Abstract
Anginex is a synthetic beta-sheet peptide with anti-angiogenic and anti-tumor activity. When added to cultured endothelial cells at concentrations ranging from 2.5 μM to 25 μM, anginex induced cell death, which was reflected by a strong increase of subdiploid cells and fragments, loss of cellular ATP, and LDH release. Cytotoxicity remained the same whether cells were treated with anginex at 4 °C or at 37 °C. At low temperatures, fluorescein-conjugated anginex accumulated on the endothelial surface, but did not reach into the cytoplasm, indicating that the cell membrane is the primary target for the peptide. Within minutes of treatment, anginex caused endothelial cells to take up propidium iodide and undergo depolarization, both parameters characteristic for permeabilization of the cell membrane. This process was amplified when cells were activated with hydrogen peroxide. Red blood cell membranes were essentially unaffected by anginex. Anginex bound lipid bilayers with high affinity and with a clear preference for anionic over zwitterionic phospholipids. Structural studies by circular dichroism and solid-state nuclear magnetic resonance showed that anginex forms a beta-sheet and adopts a unique and highly ordered conformation upon binding to lipid membranes. This is consistent with lipid micellization or the formation of pore-forming beta-barrels. The data suggest that the cytotoxicity of anginex stems from its ability to target and disrupt the endothelial cell membrane, providing a possible explanation for the angiostatic activity of the peptide.
Keywords: anginex, amyloid-beta, lipid membrane, beta-sheet, anti-angiogenic
Introduction
Anginex is a synthetic 33 residue peptide with structural features similar to those of several anti-angiogenic cytokines.1 In its bioactive form, an anti-parallel beta-sheet, anginex inhibits angiogenesis and tumor growth.2 Anginex forms complexes with fibronectin in the blood circulation, utilizing the adhesion molecule as a means to home to angiogenic blood vessels.3 It has been reported that anginex can block adhesion and migration in endothelial cells.4 However, the mechanisms by which anginex executes its in vivo and in vitro effects are largely unknown. Anginex was originally designed to reproduce the beta-sheet structure of anti-angiogenic proteins such as platelet factor 4,5 endostatin,6 tumor necrosis factor,7 and bactericidal permeability increasing protein.8 Significantly, other anti-angiogenic peptides, notably anastellin and denatured antithrombin share this structural feature.9,10 Moreover, amyloid-beta peptide, the prototypical beta-sheet peptide, was recently reported to have anti-angiogenic activity.11 This implies a general structure-function relationship of beta-sheet peptides in their interaction with cells or tissues.
Anginex is composed of numerous cationic and hydrophobic amino acids that fold as an amphiphilic beta-sheet.2 The structure-function relationships of beta-sheet peptides and beta-barrels are well established for cationic antimicrobial peptides and bacterial pore forming peptides, respectively.12,13 These peptides target prokaryotic and eukaryotic cells, and their cytotoxic effects stem primarily from their high affinity for lipids.12,13
Amphiphilic antimicrobial peptides bind membranes and disrupt lipid bilayers via micellization or pore formation.14 Their net positive charge determines their ability to bind to bacterial membranes, which contain a large amount of anionic phospholipids such as phosphatidylglycerol and cardiolipin.15,16 In mammalian cells, anionic phospholipids are normally located at the inner leaflet of the cell membrane, whereas the outer leaflet is rich in neutral and zwitterionic lipids.17 This membrane asymmetry can be reversed during apoptosis, cell stress, and cell activation. As a result, anionic phospholipids, such as phosphatidylserine, become available on the cell surface.18 Indeed, it was reported recently that phosphatidylserine, expressed on the surface of proliferating endothelial cells, is a marker for angiogenic blood vessels.18 Moreover, monoclonal antibodies raised against anionic phospholipids or annexin A1, a protein that binds to phosphatidylserine, home specifically to tumor vasculature, and have anti-tumor activity when coupled with chemo- or radiotherapeutics.19,20
Based on its structural similarities with antimicrobial peptides, we hypothesized that anginex can target the lipid bilayer of cell membranes. In this study, we report that anginex is cytotoxic to endothelial cells, causing membrane depolarization and leakiness. We show that anginex binds lipid bilayers with high avidity and specifically disrupts those composed of anionic phospholipids. Structural studies by circular dichroism (CD) and solid-state nuclear magnetic resonance (NMR) show that, upon binding to lipid membranes, anginex adopts a beta-sheet conformation with a unique orientation reminiscent of polypeptides known to cause lipid micellization and pore-formation.21 Anginex may be useful for targeting anionic phospholipids within the tumor vasculature. This study also provides a potential explanation for the anti-angiogenic and anti-tumor effects of anginex.
Results
Anginex is cytotoxic and destabilizes the endothelial cell membrane
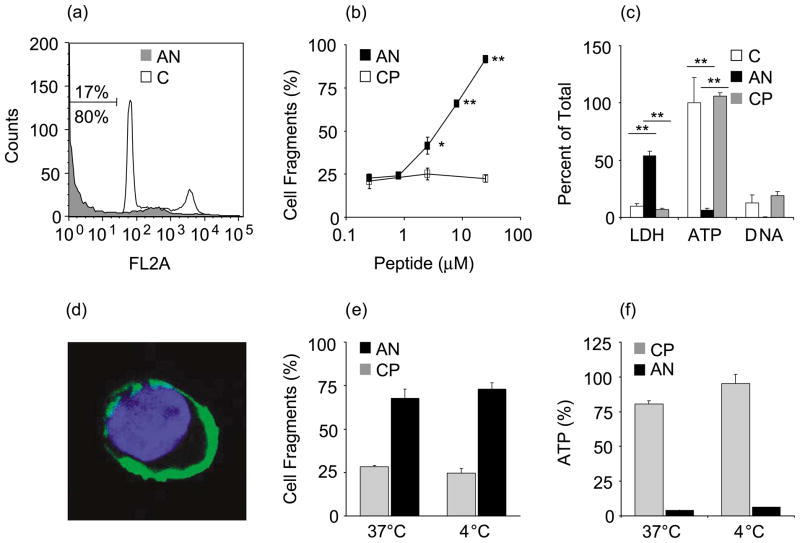
Anginex added to the culture medium of human umbilical vein endothelial cells (HUVECs) caused rapid cell death. The subdiploid cell fraction increased within 8 h from 17% in untreated HUVECs to 80% with peptide treatment (Figure 1(a)). Cell death was increased slightly in response to 2.5 μM anginex and maximal after 25 μM anginex (Figure 1(b)). HUVECs treated with anginex lost cellular ATP and leaked LDH into the medium (Figure 1(c)). The amount of LDH that was released accounted for approximately 50–60% of cell death. In addition, anginex-treated HUVECs were negative for nucleosomal DNA fragments, suggesting that the peptide induced necrotic rather than apoptotic cell death.22
Figure 1.
Anginex is cytotoxic to endothelial cells. HUVECs were analyzed for cell fragments by flow cytometry. (a) Treatment with 25 μM anginex for 8 h (AN, grey; 80% fragments) compared to control (C; 17% fragments). Representative experiments out of four are shown. (b) The subG1 fraction is increased significantly in response to 2.5 μM anginex and maximal after treatment with 25 μM anginex (AN, anginex; CP, control peptide). (c) Cytotoxicity of anginex (8 h, 25 μM) is reflected also by increased LDH release and decreased cellular ATP, whereas nucleosomal DNA fragments were not increased. (d) HUVECs were analyzed by confocal microscopy after incubation with fluorescein-conjugated anginex at 4 °C (anginex, green; nucleus, blue. Magnification: 600×). (e) SubG1 and (f) ATP analysis after 8 h treatment with 8 μM anginex or control peptide at different temperatures. Results are presented as means and SEM (*p<0.05; **p<0.01).
To determine whether anginex had to be transported across the cell membrane, HUVECs were treated with the peptide at 4 °C.23 Endosome formation was impaired at this temperature, as measured by uptake of Lysotracker® (not shown); concomitantly, fluorescein-conjugated anginex remained at the cell membrane with no fluorescence detectable inside the cell (Figure 1(d)). The cytotoxicity of anginex was not affected by the low temperature (Figure 1(e) and (f)), indicating that internalization is not necessary for anginex to be active.
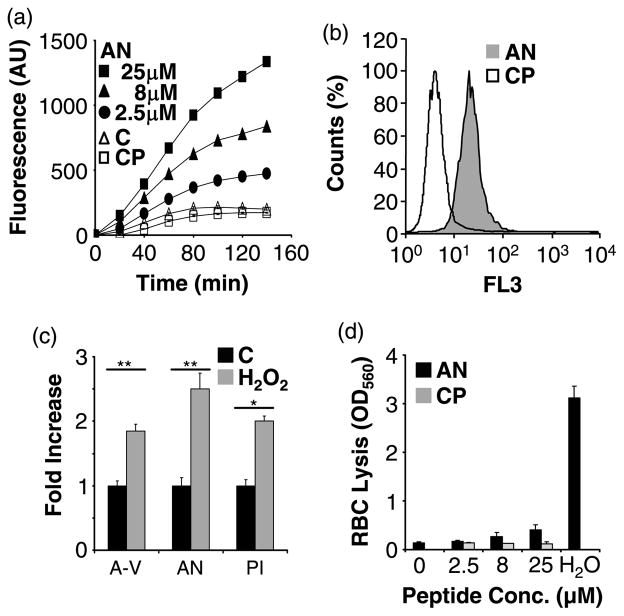
We next studied the effect of anginex on the integrity of the cell membrane. Membrane leakage causes depolarization because sodium ions can travel freely through the membrane.24 We observed membrane depolarization in response to anginex, but not in response to a control peptide (Figure 2(a)). This effect occurred at the same concentration range where anginex was cytotoxic. Membrane permeabilization of anginex-treated HUVECs was demonstrated also by propidium iodide (PI) internalization (Figure 2(b)). PI, which cannot permeate intact cell membranes, became internalized within 30 min, even when the cells were incubated on ice. Hydrogen peroxide has been shown to disturb membrane asymmetry.18 Upon activation with H2O2 HUVECs upregulated phosphatidylserine expression on the outer membrane, as indicated by increased annexin V binding (Figure 2(c)). Concomitantly, anginex binding and anginex-induced membrane permeabilization were enhanced. Normally, only a small fraction (<1%) of red blood cells expresses anionic phospholipids on the cell surface.25 The “non-activated” red blood cell membrane can be perturbed by some antimicrobial peptides, resulting in cell lysis.26 In contrast, anginex caused only minimal red blood cell lysis even at high concentration (Figure 2(d)).
Figure 2.
Anginex permeabilizes the endothelial cell membrane. (a) Anginex (AN 2.5–25 μM) induces membrane depolarization in HUVECs, whereas the control peptide (CP) has no effect over culture media (C). (b) HUVECs were treated with 25 μM anginex or control peptide for 30 min on ice. Propidium iodide uptake of anginex treated cells (grey) was assessed by flow cytometry. (c) Fold increase binding of FITC annexin V (A-V), fluorescein-conjugated anginex (AN), and propidium iodide uptake (PI) was assessed by flow cytometry after 2 h treatment with 1 mM hydrogen peroxide (H2O2) compared to untreated controls (C). (d) Red blood cells (RBC) treated with anginex show negligible lysis. Maximal lysis of RBC was induced by diluting the cells into water (H2O). Results are presented as means and SEM (*p<0.05; **p<0.01).
Anginex binds to and disrupts lipid bilayers
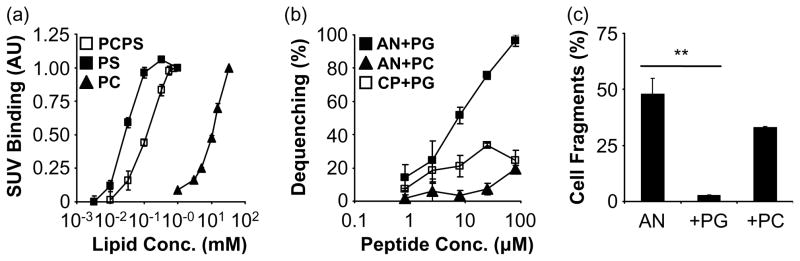
Anginex displays a hydrophobic face that facilitates binding to lipid membranes.2 In order to assess the membrane partition coefficient of anginex to lipid bilayers, peptide binding to small unilamellar vesicles (SUVs) was measured using the intrinsic tryptophan fluorescence from the sole tryptophan residue in anginex. Binding of anginex (10 μM) to zwitterionic liposomes required millimolar concentrations of dioleoyl-glycerophosphocholine (DOPC) (Figure 3(a)) and corresponded to a membrane partition coefficient (K) of 5.6×103 (Table 1). In contrast, binding of anginex to anionic dioleoyl-glycerophosphoserine (DOPS) liposomes was approximately 400-fold greater, with saturation at a lipid concentration of ca 100 μM and a membrane partition coefficient (K) of 2.2×106. Anginex binding to DOPS was approximately fivefold stronger than to liposomes made of a combination of DOPC:DOPS (molar ratio of 8:2) which closely reflects the phosphatidylserine content of the two different liposome preparations. Compared to DOPS, anginex bound to dioleoyl-glycerophosphoglycerol (DOPG) vesicles even more strongly, underscoring the high affinity of the peptide for negatively charged phospholipids (Table 1). Also, anginex binding to DOPG was fourfold greater than to DOPC:DOPG (8:2).
Figure 3.
Anginex binds to and disrupts lipid bilayers. The binding of anginex to liposomes was assessed by measuring liposome-associated tryptophan fluorescence normalized for fluorescence intensity in the absence of liposomes. (a) Binding of anginex (10 μM) to zwitterionic DOPC liposomes (PC) occurred at millimolar phospholipid concentrations, whereas binding of anginex to anionic DOPS (PS) was saturated at approximately 100 μM lipid. Anginex binding to DOPS was ca fivefold higher than to DOPC:DOPS (8:2) liposomes (PCPS). (b) Binding of anginex to liposomes leads to the disruption of lipid bilayers as assessed by carboxyfluorescein (CF) dequenching. Anginex (AN) is more effective in releasing CF from DOPC:DOPG (PG) than from DOPC (PC) liposomes. A control peptide (CP) did not cause significant CF release from the DOPC:DOPG liposomes. (c) SubG1 analysis was performed by FACS after addition of liposomes to the HUVEC medium. Anginex-induced cell death (AN) was inhibited completely by anionic DOPC:DOPG liposomes (+PG). Zwitterionic DOPC liposomes reduced anginex cytotoxicity to HUVECs by approximately 30% (+PC). Results are presented as means and SEM (*p<0.05; **p<0.01).
Table 1.
Membrane partition coefficient of anginex on liposomes
| Liposome preparation | Membrane partition coefficient (SEM) |
|---|---|
| DOPC | 5.6×103 (1341) |
| DOPS | 2.2×106 (289,589) |
| DOPC: DOPS | 4.5×105 (164,198) |
| DOPG | 3.2×106 (174,459) |
| DOPC:DOPG | 7.9×105 (437,378) |
The binding of 10 μM anginex to lipid bilayers was assessed by measuring liposome-associated tryptophan fluorescence normalized for fluorescence intensity in the absence of liposomes. The membrane partition coefficient (K) was calculated according to the best fit of the data. Results are expressed as mean values (SEM) from at least two experiments with duplicate data points.
Binding of anginex to liposomes led to the disruption of lipid bilayers, as assessed by carboxy-fluorescein (CF) dequenching (Figure 3(b)). In agreement with its high affinity for anionic phospholipids, anginex was more effective in releasing the contents from vesicles made of DOPC:DOPG (molar ratio of 8:2) than from those made of DOPC alone, which began to undergo lysis only at the highest concentration of peptide. A control peptide did not cause significant CF release from the anionic liposomes. Furthermore, when added to the HUVEC culture medium, the anionic liposomes were much more effective in antagonizing the cytotoxic effect of anginex on endothelial cells than the zwitterionic liposomes (Figure 3(c)).
Anginex adopts a unique conformation and orientation in lipid bilayers
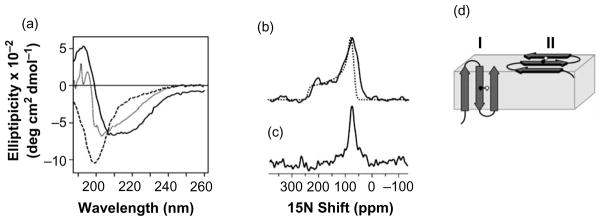
It has been shown by CD and solution NMR spectroscopy that anginex is a random-coil monomer in micromolar solutions, but adopts a tetrameric beta-sheet structure at millimolar concentrations.2,27 Our studies show that the CD spectrum of 25 μM anginex has a minimum at 200 nm, which is characteristic of unstructured polypeptides; while at 100 μM, the minimum shifts to a higher wavelength, indicating a tendency to beta-sheet conformation (Figure 4(a), broken and dotted lines). However, in the presence of dodecylphosphocholine (DPC) lipid micelles, the spectrum of anginex reflects a beta-sheet conformation even at the lower concentration of 25 μM (Figure 4(a), continuous line).
Figure 4.
(a) Anginex adopts a beta-stranded conformation in lipid micelles. CD spectra of anginex in solution at room temperature. The spectra were obtained at 25 °C and pH 5.5 for 25 μM anginex in water (broken line), 100 μM anginex in water (dotted line), and 25 μM anginex in 125 mM DPC (continuous line). The one-dimensional 15N chemical shift NMR spectrum of [15N] Val22-labeled anginex in unoriented lipid bilayers ((b) continuous line) is similar to the powder pattern calculated for a rigid 15N amide site ((b) dotted line). (c) The spectrum for anginex in oriented lipid bilayers has a single resonance frequency at 75 ppm, and (d) is consistent with two possible models for anginex membrane association. In model I, anginex traverses the membrane, while in model II it rests on the membrane surface. The Val22 NH bond is marked as black and white spheres.
To examine the conformation of anginex bound to membranes, we obtained 15N chemical shift solid-state NMR spectra of 15N-labeled anginex associated with lipid bilayers. For these samples, the methods of sample preparation and the lipid composition of 100% DOPC were identical with those of the vesicles used in the tryptophan fluorescence studies described in Figure 3. Solid-state NMR spectra of polypeptides bound to lipid bilayers that are oriented with their surface perpendicular to the magnetic field provide direct information on the structure and orientation of a polypeptide within the bilayer. For example, alpha-helices and beta-sheets give distinct solid-state NMR spectra that contain information on secondary structure, as well as tilt and rotation within the membrane.28
The 15N chemical shift spectrum of [15N]Val22-labeled anginex in unoriented lipid bilayers is a powder pattern (Figure 4(b), continuous line) that spans the full range (60–220 ppm) of the amide 15N chemical shift interaction (Figure 4(b), dotted line), and is similar to the spectrum obtained from lyophilized peptide without lipids (data not shown). The absence of intensity at the isotropic resonance frequencies (100–130 ppm) demonstrates that at Val22 the peptide is immobilized by its interaction with the lipid bilayer on the timescale of the 15N chemical shift interaction. In contrast, the spectrum of anginex in planar oriented lipid bilayers has a single resonance at 75 ppm, arising from the single 15N label at Val22 (Figure 4(c)). The absence of isotropic intensity in Figure 4(b) and the presence of a single peak in Figure 4(c) indicate that anginex associates tightly with the lipid bilayer and adopts a unique conformation and orientation in the membrane.
Discussion
In this study, we have examined the molecular basis of the anti-angiogenic properties of anginex. In agreement with earlier studies, we show that anginex is cytotoxic to endothelial cells. We then provide evidence indicating that the cytotoxicity is related to a loss of cellular ATP and membrane integrity. We show that anginex binds to lipid membranes that mimic the membrane of cells activated by growth, stress or apoptosis, which is associated with a negative surface charge of the cell membrane. Anginex elicits membrane depolarization and discharge of contents from such liposomes. Finally, CD and solid-state NMR showed that anginex adopts a beta-sheet conformation in the presence of lipids, and binds to membranes in an orientation that is compatible with membrane perturbation.
The effects of anginex reported here are reminiscent of those of bacterial beta-barrel pore-forming toxins that permeabilize eukaryotic membranes, causing irregular ion flux, ATP depletion, and ultimately necrotic cell death.29 Cellular ATP depletion, which occurs in response to anginex at concentrations as low as 2.5 μM, has been shown to sensitize tumors toward chemotherapy,30 and could be the basis of the radiosensitizing effect observed with anginex.31,32
It has been suggested that the anti-angiogenic effect of anginex is based on its ability to inhibit cell motility or to induce cell detachment and, subsequently, anoikis.4 On the basis of the results presented here, one can speculate that the anti-adhesive and anti-migratory effects result from binding to and blocking of phosphatidylinositol, an anionic phospholipid involved in cell motility and integrin activation.33 Alternatively, anginex could inhibit cell migration by allowing the uncoordinated influx of water, thus antagonizing water channels, which were shown recently to be critically important for directed cell motility and angiogenesis.34
Membrane-targeting peptides and proteins show different levels of cell-type specificity, depending on their lipid-binding properties, which are linked to a positive net charge of the compound.15 The membrane-perturbing activity of anginex is selective, as it causes only minimal red blood cell lysis, which is a measure of generalized activity on membranes.26 The key to the selectivity of anginex is likely to be its preferential binding to anionic phospholipids, which are rare in the outer leaflet of the cell membrane of resting cells.17 Anionic phoshoplipids are translocated to the cell surface in response to apoptosis and cell stress.18 Endothelial cells in tumor vasculature specifically express annexin A1, which colocalizes with anionic phospholipids on the cell surface.35,20 The affinity of anginex for anionic phospholipids may, at least in part, explain its selective anti-angiogenic activity in vivo.
Binding of anionic phospholipids does not seem to be sufficient for anginex to be anti-angiogenic in vivo, as anginex also requires the involvement of fibronectin for in vivo activity. Anginex is inactive in mice that lack plasma fibronectin, as measured by matrigel plug angiogenesis assays.3 The results indicate that anginex forms complexes with plasma fibronectin, and that these complexes assist anginex in its homing to sites of angiogenesis. The homing takes place through binding of fibronectin to integrins that are up-regulated in angiogenic endothelium. Like many cationic peptides,6,36 anginex also binds to heparan sulfate (J.P. and E.R., unpublished results). Heparan sulfate proteoglycans are up-regulated in angiogenic blood vessels,37 but do not seem to act as significant cell surface-binding sites for anginex, as treating endothelial cells with heparitinase has no significant influence on the cytotoxicity of anginex (J.P. and E.R., unpublished results). Phagocytes interact with apoptotic cells using both integrin and phospholipid receptors.38 In analogy, the anginex-fibronectin complexes may bind cooperatively to integrins and anionic phospholipids on angiogenic endothelial cells. In vitro, the cytotoxicity of anginex is unaffected by fibronectin (J.P. and E.R., unpublished results), suggesting that if such cooperativity exists, it is required only in the in vivo situation, where the binding requirements are likely to be more demanding.
Three general concepts describe the membrane-destructive effect of amphiphilic peptides. The carpet mechanism suggests that amphiphilic peptides bind with their hydrophobic face planar to the surface of the cell membrane and disrupt the membrane by forming lipid micelles.14 In contrast, the beta-barrel mechanism suggests that monomeric peptides self-associate to form pores that traverse the membrane, with polar residues lining the aqueous pore, and lipophilic residues facing the hydrophobic membrane core. In this model, the interior of the pore consists solely of lined-up peptide barrels.39 Alternatively, membrane pores can be formed by supramolecular peptide-lipid complexes, in which peptide oligomers are oriented perpendicular to the membrane surface with the lipid head groups interposing between neighboring peptide barrels.40 The model includes accelerated lateral diffusion of membrane lipids with loss of membrane asymmetry, which, in turn, would rapidly provide additional binding sites for anginex.
Our solid-state NMR spectra obtained for [15N]Val22-labeled anginex show that the peptide adopts a unique conformation and orientation in membranes that is consistent with all three models (Figure 4(d)). In model I, the anginex beta-sheet traverses the membrane (beta-barrel formation), while in model II, the beta-sheet rests on the surface of the lipid bilayer (“carpet mechanism”). Both models are compatible with the hypothesis that anginex activity is associated with membrane permeabilization, either through the formation of membrane pores in model I, or through membrane destabilization in model II, in a manner similar to the activity of antimicrobial peptides, which induce the leakage of the cell contents, disruption of the electrical potential, and ultimately cell death.41–43 Solution NMR experiments with anginex in DPC micelles show that the addition of Mn2+ significantly broadens the heteronuclear single quantum correlation (HSQC) peak from 15N-labeled Val22, indicating that Val22 is water-exposed (data not shown). This result is consistent also with both the carpet and the beta-barrel mechanisms for membrane destabilization. The amyloid beta-peptide can self-assemble spontaneously into pores in vitro,44 and anginex is capable of self-association at high concentrations (J.P. and E.R., unpublished results), but whether these aggregates could form membrane pores remains to be established.
Taken together, our results and results from other laboratories indicate that anginex possesses three significant functional properties: (i) it is cytotoxic via interactions with the cell membranes of certain cells; (ii) it homes to angiogenic vessels by binding directly to anionic phospholipids and by interacting with integrins via polymerized fibronectin; and (iii) it can sensitize target cells for other modes of cancer treatment. These properties may make anginex a useful anti-angiogenic and anti-cancer agent. Additionally, the ability of anginex to home to tumor vasculature3 could be made use of in delivering other compounds to tumors, as has been done with peptides that bind selectively to integrins or aminopeptidase N in angiogenic vessels.45–47
Anginex was originally designed to mimic the positively charged anti-parallel beta-sheet structure of anti-angiogenic proteins such as endostatin and platelet factor 4.5,6 At least two other peptides (anastellin, and the amyloid beta-peptide) have since been added to the list of anti-angiogenic, cationic beta-sheet peptides.2,9,11,48,49 Another significant similarity shared by anginex and several other members of this group of proteins is that their in vivo anti-angiogenic effect depends on the presence of plasma adhesion proteins containing the RGD cell attachment sequence;50 anastellin and anginex require plasma fibronectin, denatured anti-thrombin depends on vitronectin, while endostatin requires both.3,51 Like anginex, endostatin has been reported to bind avidly to membranes containing anionic phospholipids.52 These similarities raise the question of similarity in mechanism of action. We have found that anastellin exerts effects on endothelial cell membranes and levels of cellular ATP similar to those of anginex (J.P. and E.R., unpublished results). Endostatin, denatured antithrombin, and platelet factor 4 are thought to convey their anti-proliferative properties through heparin-binding moieties or by interference with growth factor signaling.53–55 Analyses similar to ours have not been carried out with these proteins, but it is tempting to speculate that their mechanisms of action may be, at least partially, similar to the one we propose here for anginex.
Materials and Methods
Peptide synthesis
Anginex was synthesized by N-(9-fluorenylmethoxycarbonyl)-L-amino acid (FMOC) chemistry with a solid-phase synthesizer, purified by HPLC, and conjugated with fluorescein at the N terminus by reacting with fluorescein isothiocyanate isomer (FITC, Aldrich) in dimethylformamide for 20 h in the presence of diisopropylethylamine. For NMR studies, anginex was labeled with 15N at Val22, by incorporating FMOC-[15N]Val (Cambridge Isotopes, Andover, MA) in the synthesis. A control peptide (VN peptide) comprising the central heparin-binding region of vitronectin (residues 348–361), KKQRFRHRNRKGYR, was synthesized.56 Peptide purity and homogeneity were confirmed by mass spectrometry.
Cytotoxicity assay
HUVECs cultured according to the manufacturer’s specifications (Cambrex, East Rutherford, NJ) were treated with anginex or a control peptide in culture medium for 8 h at 37 °C and 5% (v/v) CO2. To assess cell death, floating and adherent HUVECs were fixed in 50% (v/v) ethanol, and frozen at −20 °C. The genomic DNA of the cells was stained with propidium iodide (PI; Molecular Probes, Eugene, OR) in the presence of RNase (Roche). The sub-diploid cell fraction was assessed using a FACscan flow cytometer and Cell Quest software (Becton Dickinson, San Jose, CA). LDH was measured with the Cytotoxicity Detection Kit, DNA fragmentation with the Cell Death Detection ELISAplus kit, and ATP with the ATP Bioluminescence Assay Kit CLS II (Roche, Indianapolis, IN). LDH release was maximal in response to 0.1% (v/v) Triton X-100, and DNA fragmentation was maximal in response to 5 μM staurosporine (Calbiochem, San Diego, CA).
Membrane depolarization
HUVECs were grown 80% confluent in 96-well tissue culture-treated plates (Nalge Nunc International, Rochester, NY), washed, and incubated with 1 μM diSC3–5 in 50 μl of culture medium/well. diSC3-5 (Molecular Probes, Eugene, OR) is a membrane potential-sensitive dye that responds to membrane depolarization with increased fluorescence intensity.24 Anginex or the control peptide were dissolved in culture medium and added to the fluorescent-labeled cells to a final volume of 100 μl. Fluorescence intensity (λex=620 nm; λem=670 nm) was measured in a fluorescence plate reader (Biorad) and normalized for dye loading.
Flow cytometry of the endothelial membrane
HUVEC membrane binding and permeabilization was assessed using a FACscan flow cytometer and Cell Quest software (Becton Dickinson, San Jose, CA). After 2 h of incubation with 1 mM H2O2, HUVECs were harvested with trypsin/EDTA, washed with PBS, and stained with 10 μM fluorescein-conjugated anginex, FITC-conjugated annexin V, and propidium iodide (both diluted 1:100 according to the manufacturer’s protocol; Biodivision Inc.), followed by immediate flow cytometric analysis. To test membrane permeabilization, HUVECs were incubated with 25 μM anginex on ice for 30 min before addition of PI. Values were normalized for unspecific fluorescence. The effect of the treatment with H2O2 is presented as fold fluorescence over untreated cultures.
Red blood cell lysis
Red blood cells were isolated according to standard protocols,25 and were re-suspended in PBS before treatment with anginex or the control peptide for 8 h. Lysis was assessed in an absorbance plate reader at 560 nm.
Confocal microscopy
HUVECs grown on collagen-coated coverslips were incubated for 2 h with 2.5 μM fluorescein-conjugated anginex at 4 °C to prevent peptide internalization. The cells were then fixed with 4% (v/v) paraformaldehyde, mounted with 4″,6-diamidino-2-phenylindole (DAPI)-containing medium (Vectashield), and analyzed by confocal microscopy (Radiance 2100/AGR-3 (Q) multiphoton laser scanning confocal microscope). Digitized images were further processed with Adobe Photoshop 7.0.
Liposome binding, lysis, and quenching
Unilamellar vesicles were prepared by mixing phospholipids (Avanti, Alabaster AL) in chloroform, removing the solvent under vacuum, suspending the lipids in Hepes buffer, and sonicating on ice to transparency. Vesicles were composed of 100% DOPC, DOPS, DOPG, or DOPC mixed with DOPS or DOPG at a molar ratio of 8:2.
The binding of anginex to liposomes was assessed by measuring the intrinsic fluorescence of the single tryptophan residue in the anginex peptide. Fluorescence was measured with 283 nm excitation, using a MOS-250 fluorescence spectrometer equipped with Biokine-32 software (Bio-Logic Science Instruments). Fluorescence intensity (F) was divided by the fluorescence from anginex in the absence of liposomes (F0). The mole-fraction partition coefficient (K) was calculated from the best fit of the data to the equation:
where Imax is the maximum fluorescence increase upon complete binding, [L] is the average lipid concentration, and [W] is the molar concentration of water (55.3 M).57,58 For consistent presentation, F/F0 values were normalized according to the equation:
Liposome lysis was assessed by measuring carboxyfluorescein (CF; Sigma) dequenching.59 For this study, the liposomes were produced by sonicating in the presence of 50 mM CF, and then dialyzed until the extravesicular dye was entirely removed. Dequenching of 500 μM liposomes was measured at 492 nm excitation 30 min after anginex or control peptide treatment. CF release was calculated according to the equation:
where R is the CF release (in %), f0 is the fluorescence intensity before treatment; ftreatment is the fluorescence intensity after treatment; and fTriton is the fluorescence intensity after complete liposome lysis with 0.1% Triton X-100.
CD spectroscopy
Lyophilized anginex was dissolved in either water or 125 mM dodecyl-phosphocholine (DPC; Cambridge Isotopes) at concentrations of 0.1 μM and 25 μM, and the pH was adjusted by adding NaOH or acetic acid. The samples were filtered through a 0.45 μm pore size filter and transferred to a quartz cuvette (0.1 mm path length). Far-UV CD spectra were recorded at 25 °C on an Aviv model 62A-DS CD spectrometer (Aviv Instruments Inc., Lakewood, NJ) equipped with a temperature controller. A 5 s time constant and a 1.0 nm bandwidth were used during data acquisition over a wavelength range of 180–260 nm. For each sample, three spectra were recorded, averaged, and referenced by subtracting the average of three spectra obtained using either water or 125 mM DPC.
Solid-state NMR spectroscopy
Samples of [15N]Val22-labeled anginex in bilayers were prepared by adding 2 mg of peptide dissolved in 100 μl of water to 100 mg of DOPC that had been sonicated in 1 ml of water to form small unilamellar vesicles. The vesicles retained their characteristic transparency after peptide addition. The peptide-bound vesicles were distributed on the surface of 12 glass slides (11 mm×11 mm; #00, Marienfeld, Germany). To form oriented lipid bilayers, excess water was allowed to evaporate at 40 °C, and the slides were stacked and equilibrated for 24 h at 40 °C in a chamber maintained at 93% relative humidity with a saturated solution of ammonium phosphate. The samples were wrapped in Parafilm and then sealed in thin polyethylene film prior to insertion in the NMR probe. Unoriented lipid bilayers with anginex were prepared by crushing the glass slides supporting the peptide-reconstituted bilayers in additional water and resealing the sample in a plastic bag.
Solid-state NMR experiments were performed at room temperature on a Bruker AVANCE 500 spectrometer (Billerica, MA) with a 500/89 AS Magnex magnet (Yarnton, UK). The 1H/15N double-resonance probe had a square radiofrequency coil (11 mm×11 mm×3 mm) wrapped directly around the samples. The one-dimensional 15N chemical shift spectra were obtained with single contact cross-polarization,60 a contact time of 1 ms, a 1H 90° pulse-width of 5 μs, and continuous 63 kHz 1H decoupling. The 15N chemical shifts were referenced to 0 ppm for liquid ammonia. The data were processed using NMR Pipe61 and rendered in Sparky† on a Dell Precision 330 MT Linux workstation (Round Rock, TX).
Statistical analysis
Statistical analysis was performed with the Student’s t-test (*, p<0.05; **, p<0.01). Where error bars are shown, the results reflect means and standard error of the mean (SEM).
Acknowledgments
We thank Andrei Bobkov for providing support with the fluorescence spectrometry and Roslind Varghese for editing. This work was supported by National Cancer Institute Grant R01-CA-102153 (to E.R.), R01-CA-082864 (to F.M.M.) and National Cancer Institute Cancer Center Support Grant P30-CA-30199-23 and utilized the UCSD Resource for Molecular Imaging of Proteins supported by grant P41EB002031.
Abbreviations used
- DPC
dodecylphosphocholine
- DOPC
dioleoyl-glycerophosphocholine
- DOPG
dioleoyl-glycerophosphoglycerol
- DOPS
dioleoyl-glycerophosphoserine
- HSQC
heteronuclear single quantum correlation
- HUVECs
human umbilical vein endothelial cells
- SUV
small unilamellar vesicles
- CF
carboxyfluorescein
- SEM
standard error of the mean
Footnotes
References
- 1.Griffioen AW, van der Schaft DW, Barendsz-Janson AF, Cox A, Struijker Boudier HA, et al. Anginex, a designed peptide that inhibits angiogenesis. Biochem J. 2001;354:233–242. doi: 10.1042/0264-6021:3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dings RP, Arroyo MM, Lockwood NA, van Eijk LI, Haseman JR, Griffioen AW, Mayo KH. Beta-sheet is the bioactive conformation of the anti-angiogenic anginex peptide. Biochem J. 2003;373:281–288. doi: 10.1042/BJ20030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerman ME, Pilch J, Peters D, Ruoslahti E. Angiostatic peptides use plasma fibronectin to home to angiogenic vasculature. Proc Natl Acad Sci USA. 2005;102:2040–2045. doi: 10.1073/pnas.0409844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Schaft DW, Dings RP, de Lussanet QG, van Eijk LI, Nap AW, Beets-Tan RG, et al. The designer anti-angiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. FASEB J. 2002;16:1991–1993. doi: 10.1096/fj.02-0509fje. [DOI] [PubMed] [Google Scholar]
- 5.Mayo KH, Roongta V, Ilyina E, Milius R, Barker S, Quinlan C, et al. NMR solution structure of the 32-kDa platelet factor 4 ELR-motif N-terminal chimera: a symmetric tetramer. Biochemistry. 1995;34:11399–11409. doi: 10.1021/bi00036a012. [DOI] [PubMed] [Google Scholar]
- 6.Hohenester E, Sasaki T, Olsen BR, Timpl R. Crystal structure of the angiogenesis inhibitor endostatin at 1.5 Å resolution. EMBO J. 1998;17:1656–1664. doi: 10.1093/emboj/17.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeyens KJ, De Bondt HL, Raeymaekers A, Fiers W, De Ranter CJ. The structure of mouse tumour-necrosis factor at 1.4 Å resolution: towards modulation of its selectivity and trimerization. Acta Crystallog sect D. 1999;55:772–778. doi: 10.1107/s0907444998018435. [DOI] [PubMed] [Google Scholar]
- 8.Beamer LJ, Carroll SF, Eisenberg D. Crystal structure of human BPI and two bound phospholipids at 2.4 Å resolution. Science. 1997;276:1861–1864. doi: 10.1126/science.276.5320.1861. [DOI] [PubMed] [Google Scholar]
- 9.Briknarova K, Akerman ME, Hoyt DW, Ruoslahti E, Ely KR. Anastellin, an FN3 fragment with fibronectin polymerization activity, resembles amyloid fibril precursors. J Mol Biol. 2003;332:205–215. doi: 10.1016/s0022-2836(03)00890-8. [DOI] [PubMed] [Google Scholar]
- 10.Schreuder HA, de Boer B, Dijkema R, Mulders J, Theunissen HJ, Grootenhuis PD, Hol WG. The intact and cleaved human antithrombin III complex as a model for serpin-proteinase interactions. Nature Struct Biol. 1994;1:48–54. doi: 10.1038/nsb0194-48. [DOI] [PubMed] [Google Scholar]
- 11.Paris D, Townsend K, Quadros A, Humphrey J, Sun J, Brem S, et al. Inhibition of angiogenesis by Abeta peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- 12.Blazyk J, Wiegand R, Klein J, Hammer J, Epand RM, Epand RF, et al. A novel linear amphipathic beta-sheet cationic antimicrobial peptide with enhanced selectivity for bacterial lipids. J Biol Chem. 2001;276:27899–27906. doi: 10.1074/jbc.M102865200. [DOI] [PubMed] [Google Scholar]
- 13.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric trans-membrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 14.Shai Y, Oren Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22:1629–1641. doi: 10.1016/s0196-9781(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 15.Dathe M, Nikolenko H, Meyer J, Beyermann M, Bienert M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Letters. 2001;501:146–150. doi: 10.1016/s0014-5793(01)02648-5. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K. Dispensable nature of phosphatidylglycerol in Escherichia coli: dual roles of anionic phospholipids. Mol Microbiol. 2001;39:1427–1433. doi: 10.1046/j.1365-2958.2001.02320.x. [DOI] [PubMed] [Google Scholar]
- 17.Williamson P, Schlegel RA. Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol. 1994;11:199–216. doi: 10.3109/09687689409160430. [DOI] [PubMed] [Google Scholar]
- 18.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 19.Huang X, Bennett M, Thorpe PE. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res. 2005;65:4408–4416. doi: 10.1158/0008-5472.CAN-05-0031. [DOI] [PubMed] [Google Scholar]
- 20.Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 21.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62:784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matassov D, Kagan T, Leblanc J, Sikorska M, Zakeri Z. Measurement of apoptosis by DNA fragmentation. Methods Mol Biol. 2004;282:1–17. doi: 10.1385/1-59259-812-9:001. [DOI] [PubMed] [Google Scholar]
- 23.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 24.Papo N, Braunstein A, Eshhar Z, Shai Y. Suppression of human prostate tumor growth in mice by a cytolytic d-, L-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 2004;64:5779–5786. doi: 10.1158/0008-5472.CAN-04-1438. [DOI] [PubMed] [Google Scholar]
- 25.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87:1179–1187. [PubMed] [Google Scholar]
- 26.Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, et al. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Letters. 1997;403:208–212. doi: 10.1016/s0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 27.Mayo KH, Ilyina E. A folding pathway for betapep-4 peptide 33mer: from unfolded monomers and beta-sheet sandwich dimers to well-structured tetramers. Protein Sci. 1998;7:358–368. doi: 10.1002/pro.5560070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marassi FM. A simple approach to membrane protein secondary structure and topology based on NMR spectroscopy. Biophys J. 2001;80:994–1003. doi: 10.1016/S0006-3495(01)76078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Essmann F, Bantel H, Totzke G, Engels IH, Sinha B, Schulze-Osthoff K, Janicke RU. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 2003;10:1260–1272. doi: 10.1038/sj.cdd.4401301. [DOI] [PubMed] [Google Scholar]
- 30.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Dings RP, Williams BW, Song CW, Griffioen AW, Mayo KH, Griffin RJ. Anginex synergizes with radiation therapy to inhibit tumor growth by radiosensitizing endothelial cells. Int J Cancer. 2005;115:312–319. doi: 10.1002/ijc.20850. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham CC, Vegners R, Bucki R, Funaki M, Korde N, Hartwig JH, et al. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J Biol Chem. 2001;276:43390–43399. doi: 10.1074/jbc.M105289200. [DOI] [PubMed] [Google Scholar]
- 34.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 35.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 36.Schmidtchen A, Frick IM, Bjorck L. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol Microbiol. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- 37.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 38.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nature Rev Mol Cell Biol. 2001;2:627–633. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 39.Fattal E, Nir S, Parente RA, Szoka FC., Jr Pore-forming peptides induce rapid phospholipid flip-flop in membranes. Biochemistry. 1994;33:6721–6731. doi: 10.1021/bi00187a044. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 41.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 42.Marassi FM, Opella SJ, Juvvadi P, Merrifield RB. Orientation of cecropin A helices in phospholipid bilayers determined by solid-state NMR spectroscopy. Biophys J. 1999;77:3152–3155. doi: 10.1016/S0006-3495(99)77145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marassi FM, Ma C, Gesell JJ, Opella SJ. Three-dimensional solid-state NMR spectroscopy is essential for resolution of resonances from in-plane residues in uniformly (15)N-labeled helical membrane proteins in oriented lipid bilayers. J Magn Reson. 2000;144:156–161. doi: 10.1006/jmre.2000.2036. [DOI] [PubMed] [Google Scholar]
- 44.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 45.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 46.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nature Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 47.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110:475–482. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi M, Ruoslahti E. A fibronectin fragment inhibits tumor growth, angiogenesis, and metastasis. Proc Natl Acad Sci USA. 2001;98:620–624. doi: 10.1073/pnas.98.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kranenburg O, Kroon-Batenburg LM, Reijerkerk A, Wu YP, Voest EE, Gebbink MF. Recombinant endostatin forms amyloid fibrils that bind and are cytotoxic to murine neuroblastoma cells in vitro. FEBS Letters. 2003;539:149–155. doi: 10.1016/s0014-5793(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 50.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 51.Yi M, Sakai T, Fassler R, Ruoslahti E. Antiangiogenic proteins require plasma fibronectin or vitronectin for in vivo activity. Proc Natl Acad Sci USA. 2003;100:11435–11438. doi: 10.1073/pnas.1635112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H, Jutila A, Nurminen T, Wickstrom SA, Keski-Oja J, Kinnunen PK. Binding of endostatin to phosphatidylserine-containing membranes and formation of amyloid-like fibers. Biochemistry. 2005;44:2857–2863. doi: 10.1021/bi048510j. [DOI] [PubMed] [Google Scholar]
- 53.Benezra R, Rafii S. Endostatin’s endpoints—deciphering the endostatin antiangiogenic pathway. Cancer Cell. 2004;5:205–206. doi: 10.1016/s1535-6108(04)00057-1. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Swanson R, Izaguirre G, Xiong Y, Lau LF, Olson ST. The heparin-binding site of antithrombin is crucial for antiangiogenic activity. Blood. 2005;106:1621–1628. doi: 10.1182/blood-2005-02-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bikfalvi A. Platelet factor 4: an inhibitor of angiogenesis. Semin Thromb Hemost. 2004;30:379–385. doi: 10.1055/s-2004-831051. [DOI] [PubMed] [Google Scholar]
- 56.Stockmann A, Hess S, Declerck P, Timpl R, Preissner KT. Multimeric vitronectin. Identification and characterization of conformation-dependent self-association of the adhesive protein. J Biol Chem. 1993;268:22874–22882. [PubMed] [Google Scholar]
- 57.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 58.Ladokhin AS, Jayasinghe S, White SH. How to measure and analyze tryptophan fluorescence in membranes properly, and why bother? Anal Biochem. 2000;285:235–245. doi: 10.1006/abio.2000.4773. [DOI] [PubMed] [Google Scholar]
- 59.Denisova E, Dowling W, LaMonica R, Shaw R, Scarlata S, Ruggeri F, Mackow ER. Rotavirus capsid protein VP5* permeabilizes membranes. J Virol. 1999;73:3147–3153. doi: 10.1128/jvi.73.4.3147-3153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pines A, Gibby MS, Waugh JS. Proton-enhanced NMR of dilute spins in solids. J Chem Phys. 1973;59:569–590. [Google Scholar]
- 61.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]