Abstract
Proteins bearing a CaaL sequence are typically geranylgeranylated to enable their proper localization and function. We found that many of the dansyl-GCaaL peptides representing mammalian CaaL proteins can be farnesylated by FTase. This result may have important implications for prenylated protein biology.
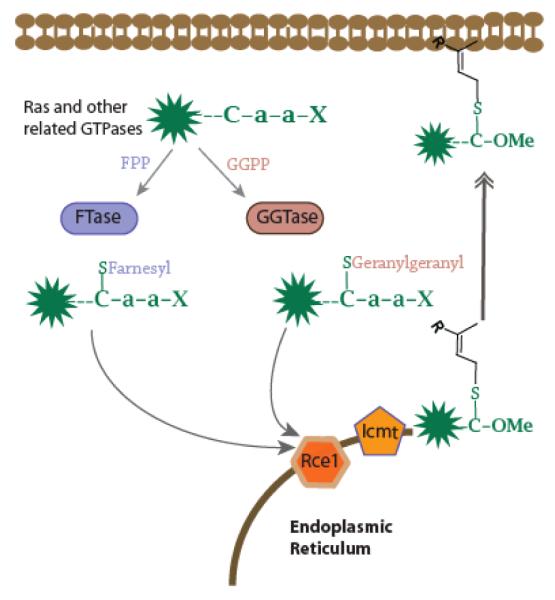
It is predicted that more than 60 mammalian proteins are farnesylated, and a similar number are monogeranylgeranylated1, 2 (Figure 1). Two enzymes catalyze these modifications: farnesyltransferase and geranylgeranyltransferase-I (FTase and GGTase-I). In both cases, the isoprenoid is attached to the cysteine in a C-terminal “Ca1a2X” sequence. While the “a” residues guide substrate ability,3, 4 the X position is the most important selectivity factor for FTase versus GGTase-I. Those peptides bearing X residues of Ser, Gln, and Met are farnesylated (Ras proteins and others) and those bearing Leu are geranylgeranylated (Rho proteins and others). However, it has been demonstrated that RhoB, with a C-terminal CKVL CaaX box, can be prenylated by either FTase or GGTase-I in mammalian cells.5, 6
Figure 1.
Prenylation (either farnesylation by FTase or geranylgeranylation by GGTase-I) of Ras-related proteins, and subsequent processing events.
FTase emerged as a drug target when it was discovered that Ras proteins are farnesylated. FTase inhibitors (FTIs) have been developed and clinically evaluated.7 Unfortunately, many tumors are resistant to FTIs, due in part to the ability of K-Ras to serve as a substrate for either FTase or GGTase-I.8, 9 Interest in GGTase-I inhibitors (GGTIs) as anti-cancer agents is gaining momentum,10 as genetic ablation of GGTase I leads to tumor regression in mice with K-Ras-driven lung tumors.11 GGTIs have been developed by several groups, and clinical trials began in 2009 with GGTI-2148.12 It is not known if the alternate farnesylation of any geranylgeranylated proteins would confer resistance to GGTIs, and this is a key question with regard to their evaluation.10 As a complement to proteomic methods,13, 14 we have employed peptide libraries to evaluate FTase substrate specificity.3 In this study, we screened 41 dansyl-GCaaL (DnGCaaL) peptides versus FTase, and found a surprising number of efficient substrates.
The DnGCaaL peptides evaluated were chosen in one of two ways. First, a number of sequences are predicted as GGTase-I substrates based on the crystal structure of this enzyme.1, 2 Second, additional GGTase I candidate substrate sequences were identified from database searches, in particular focusing on sequences possessing diverse “a” residues. The 41-member DnGCaaL library was prepared using standard Fmoc solid phase peptide synthesis protocols, with Fmoc-Leu-Wang resin, Fmoc-amino acids, dansyl-Gly, and HBTU/HOBt couplings.
A continuous spectrofluorimetric assay was used to determine FTase substrate activity for the DnGCaaL peptides.3, 15 The FTase substrate DnGCVLS, representing the CaaX box of H-Ras, was used as the control. Briefly, 3 μM DnGCaaL peptide and 9 μM FPP are combined, and farnesylation is initiated with the addition of recombinant mammalian FTase (0.05 μM). Peptide farnesylation is measured after 30 min via the relative fluorescence increase (RFI, Table 1, column 3) in emission at 535 nm. The formation of farnesylated product was confirmed by HPLC (Table 1, column 4) after 60 minutes. The prototypical FTase substrate DnGCVLS exhibits complete turnover to farnesylated product under these conditions after 15 minutes.
Table 1.
Evaluation of DnGCaaL Library versus FTase.
| CaaX1 | Protein2 | RFI3 | HPLC4 | kcat/KM5 mM−1s−1 |
Prenbase FT6 |
Prenbase GGT7 |
|
|---|---|---|---|---|---|---|---|
| 1 | CIIL | F-box and leucine-rich repeat protein 20/ G Protein γ-7 subunit*/G Protein γ–12 subunit* |
1.01 | +++ | 43 ± 8 | ++(.254) | +++(2.78) |
| 2 | CAIL | G protein γ-2 subunit** | 0.71 | +++ | 27 ± 2 | ++(.583) | +++(3.18) |
| 3 | CCIL | Rod cGMP-specific 3′5′-cyclic PDE β-subunit*/RalA* | 0.61 | +++ | 23 ± 3 | ++(.985) | +++(2.7) |
| 4 | CVIL | Rap2B*/M-Ras*/F-box & leucine-rich repeat prot. 2 | 0.52 | +++ | 47 ± 8 | ++(0.796) | +++(2.55) |
| 5 | CTIL | G protein γ-4 subunit/G protein γ-13 subunit/2′-5′- oligoadenylate synthetase 1/retinitis pigmentosa GTPase regulator isoforms 1, 2, 4/F-box protein 10 |
0.45 | ++ | 33 ± 7 | ++(.735) | +++(3.13) |
| 6 | CNIL | Interferon induced guanylate binding protein 2* | 0.22 | ++ | 12 ± 0.9 | −(−3.64) | ++(.881) |
| 7 | CSIL | NIM1 Kinase | 0.18 | +++ | 5 ± 1 | +(−1.07) | ++(0.73) |
| 8 | CILL | Rho G* | 0.15 | ++ | 8 ± 2 | +(−.311) | ++(1.86) |
| 9 | CCVL | schlafen-like1 | 0.11 | +++ | 7 ± 1 | +(−0.50) | ++(.796) |
| 10 | CLML | cone cGMP-specific 3′5′-cyclic PDE β-subunit | 0.09 | ++ | 8.1 ± 0.5 | +(−1.33) | −(−3.16) |
| 11 | CLIL | DnaJ (Hsp40)homolog, subfamily B, member | <0.05 | ++ | ++(.76) | +++(2.95) | |
| 12 | CKVL | Rho B** | <0.05 | + | +(−1.08) | +(−1.68) | |
| 13 | CRLL | Phospholipase D family, member 3 | <0.05 | + | −(−2.05) | −−(−5.03) | |
| 14 | CLVL | Rho A* | <0.05 | + | ++(.306) | +++(2.253) | |
| 15 | CLLL | Rac1*/Rif**/G protein coupled receptor kinase 7 | <0.05 | + | ++(.043) | +++(2.33) | |
| 16 | CALL | Heterotrimeric G-protein γ-3 subunit* | <0.05 | + | +(−428) | ++(1.92) | |
| 17 | CVLL | cdc42* | <0.05 | + | ++(.696) | +++(+2.95) | |
| 18 | CCLL | Ral B*/Mannose-6-phosphate isomerase | <0.05 | + | ++(.281) | ++(1.81) | |
| 19 | CPIL | Rho C* | <0.05 | + | +(−.977) | +++(2.61) | |
| 20 | CSVL | Rab 18 | 0 | + | +(−.061) | ++(.320) | |
| 21 | CTLL | Aldehyde dehydrogenase | 0 | + | +(−1.81) | +(−1.15) | |
| 22 | CSLL | Rab 8B/Rac2* | 0 | − | +(−.106) | ++(1.71) | |
| 23 | CQLL | Rap1B* | 0 | − | +(−.52) | ++(.23) | |
| 24 | CLQL | RacX | 0 | − | +(−.38) | −(−4.03) | |
| 25 | CHPL | Protein C20orf24(Rab-5-interacting protein) | 0 | − − | −−(7.89) | −−−(−13.43) | |
| 26 | CNPL | Inositol phosphatase* | 0 | − − | −−(6.57) | −−(−8.78) | |
| 27 | CGGL | Glycerol 3-P dehydrogenase | 0 | − − | −−(5.89) | −−(−8.84) | |
| 28 | CGQL | cyclophilin | 0 | − − | −(−3.05) | −−−(−11.52) | |
| 29 | CLGL | MAD prot./MAD dimer | 0 | − − | −(4.15) | −(−4.27) | |
| 30 | CTAL | Phosphate transport | 0 | − − | −(−4.85) | −−(−6.25) | |
| 31 | CSTL | CASP8 and FADD-like precursor (splice isoform 10) | 0 | − − | −(−2.67) | −−(−8.23) | |
| 32 | CAYL | Mitochondrial 28S Ribosomal protein S29 | 0 | − − | −−(−5.75) | −−(−5.80) | |
| 33 | CSFL | G-Protein γ-5 subunit* | 0 | − − | −−(−5.54) | −−(−5.16) | |
| 34 | CHAL | NUDT9 | 0 | − − | −−(−7.4) | −−(−8.3) | |
| 35 | CGCL | Mouse nodal homolog precursor | 0 | − − | −(−3.81) | −−(−8.99) | |
| 36 | CMEL | Sulfotransferase family, cytosolic, 1C, member 2-Rat | 0 | − − | −−(−6.99) | −−(−9.76) | |
| 37 | CVGL | butyrlcholinesterase | 0 | − − | −(−3.79) | −(−3.14) | |
| 38 | CEKL | Lactalbumin, alpha- | 0 | − − | −−−(−10.41) | −−−(−15.20) | |
| 39 | CQNL | RAB11B | 0 | − − | −(−2.88) | −−(−8.18) | |
| 40 | CSQL | STEAP1 | 0 | − − | −(−2.62) | −−(−8.51) | |
| 41 | CCSL | Interleukin 17 receptor B | 0 | − − | −(−2.15) | −−(−5.74) |
All peptides have the sequence DnG-CxxL
Protein(s) bearing the corresponding CaaL sequence at the carboxyl terminus.
Proteins known to be geranylgeranylated
Proteins known to be geranylgeranylated and farnesylated.
RFI = Relative Fluorescence Increase at 535 nm after 30 min in the spectrofluorimetric assay, with increase seen for DnGCVLS set at RFI = 1.0.
Formation of farnesylated peptide product after 60 min incubation with FTase as determined by HPLC analysis. +++ complete conversion of peptide to farnesylated product; ++ nearly complete conversion to product; + 25% to 50% conversion to product; − less than 25% conversion; −− no product observed.
The steady-state kinetic parameters were determined at saturating FPP (10 μM) and varying peptide concentrations (0.2-10 μM peptide) in 20-100 nM FTase. Kinetics were determined for FTase from a time-dependent increase in fluorescence (λex 340 nm, λem 520 nm) upon farnesylation of the dansylated peptide Assays were performed with 0.2-10 μM dansylated peptide, 20-100 nM FTase, 10 μM FPP, 50 mM HEPPSO pH 7.8, 5 mM tris(2-carboxyethyl)phosphine (TCEP), and 5 mM MgCl2 at 25 •C in a 96-well plate (Corning). Peptides were incubated in reaction buffer for 20 minutes prior to initiation by addition of FTase and FPP, with the FTase concentration at least 5-fold lower than the peptide concentration. Fluorescence was measured and converted to kcat values as described in our recent publication (reference 4). Under these same conditions, DnGCVLS kcat/KM = 170 ± 30 mM−1s−1.
PRENbase prediction for the FTase substrate ability of the sequence XXXXXXXXXXXCaaL, for the first protein listed.
PRENbase prediction for the GGTase-I substrate ability of the sequence XXXXXXXXXXXCaaL, for the first protein listed, as described.
The data from the DnGCaaL peptide screen are presented in Table 1. Based on their FTase substrate ability, the peptide sequences can be separated into four categories. Ten peptides (Table 1, entries 1-10) are good FTase substrates, using our previously reported screening conditions.3 They exhibit activity in the fluorescent assay between 9% and 101% that of DnGCVLS, and their substrate ability is confirmed via the HPLC assay. Nine additional peptides (Table 1, entries 11-19) are poor FTase substrates. They exhibit <5% of the activity of DnGCVLS in the fluorescence assay, and they demonstrate partial substrate conversion to product by HPLC. Five peptides (Table 1, entries 20-24) are marginal substrates, exhibiting some peptide product by HPLC but no significant activity by the fluorescence assay. The remaining 17 CaaL peptides (entries 25-41) exhibit no substrate ability with FTase. To provide more detailed information on the most effective substrates (Table 1, entries 1-10), kcat/KM values were determined for each one, using our recently reported kinetic assay.4 Each peptide was validated as an FTase substrate. However, the initial screening results overstated their relative efficiencies, with kcat/KM values ranging from 3% to 28% that of the prototypical FTase substrate DnGCVLS.4
The current understanding of FTase peptide specificity holds that the X residue of the Ca1a2X sequence is the primary determinant for FTase/GGTase-I substrate discrimination, and that CaaL peptides would likely not be FTase substrates.1, 2 This proved not to be correct with the DnGCaaL library. It was previously also predicted with regard to the a1 and a2 positions of the CaaX box that the enzyme will accept any amino acid at the a1 position, but hydrophobic residues (e.g. Ile, Leu, Val) are preferred for the a2 residue. Our results support this general a1/a2 model; of the 19 good/poor substrates, 18 contain Val, Leu, or Ile at the a2 position. However, the factors that influence CaaL processing by FTase are quite subtle. In particular, note that the Ca1IL sequence, which is found on nine peptides in the library, is preferred by FTase. The seven most active CaaL peptides (Table 1, entries 1-7) contain this sequence, and CLIL and CPIL are also confirmed substrates. Conversely, a number of poor and marginal FTase substrates bear potentially favorable Val and Leu residues at the a2 position. Another surprising finding is that DnGCLML is a good FTase substrate (Table 1, entry 10). Methionine is rare as an a2 residue in a CaaX sequence, with only four examples found among the proposed FTase or GGTase I substrates.1 Note that in our earlier study on a DnGCaaS library,3 several unusual polar residues were accepted at the a2 position, but that is not the case with the DnGCaaL library.
PRENbase is a database based on an algorithm that uses the 15 C-terminal amino acids to predict FTase and GGTase-I substrates.16 This tool proved quite effective, predicting the FTase substrate ability for 17 of the 19 good and poor CaaL substrates. The two exceptions were the CNIL and CRLL sequences (Table 1, entries 6 and 13). Another interesting feature of the PRENbase analysis is that the CaaL sequence CLML (Table 1, entry 10) is correctly predicted to be FTase substrate, but is not predicted to be a GGTase I substrate.
These data shed light on the tolerance of FTase for CaaL peptide sequences. We did not anticipate that a substantial number of these peptides would be FTase substrates. Although FTase has a second X residue binding site for hydrophobic side chains, structural analysis seemed to indicate that phenylalanine bound best and leucine bound only with steric clashes.1 However, an early study by the Merck group on the substrate activity of CaaX peptides had demonstrated that certain CaaL peptides are farnesylated by FTase.17 More recent detailed kinetic studies demonstrated that DnTKCVIL was an FTase substrate under single turnover conditions, but did not exhibit efficient turnover under steady-state conditions.18 The difference in substrate activity between DnGCVIL (Table 1, entry 4) and DnTKCVIL is presumably due to enhanced binding of the lysine-bearing peptide to FTase,19 preventing product release.
Many geranylgeranylated proteins play key roles in cancer cell growth and signaling, and thus are potential targets for GGTIs. However, alternative farnesylation of these sequences would be a complicating factor. The only CaaL protein that is a well-described FTase substrate is RhoB,5, 6 although the proteomic studies of Zhao and coworkers indicate that the CVLL and CKAL sequences may be farnesylated in vivo.13 Surprisingly, the CKVL and CVLL sequences are relatively modest FTase substrates in our assay, suggesting that other CaaL proteins may be farnesylated in the cell. Hildebrandt and coworkers have carried out an extensive proteomic examination of the post-translational modification of G-protein γ-subunits frombovine brain.20 The γ–2 subunit, bearing the CAIL CaaX motif (Table 1, entry 2) is both geranylgeranylated and farnesylated in the cell, in accord with our biochemical results. However, the γ–7 and γ–12 subunits, bearing the CIIL CaaX motif (Table 1, entry 2), did not exhibit farnesylation. Two studies have recently been carried out on the abilities of various Ras-related proteins to be alternatively prenylated.21, 22 In the first study, Sebti and coworkers reported that RalA did not exhibit cellular farnesylation by FTase.21 These results are in contrast to ours where the RalA CaaX box (DnGCCIL) is an efficient FTase substrate. This discrepancy could be due to unfavorable residues for FTase substrate ability upstream of the CCIL sequence. Very recently, Der and coworkers have published the results of a second study on the alternative prenylation of several additional CaaL-bearing Ras-related proteins.22 It was demonstrated that Rac1 is solely a GGTase-I substrate, while Rif/RhoF exhibits dual FTase/GGTase-I substrate ability behavior in cellular evaluation. Both proteins bear a CLLL CaaX box, which is a poor FTase substrate in our enzymatic assay (entry 15). Note that Rac1 bears a lysine residue adjacent to the prenylated cysteine residue, while Rif/RhoF bears a leucine residue. Taking these cellular studies together with our biochemical results, it is clear that the residues upstream of the CaaL box play an important role in the FTase substrate ability of cellular proteins.
GGTase-I catalyzes the prenylation of many crucial signaling proteins, and is also emerging as an important antitumor drug target. Our studies have shown that ten DnGCaaL peptides function as efficient substrates for FTase, and several other sequences can also be accepted as substrates. These results provide important and unexpected new insights into the biochemical substrate specificity of FTase. In combination with the recent evaluation of the prenyltransferase specificity of CaaL-bearing proteins in cells,20-22 our results provide an indication of the importance of upstream residues in FTase/GGTase-I selectivity. It may be of significant interest to evaluate the prenylation of other proteins indicated as potential FTase substrates in Table 1.
Acknowledgements
This work was supported by NIH grants CA78819 (RAG), GM40602 (CAF), GM78894 (JLH), and P30 CA21368 (Purdue Center for Cancer Research). AJK and AVA were supported in part by PRF fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reid TS, Terry KL, Casey PJ, Beese LS. J. Mol. Biol. 2004;343:417. doi: 10.1016/j.jmb.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 2.Lane KT, Beese LS. J Lipid Res. 2006;47:681. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Krzysiak AJ, Scott SA, Hicks KA, Fierke CA, Gibbs RA. Bioorg. Med. Chem. Lett. 2007;17:5548. doi: 10.1016/j.bmcl.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hougland JL, Lamphear CL, Scott SA, Gibbs RA, Fierke CA. Biochemistry. 2009;48:1691. doi: 10.1021/bi801710g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebowitz PF, Casey PJ, Prendergast GC, Thissen JA. J. Biol. Chem. 1997;272:15591. doi: 10.1074/jbc.272.25.15591. [DOI] [PubMed] [Google Scholar]
- 6.Baron R, Fourcade E, Lajoie-Mazenc I, Allal C, Couderc B, Barbaras R, Favre G, Faye J-C, Pradines A. Proc. Natl. Acad. Sci. USA. 2000;97:11626. doi: 10.1073/pnas.97.21.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basso AD, Kirschmeier PT, Bishop WR. J. Lipid Res. 2006;47:15. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai J-K. J. Biol. Chem. 1997;272:14459. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 9.Rowell CA, Kowalczyk JJ, Lewis MD, Garcia AM. J. Biol. Chem. 1997;272:14093. doi: 10.1074/jbc.272.22.14093. [DOI] [PubMed] [Google Scholar]
- 10.Philips MR, Cox AD. J. Clin. Invest. 2007;117:1223. doi: 10.1172/JCI32108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjogren A-KM, Andersson KME, Liu M, Cutts BA, Karlsson C, Wahlstrom AM, Dalin M, Weinbaum C, Casey PJ, Tarkowski A, Swolin B, Young SG, Bergo MO. J. Clin. Invest. 2007;117:1294. doi: 10.1172/JCI30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazi A, Carie A, Blaskovich M, Bucher C, Thai V, Moulder S, Peng H, Carrico D, Pusateri EE, Pledger WJ, Berndt N, Hamilton AD, Sebti SM. Mol Cell Biol. 2009;29:2254. doi: 10.1128/MCB.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kho Y, Kim SC, Jiang C, Barma D, Kwon SW, Cheng J, Jaunbergs J, Weinbaum C, Tamanoi F, Falck J, Zhao Y. Proc Natl Acad Sci U S A. 2004;101:12479. doi: 10.1073/pnas.0403413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen UTT, Guo Z, Delon C, Wu Y, Deraeve C, Franzel B, Bon RS, Blankenfeldt W, Goody RS, Waldmann H, Wolters D, Alexandrov K. Nat Chem Biol. 2009;5:227. doi: 10.1038/nchembio.149. [DOI] [PubMed] [Google Scholar]
- 15.Cassidy PB, Dolence JM, Poulter CD. Methods Enzymol. 1995;250:30. doi: 10.1016/0076-6879(95)50060-x. [DOI] [PubMed] [Google Scholar]
- 16.Maurer-Stroh S, Koranda M, Benetka W, Schneider G, Sirota FL, Eisenhaber F. PLoS Comp. Biol. 2007;3:e66. doi: 10.1371/journal.pcbi.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moores SL, Schaber MD, Mosser SD, Rands E, O’Hara MB, Garsky VM, Marshall MS, Pompliano DL, Gibbs JB. J. Biol. Chem. 1991;266:14603. [PubMed] [Google Scholar]
- 18.Hartman HL, Hicks KA, Fierke CA. Biochemistry. 2005;44:15314. doi: 10.1021/bi0509503. [DOI] [PubMed] [Google Scholar]
- 19.Fiordalisi JJ, Johnson RL, Weinbaum C, Sakabe K, Chen Z, Casey PJ, Cox AD. J. Biol. Chem. 2003;278:41718. doi: 10.1074/jbc.M305733200. [DOI] [PubMed] [Google Scholar]
- 20.Cook LA, Schey KL, Wilcox MD, Dingus J, Ettling R, Nelson T, Knapp DR, Hildebrandt JD. Mol Cell Proteom. 2006;5:671684. doi: 10.1074/mcp.M500223-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Falsetti SC, Wang D, Peng H, Carrico D, Cox AD, Der CJ, Hamilton AD, Sebti SM. Mol. Cell. Biol. 2007;27:8003. doi: 10.1128/MCB.00057-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Curring RO, Cox AD, Wilson O, Kirschmeier PT, Der CJ. J. Biol. Chem. 2008;283:25150. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]