Abstract
The cell has >60 different farnesylated proteins. Many critically important signal transduction proteins are post-translationally modified with attachment of a farnesyl isoprenoid catalyzed by protein farnesyltransferase (FTase). Recently, it has been shown that farnesyl diphosphate (FPP) analogues can alter the peptide substrate specificity of FTase. We have used combinatorial screening of FPP analogues and peptide substrates to identify patterns in FTase substrate selectivity. Each FPP analogue displays a unique pattern of substrate reactivity with the tested peptides; FTase efficiently catalyzes the transfer of an FPP analogue selectively to one peptide and not another. Furthermore, we have demonstrated that these analogues can enter cells and be incorporated into proteins. These FPP analogues could serve as selective tools to examine the role prenylation plays in individual protein function.
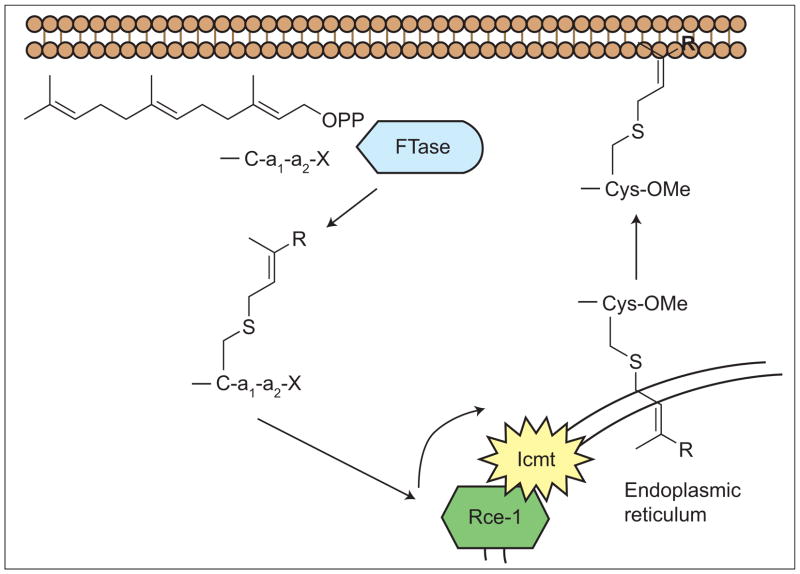
Mutant Ras proteins are one of the most important classes of oncogene products and are thus logical targets for cancer chemotherapeutics. Ras, both mutant and normal forms, must be farnesylated by protein farnesyltransferase (FTase) for proper processing, subcellular localization, and thus biological activity (Figure 1). Therefore, significant effort has been focused on the development of small-molecule FTase inhibitors (FTIs) as anticancer, anti-Ras therapeutics. Two FTIs are in advanced clinical trials (1). The clinical data have demonstrated, however, that FTIs do not function as anti-Ras agents, because K-Ras is alternatively prenylated by geranylgeranyltransferase I upon FTI treatment (23). The cellular (4) and clinical (1) efficacy of FTIs does not correlate with Ras mutational status. The FTI effectiveness observed in non-Ras-positive tumor cells is presumably elicited via inhibition of the farnesylation of other proteins crucial to the growth of tumors. This has led to significant interest in defining the entire set of mammalian prenylated proteins and determining their biological roles (5).
Figure 1.
Post-translational modification of a CaaX protein. Icmt = Isoprenylcysteine carboxyl methyltransferase; Rce-1 = Ras and a-factor converting enzyme.
Many proteins bearing a Ca1a2X sequence at their carboxyl terminus are modified by FTase using the C15 isoprenoid farnesyl diphosphate (FPP) as a co-substrate. Substrate prediction models have estimated that there are >60 farnesylated cellular proteins (6,7), containing a wide variety of C-terminal sequences, and the inhibition of farnesylation of any individual or a combination of these proteins could be responsible for the antitumor effects of FTI treatment. The investigation of potential “protein-X” FTI targets has uncovered several proteins whose inactivation upon FTI treatment led to profound cellular consequences (8). Correspondingly, the investigation of the role of the farnesyl group on cellular proteins has been aided by the development of FTIs. However, using FTIs to investigate the function of the farnesyl lipid for an individual protein is cumbersome, as they are nonspecific tools. FTIs presumably block the farnesylation of all FTase substrate proteins in mammalian cells. Chemical agents that are capable of modulating the farnesylation of selected proteins would allow for a more precise determination of their individual roles in the cell and the functions of their lipid moieties. Recent biochemical studies in our laboratory have demonstrated that a 3-methylbutenyl-modified FPP analogue can alter the peptide substrate specificity and act as a selective modulator of peptide farnesylation (9). Such FPP analogues may provide tools to inhibit the farnesylation of select proteins and thus to interrogate the function of their farnesyl moieties in a more individualized manner.
Before FPP analogues can be used as cellular tools to probe protein prenylation, several issues must be addressed.
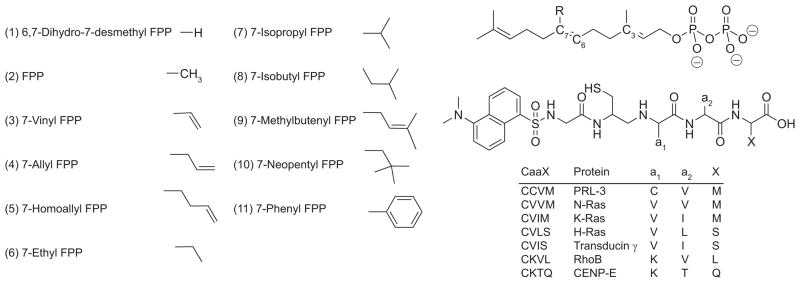
Is the phenomenon observed previously a general one? Will a similar selectivity pattern be seen with other FPP analogues and other CaaX peptide sequences? Can the FPP analogues be used to selectively modify prenylated proteins in target cells? Finally, can we rationally develop selective modulators of prenylation with one particular protein target in mind? With regard to this last question, the extensive structural studies of Beese and colleagues (10) on FTase may provide an important clue. FTase crystal structures demonstrate that the 7-position of the isoprenoid backbone exists in a conformation that interacts with the a2 side chain of the peptide substrate. Because of this interaction, we hypothesized that substituents placed at the 7-position of FPP would modulate the CaaX peptide sequence specificity of FTase in a predictable manner: FPP analogues with larger 7-substituents would favor transfer to less bulky CaaX peptides, whereas the opposite would be true for compounds with smaller 7-substituents. Therefore, we synthesized a set of FPP analogues with diverse substituents, ranging in size from hydrogen to neopentyl and phenyl, in the 7-position (11) and then screened their reactivity with CaaX peptides (Figure 2). We selected seven CaaX box sequences taken from key FTase substrates, RhoB, PRL-3, CENP-E, transducin γ, and the H-, N-, and K-Ras proteins. The analogue/CaaX pairs were screened individually using established fluorometric methods for monitoring FTase activity with dansylated-GCaaX peptides (dnGCaaX), which we have modified for a 96-well plate format (12 13). The assay measures the increase and blue shift in fluorescence that is believed to be attributable to the change in hydrophobicity surrounding the dansyl fluorophore upon farnesylation of the dnGCaaX peptide by FTase.
Figure 2.
Structures of the 7-substituted FPP analogues and dansylated CaaX box peptides.
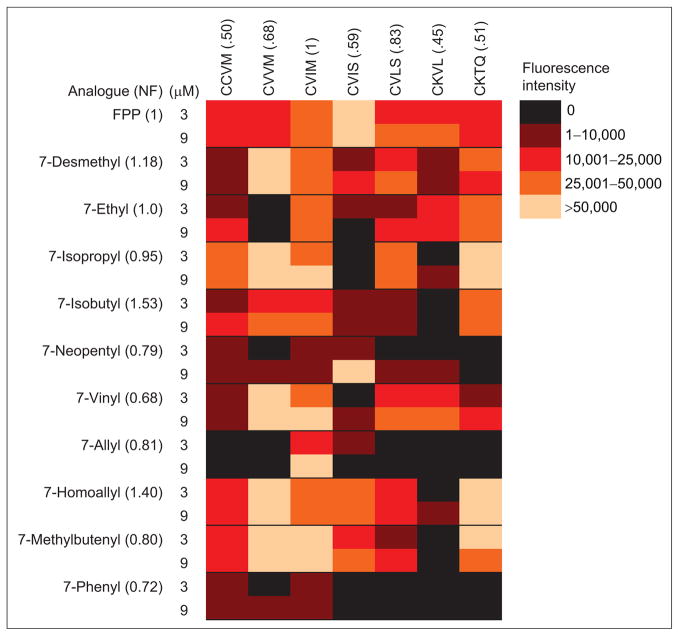
The 11 FPP analogues were qualitatively screened via the fluorescence assay with each of the seven dnGCaaX peptides to identify patterns in analogue-induced FTase substrate selectivity. Formation of farnesylated peptide was also confirmed by HPLC analysis. The screening of the analogue/CaaX peptide pairs revealed that for each FPP analogue FTase catalyzed a unique pattern of selectivity with the seven CaaX peptides (Figure 3). The analogues exhibit a wide range of reactivity. For instance, 7-desmethyl FPP reacts readily with all of the CaaX peptides tested, but 7-phenyl FPP reacts with only a limited number of these same peptides. The 7-neopentyl FPP analogue reacts efficiently with dnGCVIS but not with dnGCKTQ, whereas FTase readily catalyzes the farnesylation of dnGCKVL using the 7-vinyl and 7-ethyl FPP analogues but not other FPP analogues, including 3-methylbutenyl FPP (9). One interesting pattern seen from the screen is that FTase is able to use 7-phenyl FPP to catalyze prenylation of CaaX peptides only where X = methionine. A second intriguing result is that 7-allyl FPP reacts with CaaX peptides only where a2 is isoleucine.
Figure 3.
Heat map showing the ability of 7-substituted FPP analogues to act as substrates for prenylation of CaaX peptides catalyzed by FTase. Data shown represent the increase in fluorescence intensity 30 min after FTase addition.
Initially, we anticipated that the relative sizes of the a2 side chain and the 7-substituent of the FPP analogue would predict the reactivity of the CaaX peptide/analogue combinations. However, the data are not consistent with this hypothesis. The N-Ras and K-Ras peptides, dnGCVVM and dnGCVIM, only differ in the size of the a2 side chain, where K-Ras (CVIM) possesses the bulkier isoleucine residue and N-Ras (CVVM) has the slightly smaller valine. Our expectation was that the less bulky 7-desmethyl analogue would react preferentially with peptides bearing bulkier residues at the a2 position, whereas the converse would be observed with the bulky 7-neopentyl and 7-phenyl analogues. Instead, the opposite behavior was observed (Figure 3): the 7-desmethyl FPP analogue reacts more readily with the less bulky peptide CVVM. Furthermore, 7-neopentyl FPP reacts more readily with CVIM, although the reactivity is low with both peptides. Also, note the remarkable increased reactivity of the 7-vinyl and 7-homoallyl FPP analogues compared to that of the 7-allyl analogue with almost all of the peptides screened. The vinyl and homoallyl substituents differ from allyl by a single carbon in size, yet these analogues have very different biochemical activity compared to that of 7-allyl FPP. At this point, we cannot shed any light on the reason for this variation in activity, which is in sharp contrast to our prediction that steric bulk would play a dominant factor in controlling reactivity in a predictable manner. A second but potentially useful unexpected result is that some analogues serve as more efficient co-substrates for FTase than FPP. The Vmax/KM analogue ratio for the FTase-catalyzed reaction of dnGCVLS with 7-isopropyl FPP compared to reaction with FPP is 5.4:1 (data not shown). The factors influencing FTase substrate selectivity are not fully understood (14). Alterations in rate constants for either isoprenoid rearrangement prior to farnesylation, chemical transfer, or substrate-mediated product release could result in the observed modulation of reactivity with these analogues (15).
The unique ability of these analogues to modulate the farnesylation of FTase substrate peptides can be utilized for multiple purposes. The selective modification of peptide substrates by these FPP analogues could provide tools to study the function of protein lipidation. Because FTIs presumably block the farnesylation of all substrate proteins, the use of these FTase modulators can aid in the experimental determination of protein function, as well as the role the lipid plays in that function. To directly determine that these FTase modulators exhibit selectivity for CaaX substrates, we monitored the selective farnesylation of CaaX peptide pairs with an HPLC assay. After coincubation of 7-isopropyl FPP with dnGCVLS and dnGCKVL, FTase catalyzed the selective transfer of 7-isopropyl FPP to dnGCVLS with no detectable transfer of the analogue to the dnGCKVL peptide (Supplementary Figure 3), consistent with the results of our screen. These data suggest that FPP analogues could be used to selectively modify a particular FTase protein substrate in cells.
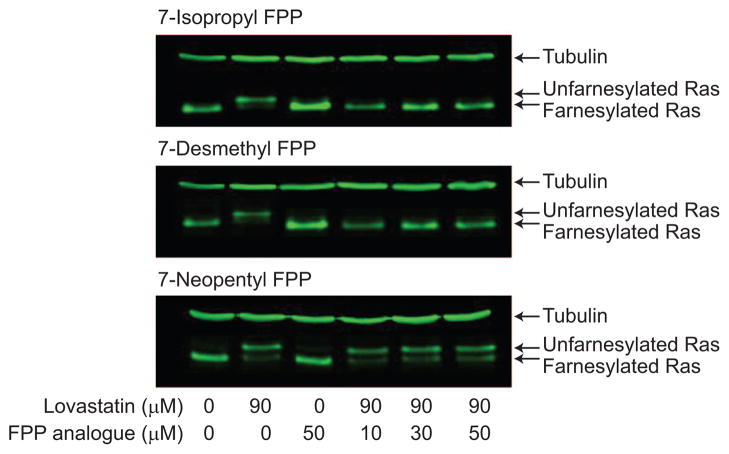
For our biochemical results to have biological relevance, these molecules must be able to modify proteins in the cell. Other FPP analogues have been shown to enter the cell and become incorporated into proteins (16 17 18). In Jurkat T cells, we examined the incorporation of 7-isopropyl FPP (predicted substrate), 7-desmethyl FPP (predicted substrate), and 7-neopentyl FPP (predicted nonsubstrate) into endogenously expressed N-Ras (Figure 4). Treatment with lovastatin alone resulted in a mobility shift in N-Ras commonly seen for unfarnesylated protein (lanes 1 and 2). After co-treatment with lovastatin and the FPP analogue, the reappearance of a farnesylated protein was seen with 7-desmethyl and 7-isopropyl FPP and can be attributed to farnesylation by the added FPP analogue (lanes 4–6). No increase in farnesylated protein was seen in 7-neopentyl FPP-treated cells. These data are consistent with the biochemical screening assays where both 7-isopropyl and 7-desmethyl FPP function as efficient isoprenoid donors for FTase-catalyzed prenylation of the N-Ras CaaX box (dnGCVVM), but 7-neopentyl FPP does not. We do not have any quantitative data on the stability of the diphosphates and on their ability to enter the cell. However, we have found that all FPP analogues that are substrates for the N-Ras peptide in vitro are incorporated on N-Ras in cells (unpublished results). This indicates that uptake and/or stability issues are not limiting with the set of analogues we have looked at thus far. Looking toward future cellular and in vivo studies, we have developed a prodrug strategy to introduce farnesyl monophosphates (potential precursors of the diphosphates described in this paper) into cells (19). The cellular incorporation data demonstrate that the 7-substituted FPP analogues show similar selectivity for CaaX peptides and proteins in both cells and in vitro. Note that a very different reactivity pattern would have been predicted with the CaaX substrate CVIS, which is a cosubstrate with 7-neopentyl FPP but not with 7-isopropyl FPP. Therefore, these analogues should be useful for studying the biological function of the lipid modification on individual proteins (20 21).
Figure 4.
Incorporation of FPP analogues onto N-Ras in Jurkat T cells.
To more directly address the issue of analogue-induced CaaX selectivity, we performed a comparison experiment on the ability of 7-allyl FPP to be incorporated into GFP-labeled K-Ras and N-Ras in Jurkat cells. After mislocalization due to statin treatment, incorporation with the unnatural FPP analogue is indicated by the restoration of the membrane localization of K- or N-Ras. From the biochemical experiments summarized in Figure 3, it would be expected that 7-allyl FPP would be much more effective at restoring the localization of K-Ras than N-Ras, and this was observed (Supplementary Figure 4). In particular, 10 μM 7-allyl FPP led to almost complete (~90%) recovery of normal localization of GFP-K-Ras in Jurkat cells, but 30 μM 7-allyl FPP led to only ~50% recovery of normal N-Ras localization. Ras isoforms have different biological functions (22), and thus a chemical tool that only modified K-Ras would allow for a more specific interrogation of this biology. Note also that Ras isoforms exhibit different cellular localization and different signaling from these diverse locations (23 24). It has become clear over the past several years that the farnesyl moieties of Ras proteins play a more complex role in their biological activity than simple membrane attachment (25). Selective incorporation of a modified lipid into one Ras isoform may alter the function of this isoform as a result of an interference with farnesyl–protein interactions (26) but not the other Ras forms.
This study provides preliminary answers to key questions regarding the substrate selectivity of FTase posed earlier. First, the phenomenon where FPP analogues can induce FTase peptide specificity is clearly a general one. Although certain CaaX peptides are inherently poor substrates, a change in the structure of the prenyldiphosphate cosubstrate can lead to a striking enhancement of farnesylation activity catalyzed by FTase. Moreover, this selective prenylation also occurs in a cellular setting, as we have demonstrated by both Western analysis and localization of GFP-Ras constructs. However, we have not yet determined the underlying mechanism for this selectivity that would allow the rational design of selective modulators of prenylation.
The 7-substituted FPP analogues were designed to alter the selectivity of prenylation of CaaX peptides catalyzed by FTase. Combinatorial screening assays demonstrate that these FPP analogues lead to diverse, interesting, and potentially useful patterns in the substrate selectivity of FTase for prenylation of CaaX peptides. These large changes in selectivity based on alteration of the structure of the co-substrate rather than the protein are unprecedented in enzymology. The FTase modulators may exert their effects through altering the rate constants for the isoprenoid rearrangement prior to chemistry, the chemical transfer, or the substrate-mediated product release. As the product release is the rate-limiting step for this enzyme at substrate concentrations where the enzyme is saturated and requires an additional isoprenoid to bind into the active site (27), this last step in farnesylation may be an excellent place to begin investigating the mechanism of these modulators. Though we do not yet understand the exact molecular basis for the different peptide selectivity patterns exhibited with FPP analogues, these analogues provide potentially valuable tools for studying the function of protein prenylation in the cell. Preliminary evidence, both biochemical and cellular, suggests that selected analogues could block the farnesylation of a small subset of cellular proteins. These chemical tools could also allow for the selective modification of proteins with structurally diverse prenyl moieties. Such unnatural lipidation may interfere with prenyl–protein interactions (21), providing a powerful chemical tool, analogous to the use of glycoengineering for the study of glycosylated proteins (28). By manipulating the available intracellular isoprenoid donor to modify FTase substrate proteins, the localization and function of a small number of farnesylated proteins can be modulated and investigated in a cell.
METHODS
Materials
Amino acids and resins were from Chem Impex, Inc. Chemicals for FPP analogue synthesis were obtained from Sigma-Aldrich.
Cell Culture
Jurkat T cells (E6.1) purchased from ATCC were maintained in log phase growth in RPMI (Roswell Park Memorial Institute) media supplemented with 7.5% inactivated fetal calf serum (Harland), 50 units mL−1 penicillin, 50 μg mL−1 streptomycin, 1 mM MEM sodium pyruvate, and 50 μM 2-mercaptoethanol at 37 °C in 6% CO2.
Chemical Syntheses
The syntheses of the 7-substituted FPP analogues 1, 3, 4, and 6–8 have been previously published (11). Analogues 5 and 9–11 were synthesized according to the same methods, and full details for their preparation will be reported later. The fluorescently labeled peptides were prepared using 9-fluorenylmethyl carbamate solid-phase peptide chemistry, as previously reported (9).
Fluorescence Assay
The fluorescence assay was based on published protocols (12, 13). Assays were performed in triplicate using FPP or FPP analogue (1, 3, or 9 μM), dansylated peptide (3 μM), and 50 nM FTase in 52 mM Tris at pH 7.5 with 5.8 μM DTT, 12 μM MgCl2, and 12 μM ZnCl2. Assays were performed in 96-well plates (Nunc F96 Microwell Plates, black) with a final reaction volume of 200 μL. Assays were initiated with 10 μL of 1 μM recombinant rat FTase (29), and the peptide fluorescence was measured at 0, 30, and 60 min on a Perkin-Elmer Fusion plate reader (excitation 335 nm, emission 485 or 535 nm). For the data presented in Figure 3, farnesylation, monitored by a fluorescence increase under kcat conditions for reaction of dnGCVIM with FPP (Supplementary Figure 1), was measured after 30 min of reaction with FTase. To compare the fluorescence increases for the CaaX peptides, the fluorescence enhancement seen for the farnesylation of each peptide was measured, and a fluorescence enhancement factor was determined and used to normalize the results (Supplementary Figure 2).
HPLC
The HPLC assays were performed in the same manner as the fluorescence assay except that the reactions were quenched with acetonitrile at 0, 5, 30, or 60 min and then analyzed using an Agilent 1100 HPLC with a Zorbax Eclipse XDB RP-C8 column. Samples were eluted with a 20–100% acetonitrile/0.025% TFA(aq) gradient over 30 min (1 mL min−1) and detected by absorbance (254 nm) and fluorescence (excitation 335 nm, emission 486 nm).
Incorporation of farnesyl analogues onto N-Ras in Jurkat T cells
Jurkat T cells (3–5 × 105 cells mL−1) were treated with lovastatin alone, lovastatin plus FPP analogues, or delivery solution alone (NH4HCO3). Cells were lysed in 1% Triton X-100 lysis buffer (25 mM Hepes, pH 7.2, 150 mM NaCl, 1% NP40, 5 mM EDTA, 10 μg mL−1 of leupeptin and aprotinin, and 1 mM sodium vanadate), resolved on SDS-PAGE gels, transferred to a nitrocellulose membrane, blocked with Odyssey Infrared Imaging System blocking buffer (Li-Cor Biosciences, 927-40000), and incubated overnight in primary antibody (1:500 anti-N-Ras, 1:100,000 antitubulin) diluted in Odyssey Infrared Imaging System blocking buffer containing 0.3% Tween. The membranes were washed with PBS containing 0.1% Tween-20 (4 × 10 min), incubated in secondary antibody (1:5000 GAM) for 1 h at RT, and washed with PBS containing 0.1% Tween-20 followed by four PBS washes (10 min each). Blots were visualized using the Odyssey Imaging System (Li-Cor Biosciences).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants CA78819 (R.A.G.), GM40602 (C.A.F.), and GM48099 (M.L.H.). A.J.K. and S.A.S. were supported by Purdue Research Foundation fellowships. J.E.P. was partially supported by a National Science Foundation predoctoral fellowship.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
References
- 1.Basso AD, Kirschmeier PT, Bishop WR. Farnesyl Transferase Inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. K- and N-Ras Are Geranylgeranylated in Cells Treated with Farnesyl Protein Transferase Inhibitors. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 3.Rowell CA, Kowalczyk JJ, Lewis MD, Garcia AM. Direct Demonstration of Geranylgeranylation and Farnesylation of Ki-Ras in Vivo. J Biol Chem. 1997;272:14093–14097. doi: 10.1074/jbc.272.22.14093. [DOI] [PubMed] [Google Scholar]
- 4.Sepp-Lorenzino L, Ma Z, Rands E, Kohl NE, Gibbs JB, Oliff A, Rosen N. A Peptidomimetic Inhibitor of Farnesyl: Protein Transferase Blocks the Anchorage-Dependent and -Independent Growth of Human Tumor Cell Lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 5.Kho Y, Kim SC, Jiang C, Barma D, Kwon SW, Chemg J, Jaunbergs J, Weinbaum C, Tamanoi F, Falck J, Zhao Y. A Tagging-via-Substrate Technology for Detection and Proteomics of Farnesylated Proteins. Proc Natl Acad Sci USA. 2004;101:12479–12484. doi: 10.1073/pnas.0403413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maurer-Stroh S, Koranda M, Benetka W, Schneider G, Sirota FL, Eisenhaber F. Towards Complete Sets of Farnesylated and Geranylgeranylated Proteins. PLoS Comput Biol. 2007;3:e66. doi: 10.1371/journal.pcbi.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid TS, Terry KL, Casey PJ, Beese LS. Crystallographic Analysis of CaaX Prenyl-transferases Complexed with Substrates Defines Rules of Protein Substrate Selectivity. J Mol Biol. 2004;343:417–433. doi: 10.1016/j.jmb.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 8.Sebti SM, Der CJ. Opinion: Searching for the Elusive Targets of Farnesyltransferase Inhibitors. Nat Rev Cancer. 2003;3:945–951. doi: 10.1038/nrc1234. [DOI] [PubMed] [Google Scholar]
- 9.Reigard SA, Zahn TJ, Haworth KB, Hicks KA, Fierke CA, Gibbs RA. Interplay of Isoprenoid and Peptide Substrate Specificity in Protein Farnesyltransferase. Biochemistry. 2005;44:11214–11223. doi: 10.1021/bi050725l. [DOI] [PubMed] [Google Scholar]
- 10.Long SB, Casey PJ, Beese LS. Reaction Path of Protein Farnesyltransferase at Atomic Resolution. Nature. 2002;419:645–650. doi: 10.1038/nature00986. [DOI] [PubMed] [Google Scholar]
- 11.Rawat DS, Gibbs RA. Synthesis of 7-Substituted Farnesyl Diphosphate Analogues. Org Lett. 2002;4:3027–3030. doi: 10.1021/ol026176i. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy PB, Dolence JM, Poulter CD. Continuous Fluorescence Assay for Protein Prenyltransferases. Methods Enzymol. 1995;250:30–43. doi: 10.1016/0076-6879(95)50060-x. [DOI] [PubMed] [Google Scholar]
- 13.Thutewohl M, Kissau L, Popkirova B, Karaguni IM, Nowak T, Bate M, Kuhlmann J, Muller O, Waldmann H. Solid-Phase Synthesis and Biological Evaluation of a Pepticinnamin E Library. Angew Chem, Int Ed. 2002;41:3616–3620. doi: 10.1002/1521-3773(20021004)41:19<3616::AID-ANIE3616>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Hartman HL, Hicks KA, Fierke CA. Peptide Specificity of Protein Prenyltransferases Is Determined Mainly by Reactivity Rather Than Binding Affinity. Biochemistry. 2005;44:15314–15324. doi: 10.1021/bi0509503. [DOI] [PubMed] [Google Scholar]
- 15.Pais JE, Bowers KE, Fierke CA. Measurement of the Alpha-Secondary Kinetic Isotope Effect for the Reaction Catalyzed by Mammalian Protein Farnesyltransferase. J Am Chem Soc. 2006;128:15086–15087. doi: 10.1021/ja065838m. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs BS, Zahn TJ, Mu YQ, Sebolt-Leopold J, Gibbs RA. Novel Farnesol and Geranylgeraniol Analogues: A Potential New Class of Anticancer Agents Directed against Protein Prenylation. J Med Chem. 1999;42:3800–3808. doi: 10.1021/jm9902786. [DOI] [PubMed] [Google Scholar]
- 17.Chehade KAH, Andres DA, Morimoto H, Spielmann HP. Design and Synthesis of a Transferable Farnesyl Pyrophosphate Analogue to Ras by Protein Farnesyltransferase. J Org Chem. 2000;65:3027–3033. doi: 10.1021/jo991735t. [DOI] [PubMed] [Google Scholar]
- 18.Roberts MJ, Troutman JM, Chehade KA, Cha HC, Kao JP, Huang X, Zhan CG, Peterson YK, Subramanian T, Kamalakkannan S, Andres DA, Spielmann HP. Hydrophilic Anilinogeranyl Diphosphate Prenyl Analogues Are Ras Function Inhibitors. Biochemistry. 2006;45:15862–15872. doi: 10.1021/bi061704+. [DOI] [PubMed] [Google Scholar]
- 19.Clark MK, Scott SA, Wojtkowiak J, Chirco R, Mathieu P, Reiners JJ, Mattingly RR, Borch RF, Gibbs RA. Synthesis, Biochemical, and Cellular Evaluation of Farnesyl Monophosphate Prodrugs as Farnesyltransferase Inhibitors. J Med Chem. 2007;50 doi: 10.1021/jm0701829. in press. [DOI] [PubMed] [Google Scholar]
- 20.Magee T, Seabra M. Fatty Acylation and Prenylation of Proteins: What’s Hot in Fat. Curr Opin Cell Biol. 2005;17:190–196. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Kloog Y, Cox AD. Prenyl-Binding Domains: Potential Targets for Ras Inhibitors and Anti-Cancer Drugs. Semin Cancer Biol. 2004;14:253–261. doi: 10.1016/j.semcancer.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Yan J, Roy S, Apolloni A, Lane A, Hancock JF. Ras Isoforms Vary in Their Ability To Activate Raf-1 and Phosphoinositide 3-Kinase. J Biol Chem. 1998;273:24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]
- 23.Hancock JF. Ras Proteins: Different Signals from Different Locations. Nat Rev Mol Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 24.Mor A, Philips MR. Compartmentalized Ras/MAPK Signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- 25.Pechlivanis M, Kuhlmann J. Hydrophobic Modifications of Ras: Proteins by Isoprenoid Groups and Fatty Acids More Than Just Membrane Anchoring. Biochim Biophys Acta. 2006;1764:1914–1931. doi: 10.1016/j.bbapap.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Thapar R, Williams JG, Campbell SL. NMR Characterization of Full-Length Farnesylated and Non-Farnesylated H-Ras and Its Implications for Raf Activation. J Mol Biol. 2004;343:1391–1408. doi: 10.1016/j.jmb.2004.08.106. [DOI] [PubMed] [Google Scholar]
- 27.Tschantz WR, Furfine ES, Casey PJ. Substrate Binding Is Required for Release of Product from Mammalian Protein Farnesyltransferase. J Biol Chem. 1997;272:9989–9993. doi: 10.1074/jbc.272.15.9989. [DOI] [PubMed] [Google Scholar]
- 28.Hang HC, Bertozzi CR. Chemo selective Approaches to Glycoprotein Assembly. Acc Chem Res. 2001;34:727–736. doi: 10.1021/ar9901570. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman KK, Scholten JD, Huang Cc, Fierke CA, Hupe DJ. High-Level Expression of Rat Farnesyl: Protein Transferase in Escherichia coli as a Translationally Coupled Heterodimer. Protein Expression Purif. 1998;14:395–402. doi: 10.1006/prep.1998.0979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.