Abstract
Carney Complex (CNC) is an inherited tumor predisposition associated with pituitary tumors, including GH-producing pituitary adenomas and rare reports of prolactinomas. This disease is caused by mutations in PRKAR1A, which encodes the type 1A regulatory subunit of the cAMP-dependent Protein Kinase, PKA. Loss of PRKAR1A causes enhanced PKA signaling, which leads to pituitary tumorigenesis. Mutations in the gene have not been detected in sporadic pituitary tumors, but there is some data to suggest that non-genomic mechanisms may cause loss of protein expression. Unlike CNC patients, mice heterozygous for Prkar1a mutations do not develop pituitary tumors, although complete knockout of the gene in the Pit1 lineage of the pituitary produces GH-secreting pituitary adenomas. These data indicate that complete loss of Prkar1a/PRKAR1A is able to cause pituitary tumors in mice and men. The pattern of tumors is likely related to the signaling pathways employed in specific pituitary cell types.
Keywords: PRKAR1A, Protein Kinase A, Pituitary Tumorigenesis, Carney Complex, Mouse Models
CARNEY COMPLEX AND PRKAR1A
Phenotype and Genetics of the Carney Complex
Carney Complex (CNC) is an autosomal dominant endocrine neoplasia syndrome characterized by spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas [1–2]. Skin lesions are present in the large majority of CNC patients, with centrofacial lentiginosis being the most common manifestation, although blue nevi and other skin findings may also be present. These findings are benign and do not require any intervention, although the inability to differentiate them from other pigmented skin lesions may require biopsy and/or excision to allow adequate pathologic characterization. Myxomas are present in over 50% of CNC patients, and can be located anywhere within the body. Cardiac myxomas are the most concerning manifestation of the disease, and require urgent resection before catastrophic complications due to embolism are seen. Cardiac myxomas are the most common cause of mortality in this disease, either from the tumors themselves, or from complications of surgical removal [2]. Myxomas can also be found elsewhere in the body where they are generally not medical issues, unless they occur in unusual places such as on the eyelid or in the ear canal where they cause problems from mass effect or from diagnostic uncertainty due to confusion with other types of tumors (e.g., breast myxomas vs. breast cancers). Endocrine overactivity in CNC is another important source of morbidity, and is observed in anywhere from 33% of patients upward. Endocrine manifestations include adrenal Cushing syndrome due to a specific pathology known as primary pigmented nodular adrenocortical disease (PPNAD), pituitary tumors (discussed in detail below), thyroid nodules/cancer, and gonadal tumors in both men and women. Pigmented schwannomas are also seen, and may become malignant in this disease. For purposes of this review, I will be discussing the situation as it applies only to the pituitary, although interested readers are referred to other more comprehensive reviews for further information [3–6].
Although significant progress towards understanding the molecular genetics of CNC has been made, the situation remains complex. CNC may be caused by mutations in PRKAR1A, which encodes the type 1A regulatory subunit of the cAMP-dependent protein kinase, or Protein Kinase A (PKA) [7]. A recent analysis of over 350 patients with this condition was able to detect mutations in PKRAR1A in 73% of patients [4]. The large majority of these were nonsense, frameshift, or splice site mutations which fail to produce a mutant protein due to mRNA degradation through the nonsense-mediated mRNA decay pathway [8]. More recently, large deletions of the gene have been detected, as have a small number of expressed mutant proteins carrying amino acid substitutions (i.e., missense mutations) [4]. For the remaining 27% of patients, no mutations have been found in PRKAR1A, and its 17q location has been excluded by genetic linkage in at least some families [8]. It seems likely that the causative genetic defect lies in the 2p16 region [9], although extensive genetic characterization of the region has yet to identify the responsible locus [10]. The situation is made more complex by the finding that patients with bilateral adrenocortical hyperplasia very similar to that observed in CNC patients have been found to carry mutations in the phosphodiesterase genes PDE11A or PDE8B [11–12]. However, these patients do not have extra-adrenal manifestations characteristic of CNC, suggesting a slightly different clinical syndrome. These findings have suggested that a gene encoding a component of the cAMP/PKA signaling pathway may cause CNC in the patients without mutations in PRKAR1A, but no such genes exist in the candidate 2p interval. Thus, identity of this second gene remains elusive.
The Pituitary Phenotype of CNC Patients
In its initial description in 1985 [13], 4/40 patients (10%) were observed to have GH-producing pituitary adenomas, causing either acromegaly or gigantism depending on the age of onset. In a subsequent analysis of a large cohort of patients [2], the numbers were similar with 33/338 (10%) of patients exhibiting GH-producing tumors at the time of presentation. However, by that time, a more thorough diagnostic approach suggested that subtle abnormalities of the GH axis, including elevated (asymptomatic) baseline GH or IGF1 or non-suppressible GH to a glucose challenge may be noted in a much larger fraction of patients [14–16]. There has been some suggestion that pituitary glands from CNC patients may also exhibit aberrant responses to TRH stimulation [17]. In the most recent analysis, the incidence of frank pituitary abnormalities was reported slightly higher, at 12% [4].
Although GH abnormalities are most common, pituitary abnormalities in CNC also appear to involve abnormal prolactin secretion. Although it is unique among CNC families reported, there is at least one family whose clinical presentation included frank prolactinomas [18]. Systematic analysis of a series of 11 CNC patients without known pituitary pathology demonstrated elevated prolactin levels in 64% of cases, although elevations were generally mild, being <100 ng/mL in all cases [16]. Additionally, in a study of 8 CNC patients with acromegaly, 2 had significant increases in prolactin, with levels of 69 and 126 ng/mL respectively [15].
At the histopathologic level, CNC-associated GH-producing pituitary adenomas may exhibit features similar to sporadic adenomas; however, these tumors also tend to have unique features. CNC-associated tumors can be multi-focal, and may demonstrate somatomammotroph hyperplasia which appears to predate frank adenomas [15]. The pattern of hormone staining is also somewhat unusual, as most tumors from CNC patients stain with multiple hormones. As might be expected from the clinical data, almost all of the GH-producing tumors also stain for prolactin. However, a significant number also stain for other hormones, including the glycoprotein subunit-α, TSH-β, LH-β, and occasionally for FSH-β. ACTH staining was not observed in pituitary adenomas from CNC patients [15,19].
At the genetic level, there has been interest in determining if loss of the normal allele is necessary for pituitary tumorigenesis in CNC patients. Data to address this specific question are somewhat sparse, although the available evidence suggests this may be the case. In the initial paper describing mutations in PRKAR1A as causing CNC, loss of heterozygosity (LOH) analysis was performed on 2 GH-producing pituitary tumors [7]. One tumor showed clear evidence for LOH of PRKAR1A, whereas the other was uninformative. A subsequent report from the same group describing a different GH-producing tumor demonstrated allelic loss of PRKAR1A using FISH for the gene [19]. On the other hand, conventional comparative genomic hybridization (CGH) of 4 CNC-associated tumors demonstrated multiple karyotypic changes in a single large pituitary adenoma, although loss of 17q was not observed. The other 3 tumors exhibited normal karyotypes [20]. However, this type of analysis has low resolution, and may not be an adequate means to detect LOH at a specific locus.
Association of mutations in PRKAR1A with sporadic pituitary tumors
In order to address the role of PRKAR1A in sporadic acromegaly, multiple groups have attempted to identify mutations in PRKAR1A in patients with non-familial GH-producing tumors. In multiple studies from a variety of groups, no mutations in PRKAR1A were detected [21–24]. The same was true in a study analyzing non-functional pituitary adenomas (NFPAs) [25], suggesting that genetic inactivation of PRKAR1A is not a significant cause of sporadic pituitary tumors. However, Spada and co-workers extended their studies to include analysis of mRNA and protein levels. Interestingly, in both GH-producing tumors and NFPAs, PRKAR1A protein levels were quite low, despite adequate mRNA. At the functional level, a GH-producing tumor model responded to reduced PRKAR1A with enhanced levels of the proliferation marker cyclin D1, whereas NFPAs did not [24–25]. These data suggest that PRKAR1A protein loss may occur through mechanisms other than genetic mutation, and that these processes may be operating during pituitary tumorigenesis. The difference between GH-producing tumors and NFPAs suggests these effects may be specific for GH-producing cells.
MOUSE MODELS OF PRKAR1A INACTIVATION
Mouse models of generalized Prkar1a KO
To date, conventional Prkar1a null alleles have been produced both in my lab [26] and in the McKnight lab [27]. Both groups have observed that mice carrying a complete knockout of Prkar1a (i.e., Prkar1a−/− animals) exhibit lethality early during embryogenesis. In careful studies from the McKnight group, it was shown that the lethality was due to a failure of mesoderm-derived structures; further, the authors showed that genetic reduction of PKA catalytic subunits provided partial rescue of the phenotype, although Prkar1a−/− pups never survived to birth [27]. Prkar1a+/− mice are born at the expected frequency, and are tumor prone. In our experience, mice tended to develop tumors in cAMP-responsive tissues [26], although others have reported that sarcomas are the major tumor type [28]. In neither model were pituitary tumors observed at a frequency above background. In a model of Prkar1a reduction caused by expression of a Prkar1a anti-sense transcript, pituitary tumors were also not observed [29].
Pituitary-specific ablation of Prkar1a causes pituitary tumorigenesis
Based on the fact that at least some pituitary tumors from CNC patients exhibit tumor-specific loss of the normal allele, we hypothesized that complete loss of Prkar1a might be necessary to cause tumor formation. In order to address this question, we decided to take advantage of cre-lox technology [30] to generate a pituitary-specific knockout (KO) of the gene. Although we already had our conditional null allele for Prkar1a (Prkar1aloxP) in hand [26], it was necessary to generate a mouse line which expressed the cre recombinase in the appropriate subset of pituitary cells. To this end, we produced a mouse line which used the rat GHRH receptor promoter to drive cre expression. This promoter, which is normally specific for the GH-producing pituitary cells [31], enabled expression of cre in cells of the Pit1 lineage, including somatotrophs, lactotrophs, and thyrotrophs [32]. These mice were then crossed with Prkar1a conditional null animals [26] to produce mice with a pituitary-specific knockout of Prkar1a (Prkar1a-pitKO) [33].
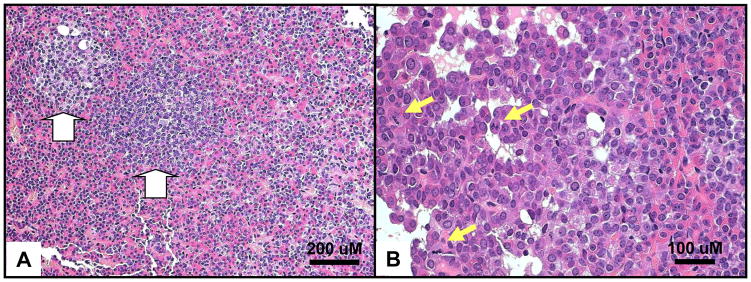
In contrast to the studies of the Prkar1a+/− mice, the Prkar1a-pitKOs developed pituitary tumors at a frequency that was significantly higher than that observed in control littermates (48% vs. 18%) [33]. Tumors in the KO’s were not clinically obvious (e.g., no increase in weight or length), but were detected by histopathological analysis which was carried out at 18 months of age in knockout animals (n=21) and non-KO animals (n=28) (Figure 1). In the control animals, almost all tumors were prolactinomas, which were observed in equal numbers in the controls and the Prkar1a-pitKO’s. Excluding the prolactinomas, pituitary tumors were observed in 6 (29%) of Prkar1a-pitKO’s mice compared to 1 (4%) of controls, a highly significant difference. In the Prkar1a-PitKO mice, multiple tumors were sometimes seen within the same gland, although they could be quite small. By immunostaining, most of this latter group of tumors were multi-hormonal tumors comprised of cells showing reactivity for GH, Prl, and TSH. As expected, the tumor cells also stained for the Pit1 transcription factor, confirming their cellular origin. Staining for ACTH, LH, or FSH was not observed in the mouse pituitary tumors in this analysis. Staining for the glycoprotein hormone α-subunit, which has been observed in some CNC-associated pituitary tumors [15,19], was not performed.
Figure 1.
Histopathology of pituitary tumors from Prkar1a-PitKO mice. A. Hematoxylin and eosin staining of a pituitary showing the presence of 2 microadenoms (white arrows). B. Mitotic figures (yellow arrows) observed within a different pituitary tumor. Scale bars are indicated for each panel.
At the biochemical level, analysis of serum from the mice demonstrated that Prkar1a-PitKO mice had >3 fold elevation in GH compared to controls (p < 0.01). GH levels did not correspond to the presence of a detectable tumor or the size of observed tumors, indicating that the biochemical abnormality pre-dated frank tumor formation. This echoes the observations in CNC patients, where biochemical changes in GH dynamics may occur as a result of pituitary hyperplasia and do not require the presence of an adenoma [15]. Interestingly, despite the fact that the mouse tumors stained for Prolactin and TSH, serum levels of these hormones were not elevated.
DISCUSSION and SUMMARY
CNC as a tumor predisposition syndrome includes acromegaly as one its significant endocrine manifestations. Although it is not completely clear, the limited data available is consistent with the hypothesis that loss of the normal allele is required for pituitary tumorigenesis in this condition. This notion is corroborated by the mouse data, where pituitary tumor formation requires loss of both alleles of Prkar1a to generate adenomas.
What does this observation mean for understanding pituitary-tumorigenesis associated with loss of PRKAR1A/Prkar1a? It is clear from multiple studies in humans [7], mice [34], and in vitro [35–36], that loss of PRKAR1A function is associated with increased signaling through the PKA pathway [37]. In the pituitary, the GHRH receptor uses the cAMP/PKA pathway to stimulate synthesis and release of GH [38]. The relevance of this biochemical signaling pathway to somatotroph tumorigenesis is corroborated in vivo by a number of mouse models, including a line with global GHRH overexpression [39] and a line expressing cholera toxin targeted to somatotrophs (rGH-CT mice) [40]. In humans, activation of this pathway, both in CNC and in sporadic cases caused by activating GNAS mutations, causes GH-secreting tumors [2,41] through the same mechanism.
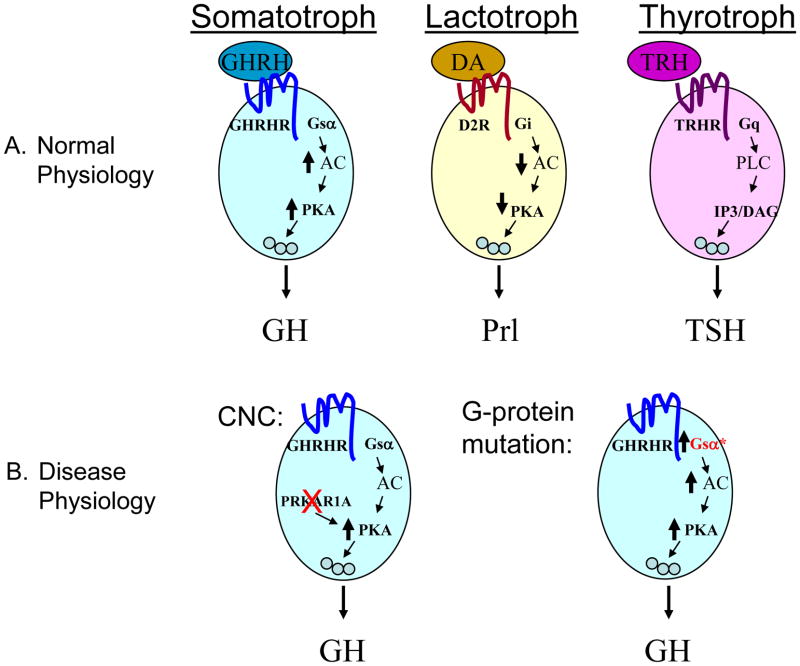
Although the GHRH-overexpressing mice and the rGH-CT mice are important models for understanding somatotroph tumorigenesis, neither is informative regarding why CNC patients (and Prkar1a-PitKO mice) typically develop GH hypersecretion without excess of other hormones. The explanation likely lies in the signaling pathways regulating hormone secretion for each of these cell types. In contrast to GH, regulation of prolactin secretion and TSH secretion occurs primary via non-PKA-mediated pathways [42–43], providing a rationale why levels of these hormones are not affected, even though the tumors may stain for these hormones. Prolactin secretion is primarily controlled by the dopamine D2 receptor, which couples to Gi to cause tonic inhibition of hormone release by reducing cAMP levels. In contrast, the TRH receptor stimulates TSH relates via the Gq/Phospholipase C (PLC) pathway (Figure 2a). In patients with mutations causing PKA activation, there is specific activation of the PKA pathway, leading to GH secretion in the absence of extracellular signals, tumor formation, and acromegaly (Figure 2b).
Figure 2.
Model for the connection between PRKAR1A mutations and acromegaly. A. The primary signaling pathways connecting hypothalamic signaling to pituitary hormone secretion are indicated for each cell type of the Pit1 lineage. See text for references. Note that Somatotrophs are primarily dependent on cAMP/PKA signaling for this process. B. Loss of PRKAR1A or activation of Gsα can cause secretion of GH independent of upstream signals.
A recent observation whose relevance to the above discussion is not yet understood is the report in 2006 that patients with mutations in the AIP gene have a familial predisposition to pituitary tumorigenesis, including acromegaly [44]. In fact, approximately 50% of families with isolated familial somatotropinomas (IFS) have mutations in this gene; conversely, of the patients with AIP mutations and pituitary tumors, over 85% have acromegaly, with the bulk of the remainder prolactinomas [45]. The AIP protein was initially identified as a cofactor for the Aryl hydrocarbon receptor transcription factor (AHR), which is thought to be important in the xenobiotic response. In the mouse, complete disruption of AIP results in embryonic lethality, and heterozygotes are not tumor prone as far as been reported [46]. However, there is data in vitro to indicate that cAMP signaling regulates function of the AHR-AIP protein complex [47]. The mechanism underlying the connection between PKA signaling and AIP mutations as it applies to acromegaly, although clearly of relevance, has not yet been explored.
The fact that mice lacking Prkar1a in the pituitary do not develop tumors until advanced age (18 months) suggests either that as yet unidentified secondary genetic hits are required (as suggested in [48] and more recently in [49]), or that the post-natal pituitary has a very low proliferative rate even in the setting of mitogenic stimulus. A third possibility is that there is active suppression of mitogenic stimuli, a role which has been attributed to somatostatin signaling [50]. The finding that the mouse pituitary tumors require a long latency is consistent with the observation in human patients that pituitary tumors, including GH-secreting tumors, grow slowly and exert their clinical effects over the course of many years.
The finding that activation of the PKA pathway is a stimulus towards the growth of a subset of pituitary tumors may have relevance for human disease given that there is a substantial portion of human patients with acromegaly who are not surgically cured of their disease. Inhibiting PKA signaling may have therapeutic implications, but this approach to therapy would need to be carefully considered because of the ubiquitous usage of the PKA signaling pathway throughout the body. Despite this caveat, this model may have usefulness as a means to begin to test new therapies aimed at acromegaly in human patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Carney JA. Carney complex: the complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Semin Dermatol. 1995;14:90–8. doi: 10.1016/s1085-5629(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 2.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–6. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 3.Bertherat J. Carney complex (CNC) Orphanet J Rare Dis. 2006;1:1–6. doi: 10.1186/1750-1172-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–91. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boikos SA, Stratakis CA. Carney complex: the first 20 years. Curr Opin Oncol. 2007;19:24–9. doi: 10.1097/CCO.0b013e32801195eb. [DOI] [PubMed] [Google Scholar]
- 6.Wilkes D, McDermott DA, Basson CT. Clinical phenotypes and molecular genetic mechanisms of Carney complex. Lancet Oncol. 2005;6:501–8. doi: 10.1016/S1470-2045(05)70244-8. [DOI] [PubMed] [Google Scholar]
- 7.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 8.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9:3037–46. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 9.Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirschner LS, Taymans SE, Pack S, Pak E, Pike BL, Chandrasekharappa SC, Zhuang Z, Stratakis CA. Genomic mapping of chromosomal region 2p15-p21 (D2S378-D2S391): integration of Genemap’98 within a framework of yeast and bacterial artificial chromosomes. Genomics. 1999;62:21–33. doi: 10.1006/geno.1999.5957. [DOI] [PubMed] [Google Scholar]
- 11.Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 12.Horvath A, Giatzakis C, Tsang K, Greene E, Osorio P, Boikos S, Libe R, Patronas Y, Robinson-White A, Remmers E, Bertherat J, Nesterova M, Stratakis CA. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet. 2008;16:1245–53. doi: 10.1038/ejhg.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–83. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T, Carney JA, Oldfield EH. Neurosurgical implications of Carney complex. J Neurosurg. 2000;92:413–8. doi: 10.3171/jns.2000.92.3.0413. [DOI] [PubMed] [Google Scholar]
- 15.Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex) J Clin Endocrinol Metab. 2000;85:3860–5. doi: 10.1210/jcem.85.10.6875. [DOI] [PubMed] [Google Scholar]
- 16.Raff SB, Carney JA, Krugman D, Doppman JL, Stratakis CA. Prolactin secretion abnormalities in patients with the “syndrome of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex) J Pediatr Endocrinol Metab. 2000;13:373–9. [PubMed] [Google Scholar]
- 17.Stergiopoulos SG, Abu-Asab MS, Tsokos M, Stratakis CA. Pituitary pathology in Carney complex patients. Pituitary. 2004;7:73–82. doi: 10.1007/s11102-005-5348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong DK, Irvine AD, Handley JM, Walsh MY, Hadden DR, Bingham EA. Carney complex: report of a kindred with predominantly cutaneous manifestations. Br J Dermatol. 1997;136:578–82. [PubMed] [Google Scholar]
- 19.Bossis I, Voutetakis A, Matyakhina L, Pack S, Abu-Asab M, Bourdeau I, Griffin KJ, Courcoutsakis N, Stergiopoulos S, Batista D, Tsokos M, Stratakis CA. A pleiomorphic GH pituitary adenoma from a Carney complex patient displays universal allelic loss at the protein kinase A regulatory subunit 1A (PRKARIA) locus. J Med Genet. 2004;41:596–600. doi: 10.1136/jmg.2004.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pack SD, Qin LX, Pak E, Wang Y, Ault DO, Mannan P, Jaikumar S, Stratakis CA, Oldfield EH, Zhuang Z, Weil RJ. Common genetic changes in hereditary and sporadic pituitary adenomas detected by comparative genomic hybridization. Genes Chromosomes Cancer. 2005;43:72–82. doi: 10.1002/gcc.20162. [DOI] [PubMed] [Google Scholar]
- 21.Kaltsas GA, Kola B, Borboli N, Morris DG, Gueorguiev M, Swords FM, Czirjak S, Kirschner LS, Stratakis CA, Korbonits M, Grossman AB. Sequence analysis of the PRKAR1A gene in sporadic somatotroph and other pituitary tumours. Clin Endocrinol (Oxf) 2002;57:443–8. doi: 10.1046/j.1365-2265.2002.01643.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandrini F, Kirschner LS, Bei T, Farmakidis C, Yasufuku-Takano J, Takano K, Prezant TR, Marx SJ, Farrell WE, Clayton RN, Groussin L, Bertherat J, Stratakis CA. PRKAR1A, one of the Carney complex genes, and its locus (17q22-24) are rarely altered in pituitary tumours outside the Carney complex. J Med Genet. 2002;39:e78. doi: 10.1136/jmg.39.12.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamasaki H, Mizusawa N, Nagahiro S, Yamada S, Sano T, Itakura M, Yoshimoto K. GH-secreting pituitary adenomas infrequently contain inactivating mutations of PRKAR1A and LOH of 17q23-24. Clin Endocrinol (Oxf) 2003;58:464–70. doi: 10.1046/j.1365-2265.2003.01740.x. [DOI] [PubMed] [Google Scholar]
- 24.Lania AG, Mantovani G, Ferrero S, Pellegrini C, Bondioni S, Peverelli E, Braidotti P, Locatelli M, Zavanone ML, Ferrante E, Bosari S, Beck-Peccoz P, Spada A. Proliferation of transformed somatotroph cells related to low or absent expression of protein kinase a regulatory subunit 1A protein. Cancer Res. 2004;64:9193–8. doi: 10.1158/0008-5472.CAN-04-1847. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani G, Bondioni S, Ferrero S, Gamba B, Ferrante E, Peverelli E, Corbetta S, Locatelli M, Rampini P, Beck-Peccoz P, Spada A, Lania AG. Effect of cyclic adenosine 3′,5′-monophosphate/protein kinase a pathway on markers of cell proliferation in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2005;90:6721–4. doi: 10.1210/jc.2005-0977. [DOI] [PubMed] [Google Scholar]
- 26.Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–14. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 27.Amieux PS, Howe DG, Knickerbocker H, Lee DC, Su T, Laszlo GS, Idzerda RL, McKnight GS. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem. 2002;277:27294–304. doi: 10.1074/jbc.M200302200. [DOI] [PubMed] [Google Scholar]
- 28.Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, La Perle K, Vaughan CJ, O’Hagan A, Bennett KR, Meyer BJ, Legius E, Karttunen M, Norio R, Kaariainen H, Lavyne M, Neau JP, Richter G, Kirali K, Farnsworth A, Stapleton K, Morelli P, Takanashi Y, Bamforth JS, Eitelberger F, Noszian I, Manfroi W, Powers J, Mochizuki Y, Imai T, Ko GT, Driscoll DA, Goldmuntz E, Edelberg JM, Collins A, Eccles D, Irvine AD, McKnight GS, Basson CT. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci U S A. 2004;101:14222–7. doi: 10.1073/pnas.0405535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos S, Robinson-White A, Lenherr S, Weinberg FD, Claflin E, Meoli E, Cho-Chung YS, Stratakis CA. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res. 2004;64:8811–5. doi: 10.1158/0008-5472.CAN-04-3620. [DOI] [PubMed] [Google Scholar]
- 30.Le Y, Sauer B. Conditional gene knockout using cre recombinase. Methods Mol Biol. 2000;136:477–485. doi: 10.1385/1-59259-065-9:477. [DOI] [PubMed] [Google Scholar]
- 31.Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J. Differential gene expression of growth hormone (GH)-releasing hormone (GRH) and GRH receptor in various rat tissues. Endocrinology. 1995;136:4147–50. doi: 10.1210/endo.136.9.7649123. [DOI] [PubMed] [Google Scholar]
- 32.Yin Z, Williams-Simons L, Rawahneh L, Asa S, Kirschner LS. Development of a pituitary-specific cre line targeted to the Pit-1 lineage. Genesis. 2008;46:37–42. doi: 10.1002/dvg.20362. [DOI] [PubMed] [Google Scholar]
- 33.Yin Z, Williams-Simons L, Parlow AF, Asa S, Kirschner LS. Pituitary-specific knockout of the Carney complex gene Prkar1a leads to pituitary tumorigenesis. Mol Endocrinol. 2008;22:380–7. doi: 10.1210/me.2006-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Z, Jones GN, Towns WH, 2nd, Zhang X, Abel ED, Binkley PF, Jarjoura D, Kirschner LS. Heart-Specific Ablation of Prkar1a Causes Failure of Heart Development and Myxomagenesis. Circulation. 2008;117:1414–22. doi: 10.1161/CIRCULATIONAHA.107.759233. [DOI] [PubMed] [Google Scholar]
- 35.Nadella KS, Kirschner LS. Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res. 2005;65:10307–15. doi: 10.1158/0008-5472.CAN-05-3183. [DOI] [PubMed] [Google Scholar]
- 36.Pavel E, Nadella K, Towns WH, 2nd, Kirschner LS. Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A. Mol Endocrinol. 2008;22:430–40. doi: 10.1210/me.2007-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirschner LS, Yin Z, Jones GN, Mahoney E. Mouse models of altered protein kinase A signaling. Endocr Relat Cancer. 2009;16:773–93. doi: 10.1677/ERC-09-0068. [DOI] [PubMed] [Google Scholar]
- 38.Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO. Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 39.Kineman RD, Teixeira LT, Amargo GV, Coschigano KT, Kopchick JJ, Frohman LA. The effect of GHRH on somatotrope hyperplasia and tumor formation in the presence and absence of GH signaling. Endocrinology. 2001;142:3764–73. doi: 10.1210/endo.142.9.8382. [DOI] [PubMed] [Google Scholar]
- 40.Burton FH, Hasel KW, Bloom FE, Sutcliffe JG. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature. 1991;350:74–7. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–63. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Lu X, Gershengorn MC. Thyrotropin-releasing hormone receptors -- similarities and differences. J Mol Endocrinol. 2003;30:87–97. doi: 10.1677/jme.0.0300087. [DOI] [PubMed] [Google Scholar]
- 44.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gundogdu S, De Menis E, Makinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–30. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 45.Cazabat L, Guillaud-Bataille M, Bertherat J, Raffin-Sanson ML. Mutations of the gene for the aryl hydrocarbon receptor-interacting protein in pituitary adenomas. Horm Res. 2009;71:132–41. doi: 10.1159/000197869. [DOI] [PubMed] [Google Scholar]
- 46.Lin BC, Sullivan R, Lee Y, Moran S, Glover E, Bradfield CA. Deletion of the aryl hydrocarbon receptor-associated protein 9 leads to cardiac malformation and embryonic lethality. J Biol Chem. 2007;282:35924–32. doi: 10.1074/jbc.M705471200. [DOI] [PubMed] [Google Scholar]
- 47.Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci U S A. 2005;102:9218–23. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira LT, Kiyokawa H, Peng XD, Christov KT, Frohman LA, Kineman RD. p27Kip1-deficient mice exhibit accelerated growth hormone-releasing hormone (GHRH)-induced somatotrope proliferation and adenoma formation. Oncogene. 2000;19:1875–84. doi: 10.1038/sj.onc.1203490. [DOI] [PubMed] [Google Scholar]
- 49.Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, Cheadle C, Watkins T, Wen F, Starost MF, Bossis I, Nesterova M, Stratakis CA. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19:1387–98. doi: 10.1093/hmg/ddq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luque RM, Soares BS, Peng XD, Krishnan S, Cordoba-Chacon J, Frohman LA, Kineman RD. Use of the metallothionein promoter-human growth hormone-releasing hormone (GHRH) mouse to identify regulatory pathways that suppress pituitary somatotrope hyperplasia and adenoma formation due to GHRH-receptor hyperactivation. Endocrinology. 2009;150:3177–85. doi: 10.1210/en.2008-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]