzia spp. A–C, only worm fragments were collected that did not have informative morphological features. Trichobilharzia sp. D is represented by cercariae from a single lymnaeid snail from Manitoba. Trichobilharzia sp. E was collected both as a posterior fragment of an immature worm from a pintail duckling, Anas acuta, and as cercariae from Stagnicola sp., both from Manitoba. Cercariae morphology and measurements of Trichobilharzia sp. E are consistent with T. elvae, but these data alone are not sufficient for accurate species discrimination. Although sufficient adult worm material for these 5 lineages was not available for a conclusive morphological study to determine if these 5 lineages represent new or previously described species, the sequence and host-use data provided here are valuable reference points for future studies as additional specimens become available (Brant et al., 2006).
Life cycle experiments
For each life cycle experiment (Table VI), cox1 and ITS sequences from experimentally obtained life cycle stages were identical with sequences from the life cycle stage used as starting material for the infection. Miracidia of each schistosome lineage were able to infect snails of only a single gastropod family, similar to results of previous experiments (Wu, 1953). The only successful infections of T. stagnicolae were Stagnicola emarginata from miracidia from M. merganser (Table VI). None of the domestic duck experiments resulted in adult worms.
Table VI.
Results of experimental infections of birds and snails with species of Trichobilharzia.
Hosts from which worms were used for exposures are on the left column; hosts exposed are along the top of the table.
| Schistosome Taxa | Stagniola emarginata | Stagnicola elodes | Physa parkeri | Physa gyrina | Physa acuta | Gyraulus parvus | Peking duck | Domestic mallard |
|---|---|---|---|---|---|---|---|---|
| Trichobilharzia stagnicolae | ||||||||
| ex. Mergus merganser | 9/10 | - | 0/10 | - | - | - | - | - |
| ex. Stagnicola emarginata | - | - | - | - | - | - | 0/6 | 0/6 |
| Trichobilharzia physellae | ||||||||
| ex. Aythya affinis | - | 0/15 | - | 10/15 | - | - | - | - |
| ex. Bucephalus albeola | - | 0/15 | - | 8/15 | - | - | - | - |
| ex. Mergus merganser | 0/10 | - | 8/10 | |||||
| Trichobilharzia querquedulae | ||||||||
| ex. Anas cyanoptera | - | 0/15 | - | - | 12/15 | 0/5 | - | - |
| ex. Anas discors | - | 0/15 | - | - | 11/15 | 0/5 | - | - |
Phylogenetic analyses
DNA sequence data was deposited in GenBank, under Accession numbers FJ174450-FJ174576, FJ711767-68 for the 18S (1776 bp), 28S (1299 bp), cox1 (824 bp), and ITS (1227–1395 bp) datasets (Table III). For the phylogenetic analysis of Trichobilharzia, 130 new sequences of Trichobilharzia were analyzed along with 55 sequences from GenBank. The numbers of individuals of each species sequenced are shown in Table III. Aligned cox1 sequences appeared to be genuine mitochondrial sequence, rather than nuclear copies: sequences contained no stop codons, overlapping fragments contained no conflicts, base compositions were homogeneous across taxa, codon positions contained expected relative divergences (3>2>1), and highly suspect relationships were not evident.
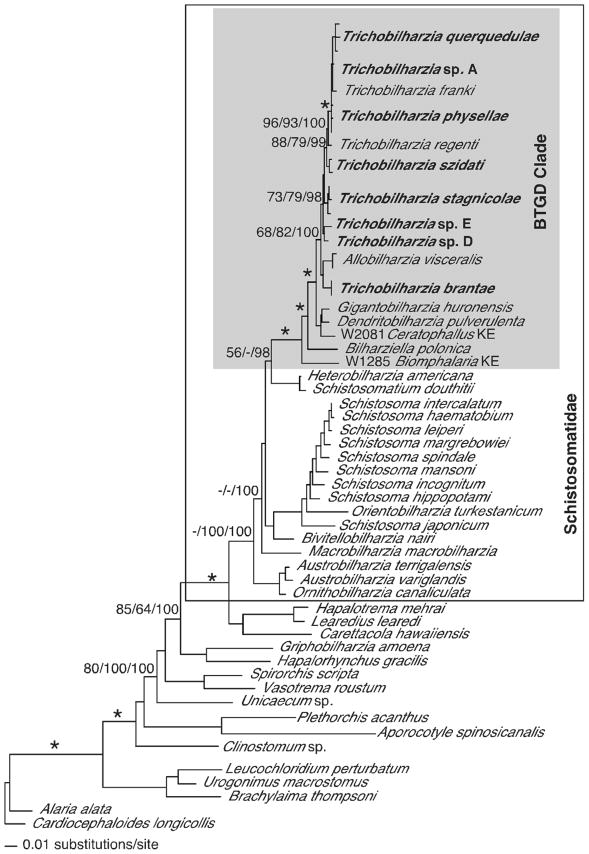
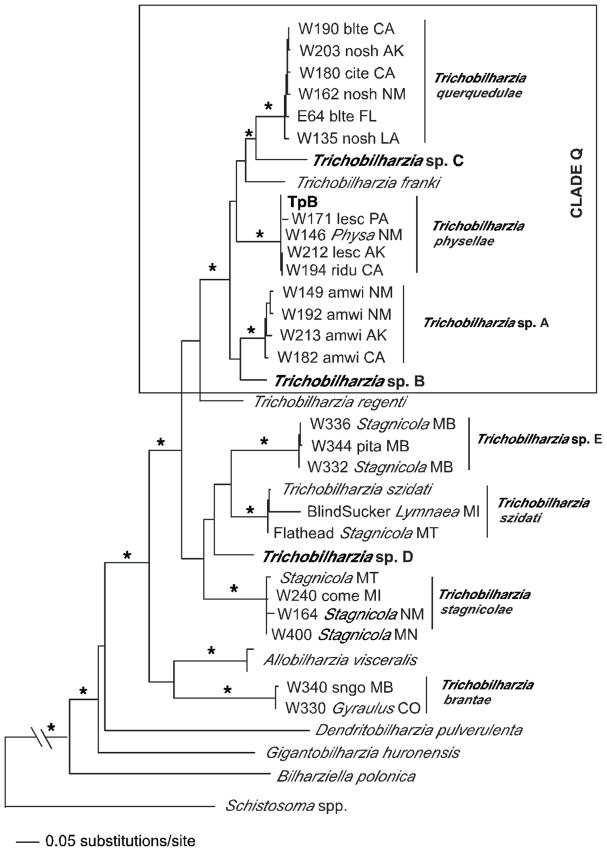
MP, ML, ME, and BI methods produced congruent results, except for some differences in the single gene analyses. Topological differences occurred, but no conflicts received high support from bootstrapping or Bayesian posterior probabilities. The 18S–28S tree supported monophyly of the Schistosomatidae and the BTGD clade (as defined in Fig. 2). Representatives of Trichobilharzia included in this study did not form a monophyletic group as T. brantae did not group with other North American or European Trichobilharzia, but rather grouped with the morphologically and genetically distinct AllobilharziaKolářová et al. 2006 from swans (Figs. 3–5). The overall relationships among the species of Trichobilharzia are shown in Figure 3. There was a basal split, albeit with low node support, between (T. stagnicolae, T. szidati, and Trichobilharzia spp. D and E) and (T. regenti and Clade Q). Here we identify Clade Q that includes species from North American and Europe that are both morphologically (Table IV) and genetically (Table VII) very similar (Fig. 3). Clade Q was recovered in all analyses and is comprised of T. franki, T. querquedulae, T. physellae, and Trichobilharzia spp. A, B, and C (Figs. 3–6, see discussion). Where known, based on our phylogenetic trees, members of Clade Q all have similar sized spindle-shaped eggs and included taxa dependent on either lymnaeid or physid snail hosts. Trichobilharzia regenti, a nasal-inhabiting species, was always recovered basal to Clade Q (Fig. 3).
FIGURE 2.
Maximum likelihood tree based on 18S–28S sequences. The schistosomatids are enclosed in the box, and the BTGD clade delimited by shading. Samples in bold are those collected from this study. Within the BTGD clade, individual specimens of Trichobilharzia were collapsed and are labeled only by the taxon name. Node support is indicated by MP and ME bootstrap values and Bayesian posterior probabilities (PP), respectively. The “*” indicates MP and ME bootstrap values of >90 and PP of 100. The ‘-’ indicates no significant node support. Branch support is designated only for the major clades.
FIGURE 3.
Maximum likelihood tree based on 18S-28S-cox1-ITS sequences. Trichobilharzia is highlighted. Samples in bold are those collected from this study. Node support is indicated by MP and ME bootstrap values and Bayesian PP, respectively. The ‘*’indicates MP and ME bootstrap values of >90 and PP of 100. The ‘-’ indicates no significant node support. Image is of Trichobilharzia physellae (USNPC# 079636).
FIGURE 5.
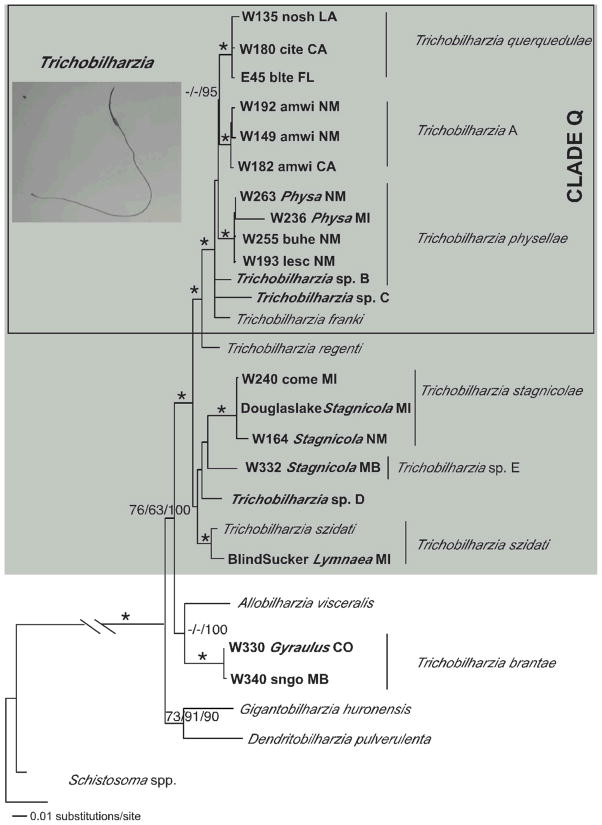
Maximum likelihood tree based on ITS sequences. The following labels apply to samples with identical haplotypes: Trichobilharzia querquedulae TqA= W135blteLA, W137blteLA, W156blteNM, W148.1citeNM, W155.3citeNM, W180citeCA, W203noshAK, W183noshCA, SDS1006noshNE. Trichobilharzia physellae TpB = W146PhysaNM, W263PhysaNM, W171lescPA, W212lescAK, W249cabaNV, W255buheNM. Trichobilharzia franki TfC = Trichobilharzia sp. C, T. franki Ra1, and T. franki RSFO1. All haplotypes of T. regenti downloaded from GenBank were identical; TrD = T. regenti Cz79, T. regenti Cz31, T. regenti Pl27, T. regenti Pl20, T. regenti Pl17, T. regenti Pl14. Trichobilharzia szidati TsE = T. szidati Tsz, T. szidati Ls5, T. szidati ToA. Trichobilharzia brantae TbF = W346GyraulusMB, W331GyraulusCO, W330GyraulusCO. Isolates of T. franki are from R. ovata (ov) and R. auricularia (Ra) snails (one sample is from Lymnaea stagnalis = Ls). The “*” indicates node support of >95% bootstrap for MP and ME and >98 Bayesian PP. The ‘-’ indicates no significant node support.
Table VII.
Genetic differences comparing ITS1, CO1 and ITS1-5.8S-ITS2 among schistosomes.
| Taxa | ITS1* | cox1* | ITS1-5.8S-ITS2 |
|---|---|---|---|
| Within Schistosoma | |||
| S. japonicum - S. malayensis | 16.40% | ||
| S. japonicum - S. mekongi | 15.80% | ||
| S. malayensis - S. mekongi | 4.60% | 9.4% | |
| S. mansoni - S. rodhaini | 1.10% | 13.30% | - |
| S. haematobium - S. mattheei | 6.60% | 16.20% | 1.40% |
| S. haematobium - S. intercalatum | 0 | 11.60% | 0.50% |
| S. hippopotami - S. edwardiense | 4.80% | 21.40% | |
| Avian genera | |||
| Allobilharzia - T. brantae | - | 14.30% | 2.70% |
| Trichobilharzia - T. brantae | - | 14.50% | 6.40% |
| Allobilharzia - Trichobilharzia | - | 14.80% | 5.50% |
| Within Trichobilharzia | |||
| T. stagnicolae - T. physellae | - | 13.40% | 2.00% |
| T. stagnicolae - T. regenti | - | 12.7% | 1.90% |
| T. stagnicolae - T. querquedulae | - | 12.00% | 2.00% |
| T. stagnicolae - T. szidati | - | 11.20% | 1.80% |
| T. stagnicolae - Trichobilharzia sp. A | - | 11.40% | 2.00% |
| T. stagnicolae - Trichobilharzia sp. B | - | 12.50% | 1.80% |
| T. stagnicolae - Trichobilharzia sp. C | - | 12.50% | 1.80% |
| T. stagnicolae - Trichobilharzia sp. D | - | 10.70% | |
| T. stagnicolae - Trichobilharzia sp. E | - | 11.60% | 1.70% |
| T. szidati -T. physellae | - | 11.70% | 3.00% |
| T. szidati -T. regenti | - | 11.50% | 2.50% |
| T. szidati - T. querquedulae | - | 10.70% | 2.00% |
| T. szidati - Blindsucker Lymnaea MT | 4.70% | 0.36% | |
| T. szidati - Flathead Stagnicola MI | 0.48% | 0.40% | |
| T. szidati - Trichobilharzia sp. A | - | 11.00% | 2.80% |
| T. szidati - Trichobilharzia sp. B | - | 10.60% | 2.70% |
| T. szidati - Trichobilharzia sp. C | - | 11.50% | 2.70% |
| T. szidati - Trichobilharzia sp. D | - | 9.80% | |
| T. szidati - Trichobilharzia sp. E | - | 10.30% | 2.60% |
| T. regenti -T. querquedulae | - | 11.50% | 1.80% |
| T. regenti -T. physellae | - | 10.60% | 1.70% |
| T. regenti - Trichobilharzia sp. A | - | 10.50% | 1.50% |
| T. regenti - Trichobilharzia sp. B | - | 9.10% | 1.40% |
| T. regenti - Trichobilharzia sp. C | - | 11.10% | 1.20% |
| T. regenti - Trichobilharzia sp. D | - | 10.8% | |
| T. regenti - Trichobilharzia sp. E | - | 12.00% | 2.30% |
| Trichobilharzia sp. D -Trichobilharzia sp. A | - | 11.10% | |
| Trichobilharzia sp. D - Trichobilharzia sp. C | - | 12.10% | |
| Trichobilharzia sp. D - Trichobilharzia sp. E | - | 11.10% | |
| Trichobilharzia sp. D - T. querquedulae | - | 11.30% | |
| Trichobilharzia sp. D - T. physellae | - | 12.30% | |
| Trichobilharzia sp. E - Trichobilharzia sp. A | - | 13.10% | 3.20% |
| Trichobilharzia sp. E - Trichobilharzia sp. B | - | 12.30% | 2.70% |
| Trichobilharzia sp. E - T. querquedulae | - | 12.10% | 3.70% |
| Trichobilharzia sp. E - T. physellae | - | 12.80% | 3.50% |
| Within T. stagnicolae | - | 0.70% | 0.17% |
| Within T. szidati | - | 3.30% | 0.35% |
| Within T. regenti | - | - | 0.00% |
| Within T. physelllae | 0.23% | 0.80% | 0.22% |
| Within T. querquedulae | 0.18% | 0.82% | 0.40% |
| Within T. franki from R. auricularia | 0.70% | - | 0.20% |
| Within T. franki from R. ovata | 0.52–2.8% | - | 0.50% |
| Within Trichobilharzia sp. A | 0.41% | 0.10% | |
| Within Trichobilharzia sp. E | - | 0.50% | 0.60% |
| Clade Q | |||
| T. querquedulae - T. physellae | 3.00% | 8.60% | 0.88% |
| T. querquedulae - Trichobilharzia sp. A | 3.10% | 9.00% | 0.32% |
| T. querquedulae - Trichobilharzia sp. B | 3.20% | 8.10% | 0.70% |
| T. querquedulae - Trichobilharzia sp. C | 3.80% | 8.50% | 0.50% |
| T. querquedulae - T. franki from R. auricularia | 3.1–3.4% | 8.10% | 0.64% |
| T. querquedulae - T. franki from R. ovata | 3.4–5.3% | - | 1.00% |
| T. physellae - Trichobilharzia sp. A | 0.60% | 9.30% | 0.76% |
| T. physellae - Trichobilharzia sp. B | 0.95% | 8.30% | 0.50% |
| T. physellae - Trichobilharzia sp. C | 3.00% | 9.40% | 0.50% |
| T. physellae - T. franki from R. auricularia | 0.82–1.3% | 9.10% | 0.60% |
| T. physellae - T. franki from R. ovata | 2.5–3.6% | - | 0.87% |
| Trichobilharzia sp. A - Trichobilharzia sp. B | 0.70% | 6.80% | 0.40% |
| Trichobilharzia sp. A - Trichobilharzia sp. C | 1.70% | 8.80% | 0.30% |
| Trichobilharzia sp. B - Trichobilharzia sp. C | 1.80% | 8.80% | 0.13% |
| Trichobilharzia sp. A - T. franki from R. auricularia | 0.60% | 8.90% | 0.40% |
| Trichobilharzia sp. A - T. franki from R. ovata | 3.10% | - | 0.70% |
| Trichobilharzia sp. B - T. franki from R. auricularia | 0.12-0-36% | 8.30% | 0.20% |
| Trichobilharzia sp. B - T. franki from R. ovata | 4.00% | - | 0.50% |
| Trichobilharzia sp. C -T. franki from R. auricularia | 2.30% | 8.60% | 0.12% |
| Trichobilharzia sp. C - T. franki from R. ovata | 4.60% | - | 0.40% |
Values for ITS1 and cox1 in Schistosoma are taken from Vilas et al., 2005.
FIGURE 6.
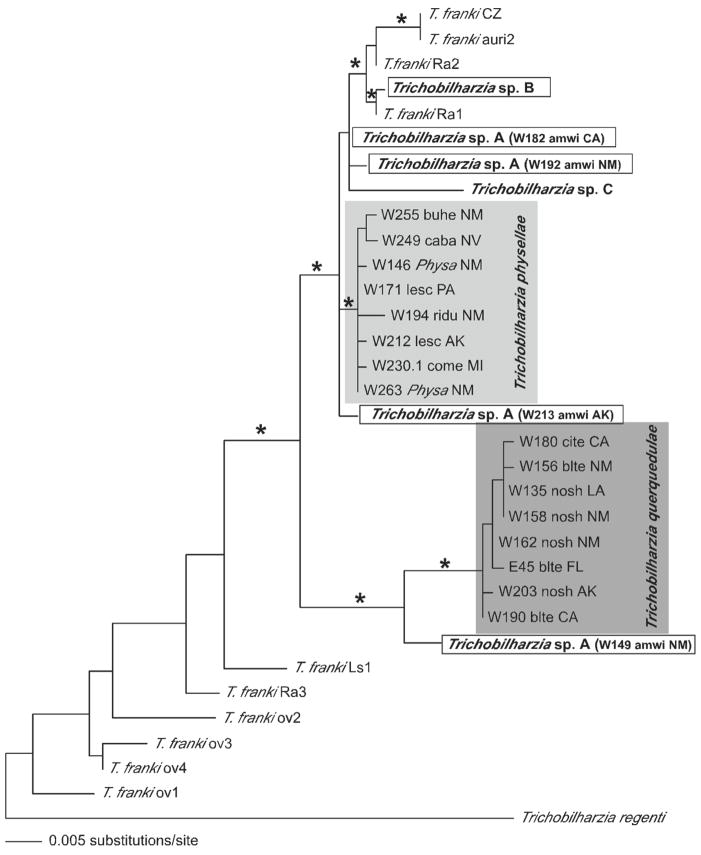
Maximum likelihood tree based on ITS1 sequences of an expanded Clade Q. Trichobilharzia A is boxed to show its variable position in the tree and paraphyly of T. franki. Isolates of T. franki are from R. ovata (ov) and R. auricularia (Ra) snails (one sample is from Lymnaea stagnalis = Ls). See Table III for label descriptions. The “*” indicates node support of >95% bootstrap for MP and ME and >98 Bayesian PP. The ‘-’ indicates no significant node support.
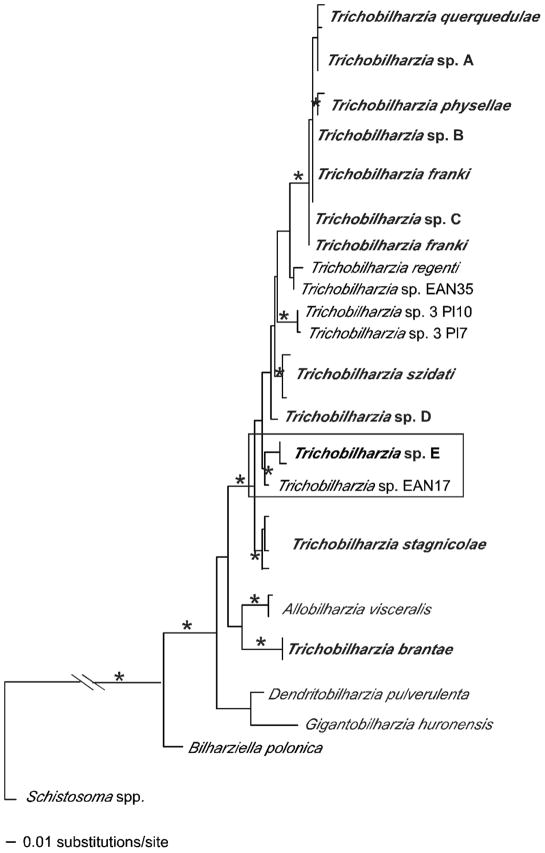
To further explore the relationships in Clade Q and to fully utilize available sequence data, separate analyses of 4 datasets (cox1, ITS1-5.8S-ITS2, ITS1, and ITS2), were completed. Additional individuals of Trichobilharzia from our collections (Table III) plus isolates of T. franki from GenBank were used. Many isolates of T. franki have been sequenced, however our analyses of these sequences revealed they did not form a monophyletic species group. Several isolates identified as T. franki were from either Radix auricularia L. snails (Ferté et al., 2005; Rudolfová et al., 2005) or R. ovata (Draparnaud, 1805) snails (Picard and Jousson 2001), but based on our analyses, isolates from these two snail species did not appear to be the same species.
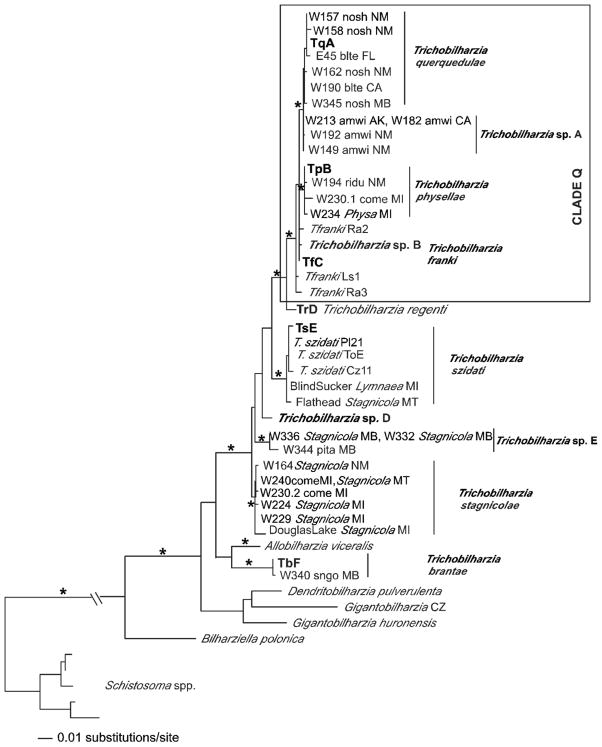
In the separate gene analyses, the topologies recovered were generally the same with some exceptions. Only the cox1 tree (Fig. 4) supported Trichobilharzia sp. A as a clade. Unfortunately, there were no additional cox1 samples of T. franki available in GenBank to include in this analysis. In the analysis of the ITS1-5.8S-ITS2 dataset (Fig. 5), T. franki from Radix auricularia was not recovered as monophyletic (see Table III for labels). Furthermore, Trichobilharzia sp. C was identical to several isolates of T. franki (Fig. 5). The ITS1 data set (Fig. 6) included additional samples of T. franki from R. ovata and our analysis indicated that not only did the T. franki from R. ovata not form a clade, but they did not group with any of the T. franki from R. auricularia (Fig. 6). Similarly, the isolates of T. franki from R. auricularia did not group together. Trichobilharzia sp. B grouped with some of the T. franki isolates from R. auricularia from the two different studies (Table III, Fig. 6) of Picard and Jousson (2001) and Rudolfová et al. (2005). Isolates of T. franki from R. ovata were basal in Clade Q (Fig. 6). Additional samples and gene regions are needed to determine definitively if T. franki as represented by available sequences is actually more than 1 species, and if our Trichobilharzia spp. B and C are the same as or distinct from the European samples labeled T. franki. Our data are suggestive that T. franki also occurs in North America. R. auricularia is found in North America, however the snail hosts for Trichobilharzia spp. A–C is not yet known.
FIGURE 4.
Maximum likelihood tree based on cox1 sequences. For the Trichobilharzia querquedulae clade only some of the worms sequenced are represented as many differed by only one base pair. The “*” indicates node support of >95% bootstrap for MP and ME and >98 Bayesian PP. The ‘-’ indicates no significant node support. Outgroup species of Schistosoma were collapsed. For convenience, the following taxa were trimmed from the tree, but were fully supported in the clade: W137blteLA, W156blteNM, W148.1citeNM, W148.2citeNM, W155.3citeNM, W158noshNM, W162noshNM, W183noshCA, SDS1006noshNE, E45blteFL (Table III). The same was done for T. physellae, except in one case there were identical haplotypes: TpB = W171lescPA, W193lescNM, W255buheNM, W263PhysaNM. Otherwise, the following with only 1–2 bp differences were removed W211olsqAK, W193lescNM, W236PhysaMI, W230.1comeMI, and W256lescNM.
The available ITS2 sequences provided yet a different perspective (Fig. 7). In this analysis 3 unidentified species of Trichobilharzia (Trichobilharzia sp. 3 Pl10, Trichobilharzia sp. 3 Pl7, Trichobilharzia sp. EAN17) in GenBank for which only ITS2 data were available were added to our dataset (Table III). The positions of Trichobilharzia sp. 3 Pl10 and Trichobilharzia sp. 3 from Anas penelope L. from Poland (Rudolfová et al., 2007), were equivocal, but in the ME analysis they aligned with Trichobilharzia sp. D, although without support. The sample Trichobilharzia sp. EAN17 from Radix peregra (Müller, 1774) from France (Jouet et al., 2008) grouped with Trichobilharzia sp. E from Manitoba with strong node support, suggesting they may be conspecific.
FIGURE 7.
Maximum likelihood tree based on ITS2 sequences showing the positions of the unidentified avian schistosome isolates from GenBank. The boxed clade highlights the relationship between the samples from North America and France. Bolded samples indicate those from this study. See Table III for label descriptions. The “*” indicates node support of >95% bootstrap for MP and ME and >98 Bayesian PP. The ‘-’ indicates no significant node support.
To provide a convenient yardstick to measure the extent of sequence difference among species of Trichobilharzia, pairwise genetic differences were calculated and compared with values obtained for the relatively well-defined species of Schistosoma (Table VII). Based on such comparisons, the lineages of Trichobilharzia are as genetically distant from each other as are the named species within both Trichobilharzia and Schistosoma (Table VII), providing good presumptive evidence that they represent distinct species (Nolan and Cribb, 2005; Vilas et al., 2005). As determined by sequence analysis, 7 of the 10 lineages of Trichobilharzia were collected from snails, 4 of which corresponded to sequences that we obtained from Trichobilharzia adults (T. physellae, T. querquedulae, T. brantae, and Trichobilharzia sp. E). The remaining 3 taxa were from cercariae that grouped with sequence data from miracidia of T. stagnicolae, with sequences for T. szidati from Europe (Figs. 4, 5; Rudolfová et al., 2005), or that did not group with any species or clade (Trichobilharzia sp. D; Figs. 4, 5).
Despite the broad geographic and host sampling for T. physellae and T. querquedulae, which were both collected from across North America (Fig. 1; Table III), we did not find indications of geographic structuring within either species (Figs. 4, 5). With the genes used in this study, haplotypes that were identical or that differed in only 1–2 base pairs were found between both eastern and western samples. The T. stagnicolae isolates collected from Minnesota, Michigan, Montana, and New Mexico, also show little evidence of geographic differentiation (Fig. 4). The collection of T. szidati related isolates from North America was unexpected. Based on the ITS1-5.8S-ITS2 region (Table VII), our samples of T. szidati, from Montana (Flathead Stagnicola MT) in Lymnaea stagnalis L., and Michigan (Blind Sucker Lymnaea MI) in Stagnicola elrodi (Baker and Henderson, 1933), were genetically very similar (0.4%) to each other, as well as to the European isolates of T. szidati (0.36%). These figures are within the range of variation noted for T. szidati from Europe based on ITS1-5.8S-ITS2 (0–1.1%). In general, the genetic differences between species pairs within continents were not less than the differences between species pairs from different continents, Europe and North America (Table VII).
DISCUSSION
General observations
This is the first molecular systematics study of species of Trichobilharzia collected from a diversity of avian and snail species collected across North America. From North America we collected 5 morphologically identifiable and genetically distinct species of Trichobilharzia. These species were T. brantae, T. physellae, T. querquedulae, T. stagnicolae, and T. szidati, one of which (T. szidati) was reported previously from Europe (Rudolfová et al., 2005). We also collected 5 additional genetically distinct lineages that group within Trichobilharzia that could not be associated with a named species. These results suggest that at least 10 genetically distinct lineages of Trichobilharzia exist in North America. How these latter 5 species relate to the remaining species of Trichobilharzia described from North American not found in this study remains to be determined. Nevertheless, the sequence database generated here for North American species of Trichobilharzia will contribute to future studies revealing the broader species diversity and the host preferences for each species.
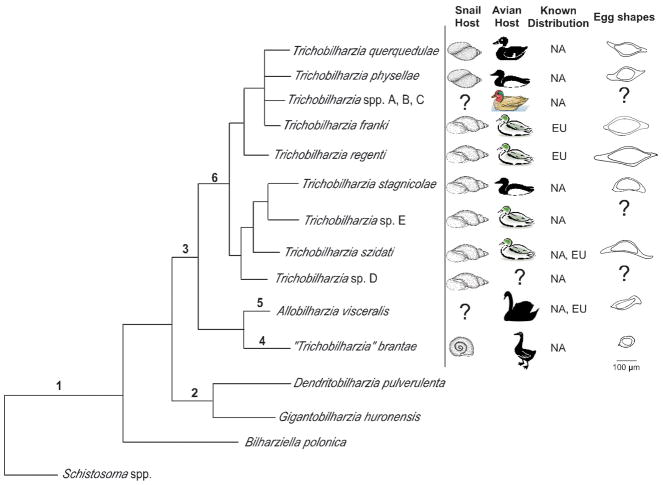
Although an analysis that includes additional specimens of putative Trichobilharzia from other continents is necessary to understand the full scope of the genus, our morphology (Table V; Fig. 8) and genetic differences strongly suggests that T. brantae should not be included as a member of Trichobilharzia. This species from geese and planorbid snails did not group within the Trichobilharzia clade, but rather aligned with Allobilharzia, a genus of schistosome collected thus far only from swans. Allobilharzia is morphologically and genetically distinct from Trichobilharzia; egg shape and position of cecal reunion are the two major differences (Table V, Fig. 8; Kolářová et al., 2006; Brant, 2007). Blair and Islam (1983) also suggested that T. brantae did not belong in the genus Trichobilharzia, but rather T. brantae should be transferred to the genus JilinobilharziaLui and Bai, 1976. However, a morphological comparison does not support inclusion of T. brantae in Jilinobilharzia or Allobilharzia. Table V compares some of the major morphological differences such as; shape of the eggs, position of the seminal vesicle and the start point and length of the gynaecophoric canal.
FIGURE 8.
Summary tree based on 28S depicting comparative features (hosts, distribution, and egg morphology) for North American and European avian schistosomes. Morphological features listed for well-supported nodes. 1– reduced sexual dimorphism, males and females flattened or thread-like, gynaecophoric canal absent or weakly developed or short (not extending to posterior), testes numerous; 2 – Absence of ventral sucker, absent or weakly developed oral sucker, uterus with numerous eggs, eggs ovoid; 3 – Well developed oral and ventral suckers, uterus usually with single egg, seminal vesicle between gynaecophoric canal and ventral sucker; 4 – cecal reunion at or anterior to seminal vesicle, >400 testes, gynaecophoric canal terminates well anterior to first testes; 5 – cecal reunion posterior to gynaecophoric canal, >400 testes, gynaecophoric canal terminates well anterior to first testes; 6 – Position of the cecal reunion overlaps the position of the seminal vesicle, gynaecophoric canal terminates at first testes, cercariae large with eyespots.
 Physidae,
Physidae,  Lymnaeidae,
Lymnaeidae,  Planorbidae,
Planorbidae,  teal,
teal,  diving ducks,
diving ducks,  Anas americana
Anas americana  most ducks,
most ducks,  swans,
swans,  geese. Eggs scaled to relative sizes.
geese. Eggs scaled to relative sizes.
The cox1 genetic difference values obtained for pairs of Trichobilharzia taxa outside of Clade Q (9–14%) were comparable to those obtained for congeners of other flatworm groups (Figs. 3, 6), including the confamilial Schistosoma (9–21%) in mammals (Vilas et al., 2005). Genetic differences among pairs of taxa within Clade Q were variable but generally low (7–9%), indicative of perhaps a more recent divergence among members of this clade (Table VII). Morphology as well as host use (definitive and intermediate) were considered relative to the molecular phylogenetic results by mapping these features onto the BTGD clade (Fig. 8). Host, morphology and DNA taken together revealed some interesting patterns discussed below, as well as highlighting the small number of distinguishing morphological features. The molecular and host use data provided here will eventually facilitate assessment of the validity of other species descriptions, assuming the specimens in question belong to a species described previously, and will help delineate new species (Štefka et al., 2009).
Life cycles of species of Trichobilharzia
Sequence markers provide an invaluable tool in connecting life cycle stages from wild hosts that have not been previously integrated into a complete life cycle through experimental infections. Given the challenges in obtaining the necessary approvals for maintenance of vertebrate animals, it may become difficult in the future to resolve life cycles through experimental infections. In our molecular survey, we found 4 sequence matches for cercariae from snails with adult worms from wild birds, thus providing strong direct inferences for the wild hosts involved in those particular life cycles. Such matches were obtained for T. physellae, Trichobilharzia sp. E, T. szidati, and T. brantae.
Snail Host Use
Excluding T. brantae, all Trichobilharzia from Europe for which life cycles are known use lymnaeid snails, whereas species from North America use lymnaeids or physids. Members of Lymnaeidae are worldwide in distribution, with their greatest species diversity occurring in North America. Interestingly, Lymnaea stagnalis and Radix auricularia, the former a host of T. szidati and the latter host to T. franki, are not considered endemic to North America (Remigio, 2002). Most of the diversity of Trichobilharzia in North America was found in species of Stagnicola (Table III). This is in contrast to studies in Europe where most of the diversity of Trichobilharzia was found in species of Radix (e.g. Picard and Jousson, 2001; Jouet et al., 2008; Aldhoun et al., 2009). Physidae is mainly a New World family, members of which have spread secondarily to other continents (Taylor, 2003). Thus far, only North America is known to have sequence-verified members of the Trichobilharzia clade that use physid snails. Although physid transmitted schistosomes are known from other continents (Ostrowski de Núñez, 1978; Rudolfová and Horák, 2001; Gerard, 2004), they have not been verified as a species of Trichobilharzia and may be representatives of Gigantobilharzia Odhner, 1910, one species of which in North America is also transmitted by physids (Brackett, 1942; Najim, 1956; Daniell, 1978). In no case did we find representatives of a single species of Trichobilharzia in snails belonging to more than 1 family, although in some cases we found more than 1 species of a particular snail family could host the same species of Trichobilharzia. For example, we found T. stagnicolae in Stagnicola emarginata and Stagnicola sp. and T. physellae in Physa parkeri and P. gyrina (Table II). In other studies, T. regenti has been collected from both Radix peregra and R. ovata snails (Picard and Jousson, 2001; Dvořák et al., 2002; Rudolfová et al., 2006). There is also an indication that T. franki occurs in more than one species of Radix, excluding those samples of T. franki from Piccard and Jousson (2001) from R. ovata snails (Jouet et al., 2008).
Physid transmitted species of Trichobilharzia were found only in Clade Q (Figs. 3,6) delineated in this study. In fact, so far as is known, Trichobilharzia is the only avian schistosome genus to use lymnaeid snails. The planorbid transmitted T. brantae, DendritobilharziaSkrjabin, 1920, and Bilharziella Looss, 1899 and the physid transmitted Gigantobilharzia huronensis Najim, 1950 are basal to Trichobilharzia within the BTGD clade, suggesting that in our results, lymnaeids are the basal hosts within species of Trichobilharzia (Fig. 8). Members of Clade Q (Fig. 6) are not strongly differentiated from one another on either morphological or genetic characters (Tables VI, VII), yet given that the clade includes 2 known physid transmitted species, T. physellae and T. querquedulae, it is suggested that at least 2 switches from one snail family to another occurred within Clade Q. Also, even though the genetic distances between physid and lymnaeid transmitted species in Clade Q (Table VII) are not great, all the available specimens for each physid transmitted species cluster together with unequivocally strong support in all analyses. Taken together, these results suggest that members of Clade Q have diverged relatively recently from one another, and that switches between 2 different snail families have occurred after which the taxa occupying different snail families remained genetically distinct from one another.
Definitive host use
In North America and Europe, all specimens of Trichobilharzia, Allobilharzia, and T. brantae for which there are molecular data, were found in avian hosts of the order Anseriformes (ducks, geese, and swans). Allobilharzia is known only from swans (Kolářová et al., 2006; Brant, 2007), and T. brantae is known only from geese (Farr and Blankemeyer, 1956; Wojcinski et al., 1987). The remaining species of Trichobilharzia collected for this study parasitize ducks of the Anatinae, Aythyinae, and Merginae. Species of Trichobilharzia from other continents have been described from other orders of birds, but thus far representatives of these species have not been available for sequencing to determine if they fall within the Trichobilharzia clade defined here.
Although there is not a strong pattern of definitive host specificity, some trends were identified (Fig. 8). Trichobilharzia querquedulae has been found only in 3 species of dabbling ducks (Table II; Anas clypeata L., A. cyanoptera, and A. discors) that are each other’s closest relatives (Johnson and Sorenson, 1999). In contrast, T. physellae utilizes mainly diving ducks (Aythyinae, mostly species of Aythya Boie, 1822) and mergansers (Merginae) as its major definitive hosts (ecological rather than phylogenetic). While these duck hosts are not each other’s closest relative, they are united ecologically by their preferred feeding habitat and style (diving). Although other duck species are also infected, prevalence is very low or there were few worms, most immature (Table II). One of the principal hosts of T. stagnicolae is a merganser (Blankespoor and Reimink, 1988; Leighton et al., 2000; Blankespoor et al., 2001; Coady et al., 2006), corroborated by our survey. It is interesting to note that 2 of the 3 unidentified lineages in Clade Q came from Anas americana, the American widgeon.
Some biogeographical remarks regarding North American Trichobilharzia
North American species of Trichobilharzia that we collected have broad geographic ranges and, at least as suggested by the markers used here, show little evidence of intraspecific genetic structure (Figs. 4–6). This is true for T. stagnicolae, which has been collected from Michigan, Minnesota, Montana, and New Mexico (Table III), and for specimens of T. physellae and T. querquedulae were collected from all the major avian migratory flyways, and from latitudes as distant as Alaska and Manitoba to Louisiana and Florida (Fig. 1; Table III). The latter 2 species have yet to be collected outside of North America.
Using sequence similarity as the criterion to designate species as outlined in Vilas et al. (2005), 4 avian schistosome lineages from North America have presumptive representatives in Europe (Table VII): (1) T. szidati, which is considered a European species (Rudolfová et al., 2005), was collected from North American snails (Table III, Fig. 5); (2) Trichobilharzia sp. B grouped with the European T. franki from R. auricularia (Fig. 6); (3) Trichobilharzia sp. E was closely aligned with Trichobilharzia sp. EAN17 from the snail, Radix peregra, collected in France (Fig. 7); and (4) although not the specific subject of this paper, specimens of Allobilharzia visceralis collected from the North American swans as part of this survey were indistinguishable from worms collected from swans in Iceland (see Kolářová et al., 2006; Brant, 2007). Thus, the continent of origin by no means represents an infallible indicator for species designations either for species of Trichobilharzia or other genera of avian schistosomes.
Diversification of Trichobilharzia
Incomplete taxon sampling and uncertainty among the basal nodes of the Trichobilharzia spp. radiation continue to challenge our understanding of the global diversification of this genus. The 40 named species of Trichobilharzia have been described from multiple locations in Europe and North America, Brazil (Leite et al., 1978), Australia (Blair and Islam, 1983; Islam, 1986; Islam and Copeman, 1986), New Zealand (Davis, 2006), China (Pao and Yung, 1957; Tang and Tang, 1976; Lui et al., 1977; Tsai et al., 1979), India (Baugh, 1963; Chauhan et al., 1973), Malaya (Basch, 1966), Japan (Ito, 1960; Yamaguti, 1971), Congo-Rwanda (Fain, 1955, 1956, 1959) and South Africa (Appleton, 1982, 1986). Most of these species were reported from ducks, geese and swans, however a few were reported from passerine birds, kingfishers, grebes and ibises (Fain, 1955, 1956; Ito, 1960; Tsai et al., 1979). Snail hosts where known, with one exception (Ito, 1960), are physid or lymnaeid snails. Reports of species of Trichobilharzia in North America, including this study, were all collected from ducks and/or physid or lymnaeid snails. The exception is T. brantae, which we now know occurs in geese and uses a planorbid snail as an intermediate host. A true global definition of Trichobilharzia awaits inclusion of genetically verified species from South America, Asia, and Africa.
Recent studies have shown that uncovering additional diversity among avian schistosomes is a frequent occurrence, particularly when snails are surveyed and molecular approaches are applied (Larsen et al., 2004; Brant et al., 2006; Rudolfová et al., 2007; Jouet et al., 2008; Skirnisson and Kolářová, 2008; Aldhoun et al., 2009). This suggests there is more diversity to discover with respect to Trichobilharzia, already considered the most speciose genus in the family. The second most speciose genus is Schistosoma, currently comprised of 22 species.
Perhaps what is more noteworthy is the relatively large number of distinct lineages for a parasite group that colonizes vagile, migratory definitive hosts. This is particularly so considering the overlaps in host species use and spatial and temporal sympatry among host species that regularly occurs on their breeding/wintering grounds and in other wetland habitats. The lack of host isolation coupled with the mobility of their host species would seem to weaken barriers to gene flow among the avian schistosomes. Moreover, lymnaeid and physid snails are both common, occur in large numbers, and are widely distributed, seemingly further reducing opportunities for regional diversification. The extent to which mating behavior/preferences or temporal or spatial separation within definitive hosts may disrupt gene flow and isolate species is not well known for avian schistosomes and will be excellent model systems for future investigations. Also, the acquisition of new molluscan hosts (for example, a switch from lymnaeid to physid snails as seems to have occurred in Clade Q) may also serve as a major isolating mechanism.
The relationships among T. franki, T. physellae, and Trichobilharzia spp. A, B, and C in Clade Q provide an interesting opportunity to address questions about gene flow, and speciation, and ultimately, diversification. There are several hypotheses, not necessarily mutually exclusive, that might explain the patterns observed in this clade that includes geographically distant, yet closely related North American and European species. It may be that (1) given the genetic and morphological similarities, these taxa are not fully differentiated as species because they have only recently diverged; (2) there may be isolation and incipient diversification among populations or species of Trichobilharzia that is diminished by ongoing gene flow that is maintained by the mobility of their hosts; (3) even though they are found in hosts considered mobile and that migrate long distances, the different taxa of Clade Q actually have subtle patterns of host use, or different geographical preferences that are not yet differentiated or require more sensitive genetic markers (like microsatellites) to reveal cryptic variation (Štefka et al., 2009); (4) hybridization may have occurred (Morgan et al., 2003; Fan and Lin, 2005; Steinauer et al., 2008); (5) the equivocal positions, or low branch support of individuals like Trichobilharzia spp. A, B, and C might imply that there remains undiscovered diversity (missing taxa) that, if available, would clarify relationships in this clade. Future work to increase the sample size within Clade Q and selection of alternative, faster evolving, markers to estimate gene flow will help address which of these processes have been important to shaping the diversity we find. We also need to accumulate more morphological data and understand how it correlates with genetic variation, to better define the status of species such as T. szidati and T. franki in North America.
Medical significance including cercarial dermatitis
None of the North American adult worms was found in host nasal turbinates, a location inhabited by some Trichobilharzia species in Europe (T. regenti), Australia (T. australisBlair and Islam, 1983, T. arcuata Islam, 1986), and Africa (T. spinulata Fain, 1955, T. rodhaini Fain, 1955, T. nasicola Fain, 1955, T. aureliani Fain, 1956, T. duboisi, Fain, 1959). This is of note from a public health perspective because the nasal-dwelling T. regenti migrates via both peripheral nerves and the central nervous system to reach its preferred site of infection. This species has been shown to cause anomalous behavior in both experimentally infected birds and mammals (Horák et al., 1999; Hrádková and Horák, 2002; Kouřilová et al., 2004) and has the potential to present similar consequences in humans.
Although most North American outbreaks of cercarial dermatitis are ascribed to T. physellae or T. stagnicolae (Swales, 1936; Cort, 1950; McMullen and Brackett, 1941; McLeod, 1940; Hunter, 1960; Zischke and Zischke, 1968; Leighton et al., 2000; Blankespoor et al., 2001; Coady et al., 2006), such identifications typically reflect whether the cercariae were shed from a physid or lymnaeid snail, respectively. The framework incorporating molecular markers developed here will be of immediate use in making more precise determinations. For example, although T. physellae was the taxon we most frequently collected from snails, at least 3 additional avian schistosome taxa from physid snails were collected, including representatives of other genera; accordingly, caution is required in ascribing physid transmitted outbreaks of dermatitis to T. physellae (S. Brant pers. obs.). The extent to which each of the 10 different taxa of Trichobilharzia noted here is actually involved in causing cercarial dermatitis in North America is an important priority for future study.
Acknowledgments
For help collecting birds and snails - Alaska: Kevin McCracken, Robert Wilson, Chris Barger at University of Alaska Fairbanks, Toolik Field Station; California: Stacy Frietas and staff at Salton Sea Wister Unit, Ryan Hechinger University California Santa Barbara, Andy Cohen San Francisco Estuary Institute, Neal Fujita East Bay Regional Park District; Florida: Jason M. Garvon Lake Superior State University; Louisiana: Steve Cardiff, Donna Dittman at Louisiana State University Museum of Natural History; Michigan: Harvey Blankespoor Hope College, Jitka Rudolfová Charles University Czech Republic; Minnesota: Jeff Lawrence and Steve Cordts MN Dept. Natural Resources; Pennsylvania: Jerry Bish Pennsylvania Game Commission, Tony Marich; Montana: Paul Watson University of New Mexico; Nebraska: Scott Snyder University of Nebraska-Omaha; Nevada: Chris Nicolai University of Reno, Dan Rabbers NV Fish and Wildlife Service, Kevin Wier; New Mexico: Loker Lab, Bob Dickerman, Andy Johnson at Museum of Southwestern Biology, Gordon Warrick Bitter Lake NWR; Canada, Manitoba LeeAnn Fishback, Carley Basler at Churchill Northern Studies Center. Duck hunters of Cameron and Ascension Parish Louisiana, Salton Sea California, Presque Isle and Pymatuning Pennsylvania; Stillwater Wildlife Management Area Nevada. For much help in the lab and perfecting techniques, Ben Hanelt. Appreciation extended to the United States National Parasite Collection, Eric Hoberg and Patricia Plitt for museum specimen loans. We thank two anonymous reviewers for their comments to help improve this paper. We acknowledge technical support from the University of New Mexico’s Molecular Biology Facility, which is supported by NIH Grant Number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research Resources. This study was supported by funds provided by the College of Arts and Sciences at UNM and NIH grant RO1 AI44913.
LITERATURE CITED
- Aldhoun JA, Kolářová L, Horák P, Skirnisson K. Bird schistosome diversity in Iceland: molecular evidence. Journal of Helminthology. 2009;83:173–180. doi: 10.1017/S0022149X09289371. [DOI] [PubMed] [Google Scholar]
- Appleton CC. The eggs of some blood-flukes (Trematoda: Schistosomatidae) from South African birds. South African Journal of Zoology. 1982;17:147–150. [Google Scholar]
- Appleton CC. Occurrence of avian Schistosomatidae (Trematoda) in South African birds as determined by faecal survey. South African Journal of Zoology. 1986;21:60–67. [Google Scholar]
- Basch PF. The life cycle of Trichobilharzia brevis, n. sp., an avian schistosome from Malaya. Zeitschrift fur Parasitenkunde. 1966;27:242–251. doi: 10.1007/BF00260344. [DOI] [PubMed] [Google Scholar]
- Baugh SC. Contributions to our knowledge of digenetic trematodes VI. Zeitschrift fur Parasitenkunde. 1963;22:303–315. doi: 10.1007/BF00260191. [DOI] [PubMed] [Google Scholar]
- Bayssade-Dufour C, Jouet D, Rudolfová J, Horák P, Ferté H. Seasonal morphological variations in bird schistosomes. Parasite. 2006;13:205–214. doi: 10.1051/parasite/2006133205. [DOI] [PubMed] [Google Scholar]
- Blair D, Islam KS. The life cycle and morphology of Trichobilharzia australis n. sp. (Digenea: Schistosomatidae) from the nasal blood vessels of the black duck (Anas superciliosa) in Australia, with a review of the genus Trichobilharzia. Systematic Parasitology. 1983;5:89–117. [Google Scholar]
- Blankespoor HD, Reimink RL. Control of swimmer’s itch in Michigan: Past, present and future. Michigan Riparian. 1988;10:10–19. [Google Scholar]
- Blankespoor CL, Reimink RL, Blankespoor HD. Efficacy of praziquantel in treating natural schistosome infections in common mergansers. Journal of Parasitology. 2001;87:424–246. doi: 10.1645/0022-3395(2001)087[0424:EOPITN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bouree P, Caumes E. La dermatitie cercarienne. Presse Medicale. 2004;33:490–493. doi: 10.1016/s0755-4982(04)98638-1. [DOI] [PubMed] [Google Scholar]
- Bowles J, McManus DP. Rapid discrimination of Echinococcus species and strains using a PCR-based RFLP method. Molecular and Biochemical Parasitology. 1993;57:231–239. doi: 10.1016/0166-6851(93)90199-8. [DOI] [PubMed] [Google Scholar]
- Bowles J, Blair D, McManus DP. A molecular phylogeny of the human schistosome. Molecular Phylogenetics and Evolution. 1995;4:103–109. doi: 10.1006/mpev.1995.1011. [DOI] [PubMed] [Google Scholar]
- Brackett S. Five new species of avian schistosomes from Wisconsin and Michigan with the life cycle of Gigantobilharzia gyrauli (Brackett, 1940) Journal of Parasitology. 1942;28:25–42. [Google Scholar]
- Brant SV. The occurrence of the avian schistosome Allobilharzia visceralis Kolářová, Rudolfová, Hampl et Skirnisson, 2006 (Schistosomatidae) in the tundra swan, Cygnus columbianus (Anatidae), from North America. Folia Parasitologica. 2007;54:99–104. doi: 10.14411/fp.2007.013. [DOI] [PubMed] [Google Scholar]
- Brant SV, Loker ES. Can specialized pathogens colonize distantly related hosts? Schistosome evolution as a case study. PLoS Pathogens. 2005;1:e38. doi: 10.1371/journal.ppat.0010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant SV, Morgan JAT, Mkoji GM, Snyder SD, Rajapakse RPVJ, Loker ES. An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including DNA sequence from single life cycle stages. Journal of Parasitology. 2006;92:77–88. doi: 10.1645/GE-3515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JJC. On a dermatitis in Malays caused by the cercariae of Schistosoma spindale Montgomery, 1906. Journal of Helminthology. 1938;16:117–120. [Google Scholar]
- Carmichael AC. PhD Dissertation. Michigan State University; East Lansing, Michigan: 1984. Phylogeny and historical biogeography of the Schistosomatidae; p. 246. [Google Scholar]
- Chauhan AS, Srivastava CB, Chauhan BS. Studies on the trematode fauna of India. Part 6. Digenea: Schistosomatidae a monographic aid to the identification of Indian schistosomes. Journal of the Zoological Society of India. 1973;25:83–128. [Google Scholar]
- Chu GWTC. Pacific area distribution of freshwater and marine cercarial dermatitis. Pacific Science. 1958;12:299–312. [Google Scholar]
- Coady NR, Muzzall PM, Burton TM, Snider RJ, Saxton J, Sergeant M, Sommers A. Ubiquitous variability in the prevalence of Trichobilharzia stagnicolae (Schistosomatidae) infecting Stagnicola emarginata in three northern Michigan lakes. Journal of Parasitology. 2006;92:10–15. doi: 10.1645/GE-3336.1. [DOI] [PubMed] [Google Scholar]
- Combes C. Corrélations entre les cycles sexuels des amphibiens Anoures et des Polystomatidae (Monogenea) Comptes Rendus de l’Academie des Sciences Series D. 1967;264:1051–1052. [Google Scholar]
- Cort WW. Schistosome dermatitis in the United States (Michigan) Journal of the American Medical Association. 1928;90:1027–1029. [Google Scholar]
- Cort WW. Studies on schistosome dermatitis XI. Status of knowledge after more than twenty years. American Journal of Hygiene. 1950;52:251–307. [PubMed] [Google Scholar]
- Daniell DL. PhD Dissertation. Iowa State University; Ames, Iowa: 1978. Biology and host-parasite relationships of Gigantobilharzia huronensis (Trematoda: Schistosomatidae) p. 166. [Google Scholar]
- Davis NE. Identification of an avian schistosome recovered from Aythya novaeseelandia and infectivity of its miracidia to Lymnaea tomentosa snails. Journal of Helminthology. 2006;80:225–233. [PubMed] [Google Scholar]
- Desalle R, Egan MG, Siddall M. The unholy trinity: taxonomy, species delimitation, and DNA bar-coding. Philosophical Transactions of the Royal Society, London B. 2005;360:1905–1916. doi: 10.1098/rstb.2005.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák J, Vanacova S, Hampl V, Flegr J, Horák P. Comparison of European Trichobilharzia species based on ITS1 and ITS2 sequences. Parasitology. 2002;124:307–313. doi: 10.1017/s0031182001001238. [DOI] [PubMed] [Google Scholar]
- Edwards DK, Jansch ME. Two new species of dermatitis producing schistosome cercariae from Cultus Lake, British Columbia. Canadian Journal of Zoology. 1955;33:182–194. [Google Scholar]
- Elliot AM. The present status of “swimmer’s itch” in northern Minnesota. Proceedings of the Minnesota Academy of Science. 1942;10:15–16. [Google Scholar]
- Fain A. Recherches sur les schistosomes d’oiseaux au Ruanda-Urundi (Congo belge) Revue de Zoologie et de Botanique Africaines. 1955;51:373–387. [Google Scholar]
- Fain A. Les schistosomes d’oiseaux du genre Trichobilharzia Skrjabin et Zakharov, 1920 au Ruanda Urundi. Revue de Zoologie et de Botanique Africaines. 1956;54:147–178. [Google Scholar]
- Fain A. Un nouveau schistosome du genre Trichobilharzia dans les fosses nasales du canard nain. Revue de Zoologie et de Botanique Africaines. 1959;60:227–232. [Google Scholar]
- Fan PC, Lin LH. Hybridization of Schistosoma mansoni and Schistosoma japonicum in mice. Southeast Asian Journal of Tropical Medicine and Public Health. 2005;36:89–96. [PubMed] [Google Scholar]
- Farahnak A, Essalat M. A study on cercarial dermatitis in Khuzestan province, south western Iran. BMC Public Health. 2003;3:35–38. doi: 10.1186/1471-2458-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr MM, Blankemeyer VG. Trichobilharzia brantae n. sp. (Trematoda: Schistosomatidae) from the Canada goose (Branta canadensis L.) Journal of Parasitology. 1956;42:320–325. [PubMed] [Google Scholar]
- Ferté H, Depaquit J, Carré S, Villena I, Léger N. Presence of Trichobilharzia szidati in Lymnaea stagnalis and T. franki in Radix auricularia in northeastern France: molecular evidence. Parasitology Research. 2005;95:150–154. doi: 10.1007/s00436-004-1273-7. [DOI] [PubMed] [Google Scholar]
- Gerard C. First occurrence of Schistosomatidae infecting Aplexa hypnorum (Gastropoda, Physidae) in France. Parasite. 2004;11:231–234. doi: 10.1051/parasite/2004112231. [DOI] [PubMed] [Google Scholar]
- Harkema R. Further studies of Alaskan schistosomes. Arctic Aeromedical Laboratory, Ladd AFB. 1955;3:1–15. [Google Scholar]
- Harkema R. Further studies of Alaskan schistosomes. Arctic Aeromedical Laboratory, Ladd AFB. 1960;57–61:iii-23. [Google Scholar]
- Horák P, Kolářová L, Dvořák J. Trichobilharzia regenti n. sp. (Schistosomatidae, Bilharziellinae) a new nasal schistosome from Europe. Parasite. 1998;5:349–357. doi: 10.1051/parasite/1998054349. [DOI] [PubMed] [Google Scholar]
- Horák P, Dvořák J, Kolářová L, Trefil L. Trichobilharzia regenti, a pathogen of the avian and mammalian central nervous system. Parasitology. 1999;119:577–581. doi: 10.1017/s0031182099005132. [DOI] [PubMed] [Google Scholar]
- Horák P, Kolářová L, Adema CM. Biology of the schistosome genus Trichobilharzia. Advances in Parasitology. 2002;52:155–233. doi: 10.1016/s0065-308x(02)52012-1. [DOI] [PubMed] [Google Scholar]
- Hrádková K, Horák P. Neurotrophic behaviour of Trichobilharzia regenti in ducks and mice. Journal of Helminthology. 2002;76:137–141. doi: 10.1079/JOH2002113. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hunter GW. Studies on schistosomiasis. XIII. Schistosome dermatitis in Colorado. Journal of Parasitology. 1960;46:231–233. [PubMed] [Google Scholar]
- Islam KS. The morphology and life-cycle of Trichobilharzia arcuata n. sp. (Schistosomatidae: Bilharziellinae) a nasal schistosome of water whistle ducks (Dendrocygna arcuata) in Australia. Systematic Parasitology. 1986;8:117–128. [Google Scholar]
- Islam KS, Copeman DB. The morphology and life cycle of Trichobilharzia parocellata (Johnston and Simpson, 1939) Islam and Copeman, 1980 from the visceral blood vessels of Australian anatids. Systematic Parasitology. 1986;8:39–49. [Google Scholar]
- Ito J. Contributions to the morphology of cercariae obtained from a snail host, Semisulcospira libertina in Japan. Japanese Journal of Medical Science and Biology. 1960a;13:59–72. doi: 10.7883/yoken1952.13.59. [DOI] [PubMed] [Google Scholar]
- Ito J. Studies on the morphology and life cycle of Pseudobilharziella corvi Yamaguti, 1941 (Trematoda: Schistosomatidae) Japanese Journal of Medical Science and Biology. 1960b;13:53–58. doi: 10.7883/yoken1952.13.53. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Sorenson MD. Phylogeny and biogeography of dabbling ducks (genus Anas): a comparison of molecular and morphological evidence. The Auk. 1999;116:792–805. [Google Scholar]
- Jouet D, Ferté H, Depaquit J, Rudolfová J, Latour P, Zanella D, Kaltenback ML, Léger N. Trichobilharzia spp. in natural conditions in Annecy Lake, France. Parasitology Research. 2008;103:51–58. doi: 10.1007/s00436-008-0926-3. [DOI] [PubMed] [Google Scholar]
- Keas BE, Blankespoor HD. The prevalence of cercariae from Stagnicola emarginata (Lymnaeidae) over 50 years in northern Michigan. Journal of Parasitology. 1997;83:536–540. [PubMed] [Google Scholar]
- Kolářová L, Horák P, Sitko J. Cercarial dermatitis in focus: schistosomes in the Czech Republic. Helminthologia. 1997;34:127–139. [Google Scholar]
- Kolářová L, Rudolfová J, Hampl V, Skirnisson K. Allobilharzia visceralis gen. nov. sp. nov. (Schistosomatidae-Trematoda) from Cygnus cygnus (L.) (Anatidae) Parasitology International. 2006;55:179–186. doi: 10.1016/j.parint.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Kouřilová P, Syrůček M, Kolářová L. The severity of mouse pathologies caused by the bird schistosome Trichobilharzia regenti in relation to host immune status. Parasitology Research. 2004;93:8–16. doi: 10.1007/s00436-004-1079-7. [DOI] [PubMed] [Google Scholar]
- Larsen AH, Bresciani J, Buchmann K. Increasing frequency of cercarial dermatitis at higher latitudes. Acta Parasitologica. 2004;92:30–35. [Google Scholar]
- Leighton BJ, Zervos S, Webster JM. Ecological factors in schistosome transmission, and an environmentally benign method for controlling snails in a recreational lake with a record of schistosome dermatitis. Parasitology International. 2000;49:9–17. doi: 10.1016/s1383-5769(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Leite ACR, Costa HMA, Costa JO. Trichobilharzia jequitibaensis n. sp. (Trematoda: Schistosomatidae) in Cairina moschata domestica (Anatidae) Revista Brasileira de Biologia. 1978;38:843–846. [Google Scholar]
- Lindblade KA. The epidemiology of cercarial dermatitis and its association with limnological characteristics of a northern Michigan Lake. Journal of Parasitology. 1998;84:19–23. [PubMed] [Google Scholar]
- Loken BR, Spencer CN, Granath WR., Jr Prevalence and transmission of cercariae causing schistosome dermatitis in Flathead Lake, Montana. Journal of Parasitology. 1995;81:646–649. [PubMed] [Google Scholar]
- Lockyer AE, Olsen PD, Ostergaard P, Rollinson D, Johnston DA, Attwood SW, Southgate VR, Horák P, Snyder SD, Le TH, Agatsuma T, McManus DP, Carmichael AC, Naem S, Littlewood DTJ. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126:203–224. doi: 10.1017/s0031182002002792. [DOI] [PubMed] [Google Scholar]
- Lui Z, Bai G. On bird schistosomes from Jilin Province: Jilinobilharzia crecci gen. nov., sp. nov. (Schistosomatidae: Bilharziellinae) with a discussion on the taxonomy of the subfamily Bilharziellinae. Acta Zoologica Sincia. 1976;22:385–392. [Google Scholar]
- Lui Z, Chen M, Jin G, Tan Y, Yang F. A survey of the aetiological agent of paddy field dermatitis in Ji’an Xian, Jilin Province, with preliminary observations of the life history of Trichobilharzia jianensis sp nov (Trematoda: Schistosomatidae) Acta Zoologica Sincia. 1977;23:161–174. [Google Scholar]
- Macfarlane DG, Macy RW. Cercaria oregonensis, n. sp., a dermatitis-producing schistosome cercaria from the Pacific Northwest. Journal of Parasitology. 1946;32:281–285. [PubMed] [Google Scholar]
- Macy RW, Moore DJ, Price WS., Jr Studies on dermatitis producing schistosomes in the Pacific Northwest, with special reference to Trichobilharzia oregonensis. Transactions of the American Microscopical Society. 1955;74:235–251. [Google Scholar]
- Martin FS, Vicente FS. The life cycle of Trichobilharzia salmanticensis n. sp. (Digenea: Schistosomatidae), related to cases of human dermatitis. Research and Reviews in Parasitology. 1999;59:13–18. [Google Scholar]
- McLeod JA. Two new schistosomid trematodes from water birds. Journal of Parasitology. 1937;23:456–466. [Google Scholar]
- McLeod JA. Studies on cercarial dermatitis and the trematode family Schistosomatidae in Manitoba. Canadian Journal of Research. 1940;18:1–18. [Google Scholar]
- McMullen DB, Brackett S. The distribution and control of schistosome dermatitis in Wisconsin and Michigan. American Journal of Tropical Medicine. 1941;s1–21:725–729. [Google Scholar]
- McMullen DB, Beaver PC. Studies on schistosome dermatitis. IX. The life cycles of three dermatitis-producing schistosomes from birds and a discussion of the subfamily Bilharziellinae (Trematoda: Schistosomatidae) American Journal of Hygiene. 1945;42:128–154. [Google Scholar]
- Miller HM. Notes on some furcocercous larval trematodes. Journal of Parasitology. 1923;10:35–46. [Google Scholar]
- Morgan JAT, DeJong RJ, Kazibwe F, Mkoji GM, Loker ES. A newly- identified lineage of Schistosoma. International Journal for Parasitology. 2003;33:977–985. doi: 10.1016/s0020-7519(03)00132-2. [DOI] [PubMed] [Google Scholar]
- Morgan JAT, DeJong RJ, Lwambo NJS, Mungai BN, Mkoji GM, Loker ES. First report of a natural hybrid between Schistosoma mansoni and S. rodhaini. Journal of Parasitology. 2003;89:416–418. doi: 10.1645/0022-3395(2003)089[0416:FROANH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Müller V, Kimmig P. Trichobilharzia franki n. sp. a causative agent of swimmer’s itch in south-western Germany. Applied Parasitology. 1994;35:12–31. [PubMed] [Google Scholar]
- Najim AT. Life history of Gigantobilharzia huronensis Najim, 1950. A dermatitis-producing bird blood-fluke (Trematoda-Schistosomatidae) Parasitology. 1956;46:443–469. doi: 10.1017/s0031182000026597. [DOI] [PubMed] [Google Scholar]
- Nassi H. Sur quatre furcocercaires emises par Biomphalaria glabrata en Guadeloupe. Annales Parasitologie Humaine et Compareé. 1987;62:17–35. [Google Scholar]
- Neuhaus W. Biologie und Entwicklung von Trichobilharzia szidati n. sp. (Trematoda: Schistosomatidae), einem Erreger von Dermatitis beim Menschen. Zeitschrift fur Parasitenkunde. 1952;15:203–266. doi: 10.1007/BF00260453. [DOI] [PubMed] [Google Scholar]
- Nolan MJ, Cribb TJ. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Advances in Parasitology. 2005;60:101–163. doi: 10.1016/S0065-308X(05)60002-4. [DOI] [PubMed] [Google Scholar]
- Oda T. Schistosome dermatitis in Japan. Progress in Medical Parasitology in Japan. 1973;5:5–63. [Google Scholar]
- Ostrowski de Núñez M. Fauna de agua dulce de la República Aregentina. VII. Cercarias de la familia Schistosomatidae (Trematoda, Digenea) Revista del Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’ e Instituto Nacional de Investigación de las Ciencias Naturales. 1978;2:65–76. [Google Scholar]
- Pao TC, Yung YL. The discovery of an avian schistosome, Pseudobilharzia sp. (Family Schistosomatidae, subfamily Bilharziellinae) in Chung-Ching Szechwa Province China. Acta Zoologica Sincia. 1957;9:291–297. [Google Scholar]
- Picard D, Jousson O. Genetic variability among cercariae of the Schistosomatidae (Trematoda: Digenea) causing swimmers’ itch in Europe. Parasite. 2001;8:237–242. doi: 10.1051/parasite/2001083237. [DOI] [PubMed] [Google Scholar]
- Posada D. jModeltest: Phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Pritchard MH, Kruse GOW. The collection and preservation of animal parasites. University of Nebraska Press; Lincoln: 1982. p. 141. [Google Scholar]
- Remigio EA. Molecular phylogenetic relationships in the aquatic snail genus Lymnaea, the intermediate host of the causative agent of fascioliasis, insights from a broader taxon sampling. Parasitology Research. 2002;88:687–696. doi: 10.1007/s00436-002-0658-8. [DOI] [PubMed] [Google Scholar]
- Rind S. Three ocellate schistosome cercariae (Trematoda: Schistosomatidae) in Gyraulus corinna, with reference to Cercaria longicauda MacFarlane, 1944 in Lymnaea tomentosa. New Zealand Journal of Zoology. 1991;18:53–62. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rudolfová J, Horák P. Occurrence of bird schistosomes in the Czech Republic. Helminthologia. 2001;38:175. [Google Scholar]
- Rudolfová J, Hampl V, Bayssade-Dufour C, Lockyer AE, Littlewood DTJ, Horák P. Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitology Research. 2005;95:79–89. doi: 10.1007/s00436-004-1262-x. [DOI] [PubMed] [Google Scholar]
- Rudolfová J, Littlewood DTJ, Sitko J, Horák P. Bird schistosomes of wildfowl in the Czech Republic and Poland. Folia Parasitologica. 2007;54:88–93. [PubMed] [Google Scholar]
- Sheng SC, Qin ZH, Zhang MQ, Tai Y, Ni SG, Wen JY. Preliminary study on the ecology of Trichobilharzia cercariae in the Huaihe river system. Chinese Journal of Parasitology and Parasitic Disease. 2004;22:349–352. [PubMed] [Google Scholar]
- Skirnisson K, Kolářová L. Diversity of bird schistosomes in anseriform birds in Iceland based on egg measurements and egg morphology. Parasitology Research. 2008;103:43–50. doi: 10.1007/s00436-008-0925-4. [DOI] [PubMed] [Google Scholar]
- Skrjabin KI, Zakharov NP. Zwei neue Trematodengattungen aus den Blutgefässen der Vögel. Izvestnik Donskovo Veterinarnovo Instituta. 1920;2:1–6. [Google Scholar]
- Snyder SD. Phylogeny and paraphyly among tetrapod blood flukes (Digenea: Schistosomatidae and Spirorchiidae) International Journal for Parasitology. 2004;34:1385–1392. doi: 10.1016/j.ijpara.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Štefka J, Hypša V, Scholz T. Interplay of host specificity and biogeography in the population structure of a cosmopolitan endoparasite: microsatellite study of Ligula intestinalis (Cestoda) Molecular Ecology. 2009;18:1187–1206. doi: 10.1111/j.1365-294X.2008.04074.x. [DOI] [PubMed] [Google Scholar]
- Steinauer ML, Hanelt B, Mwangi IN, Maina GM, Lelo EL, Kinuthia JM, Mutuku MW, Mungai BN, Wilson WD, Mkoji GM, Loker ES. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Molecular Ecology. 2008;17:5062–5074. doi: 10.1111/j.1365-294X.2008.03957.x. [DOI] [PubMed] [Google Scholar]
- Stunkard HW. Induced gametogenesis in a monogenetic trematode, Polystoma stellai Vigueras, 1955. Journal of Parasitology. 1959;45:389–394. [PubMed] [Google Scholar]
- Stunkard HW, Hinchliffe MC. The morphology and life history of Microbilharzia variglandis (Miller and Northup, 1926) Stunkard and Hinchliffe, 1951, avian blood flukes whose larvae cause “swimmer’s itch” of ocean beaches. Journal of Parasitology. 1952;38:248–265. [PubMed] [Google Scholar]
- Swales WE. Schistosome dermatitis in Canada. Notes on two causative agents and their snail hosts in Manitoba. Canadian Journal of Research. 1936;14:6–10. [Google Scholar]
- Swofford DL. PAUP*: Ver 4.0. Phylogenetic Analysis Using Parsimony (*and other methods) Sinauer Associates, Inc; Sunderland, Massacusetts: 2000. [Google Scholar]
- Talbot SB. Studies on schistosome dermatitis. II. Morphological and life history studies on three dermatitis-producing schistosome cercariae, C. elvae Miller, 1923, C. stagnicolae n. sp., and C. physellae n. sp. American Journal of Hygiene. 1936;23:372–384. [Google Scholar]
- Tang Z, Tang C. Dermatitis producing schistosomes of birds and mammals in China. Acta Zoologica Sincia. 1976;22:341–360. In Chinese. [Google Scholar]
- Taylor DW. Introduction to Physidae (Gastropoda: Hygrophila): Biogeography, Classification, Morphology. Revista de Biologia Tropical. 2003;51(suppl):1–287. [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and Tris (Hotshot) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Tsai ST, et al. Cercarial dermatitis and its causative agents (schistosome trematodes) in Guangdong Province. Annual Bulletin of the Society of Parasitology, Guangdong Province. 1979;1:44–55. In Chinese. [Google Scholar]
- Vilas R, Criscione C, Blouin M. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology. 2005;131:839–846. doi: 10.1017/S0031182005008437. [DOI] [PubMed] [Google Scholar]
- Voronin MV, Beer SA. Morphological peculiarities of Schistosomatidae cercariae of Trichobilharzia cf. ocellata group occurring in Moscow and Saint-Petersburg populations. Parazitologiia. 2002;36:60–70. [PubMed] [Google Scholar]
- Weinland DF. Human Cestoides. Cambridge; Massachusetts: 1858. p. 93. [Google Scholar]
- Wojcinski ZW, Barker IK, Hunter DB, Lumsden H. An outbreak of schistosomiasis in Atlantic Brant geese, Branta bernicla hrota. Journal of Wildlife Disease. 1987;23:248–255. doi: 10.7589/0090-3558-23.2.248. [DOI] [PubMed] [Google Scholar]
- Wu L. A study of the life history of Trichobilharzia cameroni sp. nov. (Family Schistosomatidae) Canadian Journal of Zoology. 1953;31:151–173. [Google Scholar]
- Yamaguti S. Synopsis of Digenetic Trematodes of Vertebrates. Keigaku Publishing Co; Tokyo, Japan: 1971. p. 1074. [Google Scholar]
- Żbikowska E. Infection of snails with bird schistosomes and the threat of swimmer’s itch in selected Polish lakes. Parasitology Research. 2004;92:30–35. doi: 10.1007/s00436-003-0997-0. [DOI] [PubMed] [Google Scholar]
- Zischke JA, Zischke DP. Schistosome dermatitis at Basswood Lake, Minnesota. Journal of the Minnesota Academy of Science. 1968;35:29–32. [Google Scholar]