Abstract
Purpose
To investigate the relationship among microfluctuations in accommodation, resting tension on the crystalline lens, ciliary body thickness, and refractive error in children.
Methods
Subjects were 49 children, aged 8 to 15 years. Subjects wore habitual correction over their left eye and an infrared filter over the right eye during accommodative measurements. Monocular accommodation was measured continuously for two, 30-second periods using a PowerRef I at a sampling rate of 25 Hz while subjects viewed a high-contrast target at 0.25 m. The high (1.0 to 2.3 Hz) and low- (0 to 0.6 Hz) frequency components of the power spectrum from a fast Fourier transform of the accommodative response were used in analysis. Resting tension on the crystalline lens was assessed by measuring the amplitude of the oscillations of the crystalline lens after a rightward 20° saccadic eye movement. Ciliary body thickness was measured 2 mm posterior to the scleral spur from images obtained with a Zeiss Visante optical coherence tomography (OCT). Cycloplegic spherical equivalent refractive error was obtained with the Grand Seiko autorefractor.
Results
The mean ± SD spherical equivalent refractive error was −1.00 D ± 2.25 (range, −6.00 D to +3.44 D). Greater power in the log of the high-frequency component of accommodative microfluctuations was associated with thinner ciliary bodies (p = 0.03) and lower ages (p = 0.0004). More hyperopic refractive errors with greater power in the high-frequency component (p = 0.0005) and the low-frequency component (p = 0.02). No statistically significant relationship was found for the low-frequency component or root mean square of accommodative microfluctuations and refractive error.
Conclusions
High-frequency microfluctuations of accommodation appear to be suppressed with thicker ciliary bodies. These variations in accommodation need to be observed in a longitudinal study to better assess the functional significance of their relationship to ciliary body size and refractive error.
Keywords: myopia, accommodation, crystalline lens, ciliary body, children
Microfluctuations are small variations in the refractive power of the eye. The temporal characteristics of the oscillations were first described in detail using Fourier analysis almost 50 years ago.1 A high-frequency component (1.3 to 2.2 Hz) and low-frequency component (<0.5 Hz) were identified by Campbell et al.1–3 and confirmed by other laboratories. Further study has shown that the location of the high-frequency component peak varies between subjects within the range of high frequencies.1–3 It has been correlated with the arterial pulse rate.4,5 The peak frequency has been shown to decrease with application of a topical β-adrenergic receptor antagonist that also reduces arterial pulse,6 and it increases with elevated arterial pulse such as during exercise.7 The high-frequency component seems to reflect noise from the arterial pulse in the accommodative plant, and it is not caused by optical fluctuations in the crystalline lens.7,8 Although a small amplitude high-frequency component was found to coincide with small fluctuations in vitreous chamber depth and axial length, both were found to correspond with heart rate.8 The power of the high-frequency component reaches a maximum near the center of the accommodative range or around −3.00 to −5.00 D and then decreases approaching the near point.9,10
The amplitude of the microfluctuations, however, increases with the amount of accommodation exerted up to around −4.00 D,3,11 particularly in the increasing power of the low-frequency component.12,13 Low-frequency component accommodative microfluctuations are maintained approaching the near point.9 Increases in power of the low-frequency component have also been associated with conditions leading to a larger ocular depth-of-focus such as decreasing pupil size14 and increasing blur,15 indicating that the low-frequency component may play a role in control of the accommodative response.7,16,17 The low-frequency component appears to be due to optical fluctuations in the crystalline lens. Ultrasound techniques found a low-frequency fluctuation in the anterior and posterior lens surface position but not to changes in the axial length.8
More recently, differences in the characteristics of microfluctuations have been observed between refractive error groups. Seidel et al.13 found for a −4.00 D stimulus in a Badal system that subjects with late-onset myopia had a significant increase in low-frequency component power when compared with subjects with emmetropia and early-onset myopia. However, these differences were not found in free space viewing.18 Using a Badal system and several stimuli levels, subjects with late-onset myopia had more power in the high-frequency component unrelated to stimulus level and larger microfluctuations during distance viewing. The low-frequency component power did not increase as rapidly while viewing accommodative stimuli more distant than −3.00 D for subjects with late-onset myopia as it did for other refractive groups.12 Harb et al.11 found that after sustained −3.50 D accommodation, the power in all frequency components increased with increasing myopia. It has also been shown that there is more accommodative variability in children with early-onset myopia.19
Recently, there have been reports showing that the thickness of the ciliary body is related to refractive error in both children and adults.20,21 There have been relatively few studies regarding accommodative microfluctuations in school-aged children, and none of those studies have evaluated mechanical factors related to the crystalline lens or whether ciliary body anatomy affects microfluctuations. The aim of this study was to determine whether there is a relationship between accommodative microfluctuations and tension on the crystalline lens or ciliary body size in children.
METHODS
Subjects
Subjects were recruited via electronic mail sent to faculty, staff, and students at The Ohio State University College of Optometry, flyers given to the parents of eligible patients that visited the Ohio State Optometric Services Clinic, subjects who completed participation in the Adolescent and Child Health Initiative to Encourage Vision Empowerment study, letters sent through the mail to parents of recent Ohio State Optometric Services Clinic patients who met inclusion criteria, and word of mouth.
Forty-nine subjects aged 8 to 15 years (mean = 11.4 years, SD = 2.2 years) participated in the study. Exclusion criteria were a history of ocular surgery or amblyopia, corrected vision with habitual correction of worse than 20/40, medications that interfere with eye movement or accommodation, and symptoms or diagnoses of an accommodative disorder. Written informed consent was obtained from a parent or guardian of each subject, and written assent was obtained from each subject. The study was approved by the Institutional Review Board of The Ohio State University.
All measurements were made on right eyes only. Cycloplegia was achieved by instilling 1 drop of 0.5% proparacaine followed by 2 drops of 1% tropicamide separated by 5 minutes. Cycloplegic measurements were made 25 minutes after the 2nd drop of tropicamide. Cyclopegic, spherical equivalent refractive error in the right eye was obtained from the mean of five readings with the Grand Seiko WR-5100K (Grand Seiko Co., Hiroshima, Japan) autorefractor. The mean ± SD spherical equivalent refractive error was −1.00 D ± 2.19 (range, −6.00 D to +3.44 D).
Accommodation Measurements
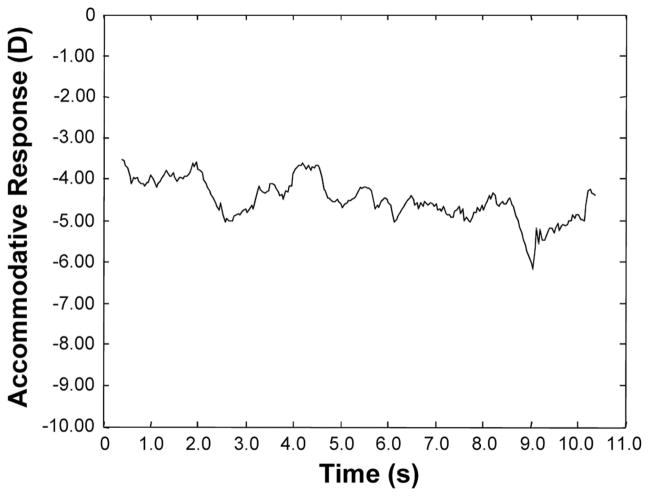
Throughout the experiment, subjects wore their habitual correction over their left eye and an infrared filter over their right eye that occluded vision while still allowing measurements to be taken. Monocular accommodative response for measurements of microfluctuations was measured using a PowerRefractor (MultiChannelSystems, Reutlingen, Germany) at a sampling rate of 25 Hz and a test distance of 1 m. The instrument has a range of −8.75 to +4.00 D22 and requires a pupil size larger than 3.7 mm.23 Its use has been established for measuring refractive error in children24 and for the measurement of accommodative microfluctuations.11,25 While positioned in a head and chin rest to minimize the head movement, subjects viewed a high-contrast Maltese cross target (angular subtense: 1.4°) at a distance of 0.25 m (−4.00 D) continuously for two, 30-second periods while measurements were taken. The luminance of the targets was approximately 200 lux. A sample measurement is shown in Fig. 1. Pupil size data were also recorded.
FIGURE 1.
A typical accommodative response sample from PowerRefractor measurement using a −4.00 D stimulus with a Maltese Cross target.
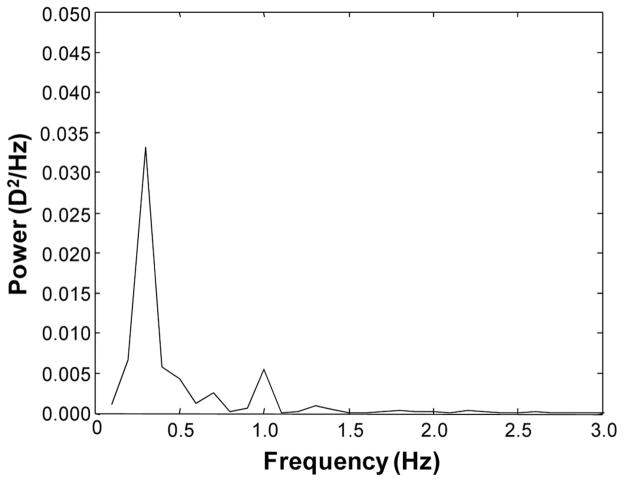
To improve the confidence in the analyses, five 10-second segments of data were used for a frequency resolution of 0.1 Hz.26 MatLab Version 7.1 (Mathworks, Natick, MA) was used to write a program that identified blinks by missing data points and changes >10 D/s that are faster than physiologically possible11 and filtered erroneous data points to form a line using the average of the points before and after the erroneous data to connect the valid data points. For each segment, the fast Fourier transform was calculated and then averaged for the five segments. The power spectrum was directly obtained from the fast Fourier transform using MatLab. The area under the curve of the power spectrum was integrated to find the component power in the high frequency (1.0 to 2.3 Hz) and low-frequency (0.0 to 0.6 Hz) ranges according to previous classifications.2,11 A sample power spectrum is shown in Fig. 2.
FIGURE 2.
A sample power spectrum obtained from Fourier analysis of the accommodative response.
Accommodative lag was also measured using the Grand Seiko autorefractor. Subjects viewed a 4.00-D stimulus, a single row of 20/100 letters, through a Badal lens system. The mean of five measurements was used in analysis.
Crystalline Lens Tension Measurement
In a 1995 report, Deubel and Bridgeman27 described oscillations of the crystalline lens after saccadic eye movements. Their work documents the idea that the eye is not “inelastic” and that the crystalline lens moves under both viscous and elastic forces during high-speed, saccadic eye movements, behaving like a “mass-spring” system. The authors demonstrated that the Purkinje tracking system will be contaminated by these oscillations when studying the main sequence of saccade dynamics. They also demonstrated that the oscillations of the crystalline lens are dependent upon the “stiffness” of the accommodative system. Two conditions that should lead to less tension on the crystalline lens, increased accommodation and younger age, were both associated with larger, postsaccadic oscillations of the crystalline lens.27 It is unknown whether the oscillations are related to elasticity with the crystalline lens or elasticity within other components of the accommodative plant.
Purkinje images I and IV were created with a single pipe fiber optic light source with an infrared pass filter. Oscillations in the right eye, which was occluded with a Wratten 89 B infrared filter, were videotaped. The left eye was unoccluded to allow for fixation between the two saccadic targets. Digital video files of the saccadic eye movements were recorded with a digital video camera (pco.1200hs, The COOKE Corporation, Romulus, MI) at the rate of 1000 frames per second.
All saccadic eye movements were 20° in magnitude to maximize velocity of the saccade while preserving accuracy and therefore maximizing the oscillations of the crystalline lens.28 Measurements from five saccadic eye movements made in a rightward direction under cycloplegic conditions were included in analysis. On review of each video file, if the subject was found not to have completed a smooth, full saccadic eye movement then that video file was discarded and another trial was completed and recorded. The saccadic targets at distance were “+” symbols at 4.0 m.
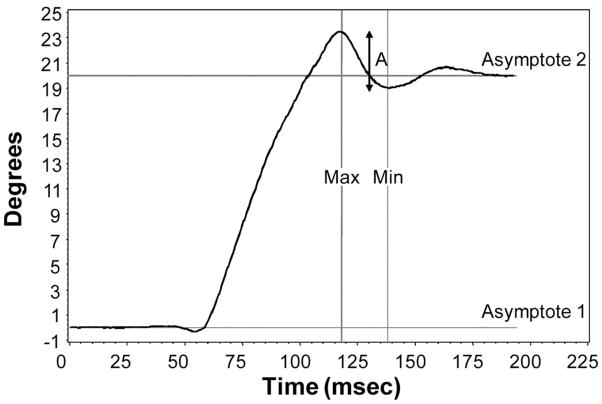
All video files were imported into Matlab for analysis. Files were “batch processed” in a Matlab program. Briefly, the Matlab program identified Purkinje images I and IV through a routine that isolated the two Purkinje images based on a texture analysis, intensity analysis, and finally a “roundness” and size analysis. Once Purkinje images I and IV were isolated in the individual frames of the video, the horizontal position of Purkinje images I and IV were identified through a center of mass function and recorded in separate arrays for each Purkinje image in the video file. Arrays were exported from Matlab as Microsoft Excel spreadsheets. The difference in the horizontal position between Purkinje images I and IV was calculated through subtraction of x axis coordinates. Graphs of the difference in Purkinje images I and IV (Fig. 3) were created for each video file. Specific features were extracted from each of the curves to serve as measurements of the crystalline lens oscillations. The amplitude of the crystalline lens oscillation at the end of the saccade was termed “A,” which was the difference between max and min in Fig. 3. The value of A served as our primary measurement of crystalline lens oscillation.
FIGURE 3.
Difference in horizontal position (degrees) between Purkinje images I and IV. The points between the first and the second asymptotes represent the extent of the 20° saccadic eye movement. A slight lag or delay in the movement of the crystalline lens at the beginning of the saccade is evident in the deviation of the curve between 50 and 60 milliseconds. Deviations/oscillations in the curve above and below the second asymptote represent oscillations of the crystalline lens at the end of the saccade. The amplitude of the oscillation (A) is denoted by the largest arrow and is the difference in degrees between max and min.
Ciliary Body Measurements
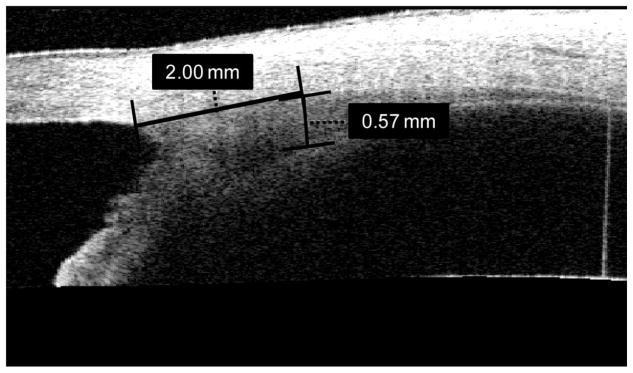
Images of the nasal ciliary body of the right eye were obtained with the Zeiss Visante Anterior Segment OCT. Measurements were made under cycloplegia as previously described.20 A radial thickness measurement at 2.0 mm posterior to the scleral spur (Fig. 4) was used in analysis [ciliary body thickness (CBT) 2], as the thickness at this location has been previously shown to be negatively correlated with refractive error.20 F5
FIGURE 4.
Representative Visante image of the nasal ciliary body while the subject viewed an external fixation target. Thickness measurement of 0.57 mm was taken 2 mm posterior to the scleral spur (CBT2).
Statistical Analyses
There were three dependent variables of interest: high-frequency component, low-frequency component, and root mean square of accommodative microfluctuations. The distributions of the high- and low-frequency components were skewed toward higher powers. To make them less skewed for the purpose of modeling them as outcomes in a regression, the variables underwent a logarithmic transformation and were tested with a Shapiro-Wilk test for normality.
The independent variables of interest were ciliary body thickness at 2.0 mm posterior to the scleral spur (CBT2), the amplitude of the crystalline lens tension measurements after saccadic eye movements (A), and refractive error. Saccadic velocity, mean refractive measurement during accommodation (mu), pupil size, gender, and age were independent variables used as controls. Using multiple regression, each dependent variable (high-frequency component, low-frequency component, and root mean square) was regressed separately on CBT2, A, refractive error, saccadic velocity, mu, pupil size, gender, and age. A variance inflation factor (VIF) was calculated for each of the independent variables in both the models. The VIF values ranged from 1.0 to 2.8. A VIF <10 is considered an indication that multicollinearity is not unduly influencing least squares estimates.29
RESULTS
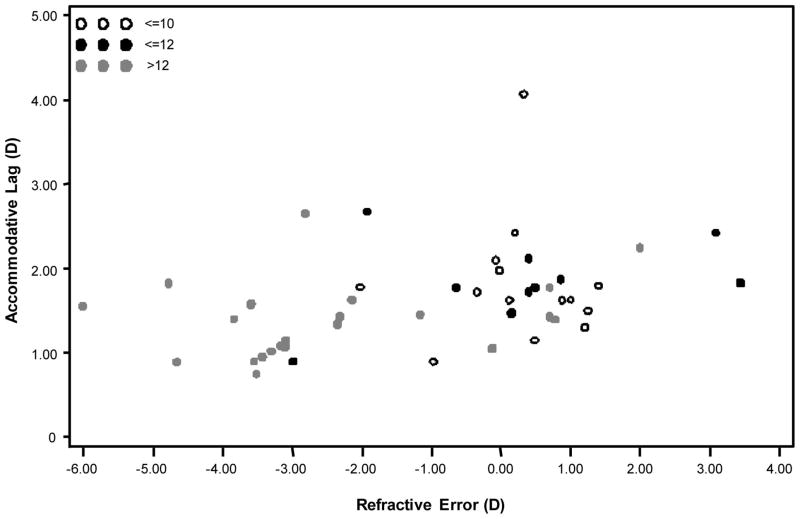
The general characteristics of the study sample are listed in Table 1. There was a strong, negative correlation between refractive error and age (r = −0.55, p < 0.0001), i.e., older subjects tended to be myopic, whereas younger subjects tended to be emmetropic or hyperopic. Refractive error was also negatively correlated with ciliary body thickness (r = −0.31, p = 0.03). There were also correlations between age and accommodative microfluctuations in the log of the high-frequency component (r = −0.38, p = 0.01), log of the low-frequency component (r = −0.29, p = 0.05), and log root mean square (r = −0.36, p = 0.01). Mean refractive measurement during accommodation was correlated with refractive error (r = 0.33, p = 0.02). Amplitude of crystalline lens oscillations, saccadic velocity, pupil size, and gender did not have statistically significant correlations with any outcome or predictor variables. The relationship between accommodative lag and refractive error in this sample is shown in Fig. 5. Note that the myopes in this sample do not have the characteristically higher accommodative lag when compared with emmetropes.
TABLE 1.
General characteristics of the study sample
| Measurement | Mean | STD |
|---|---|---|
| Age (yr) | 11.43 | 2.23 |
| Cycloplegic refractive error (D) | −1.00 | 2.19 |
| Ciliary body thickness 2 mm posterior to the scleral spur (CBT2) (μm) | 604.06 | 104.28 |
| Crystalline lens oscillations (degrees) | 2.07 | 0.67 |
| Saccadic velocity (degrees/s) | 0.37 | 0.06 |
| Pupil size (mm) | 5.87 | 0.77 |
| Mean measurement of refraction during accommodation | −3.80 | 1.96 |
| Low-frequency component (D2/Hz) | 0.0422 | 0.0608 |
| High-frequency component (D2/Hz) | 0.0049 | 0.0047 |
| Root mean square (D) | 0.49 | 0.26 |
FIGURE 5.
The distribution of subject refractive error and accommodative response to a 4.00-D stimulus using a Badal lens system. Note that myopic subjects were older and did not appear to have the characteristically higher accommodative lag.
Multiple Regression Models of Accommodative Microfluctuations and Ciliary Body Thickness or Crystalline Lens Tension
In the multiple regression model, there was a negative, statistically significant relationship between the log of the high-frequency component and ciliary body thickness (Table 2). There was also a negative statistically significant relationship between the log of the high-frequency component and age. This indicates that older subjects and subjects with thicker ciliary bodies have smaller microfluctuations of accommodation in the high-frequency component. Only the age control variable was statistically significant in the log low-frequency component or log root mean square model (Tables 3 and 4).
TABLE 2.
Multiple linear regression model for the log of the high-frequency component accommodative microfluctuations as a function of ciliary body thickness and other independent variables in children
| Predictor | p | Parameter estimate |
|---|---|---|
| Intercept | −5.76 | |
| Age (relative to 8 yr) | 0.004 | −0.19 |
| Gender (female = 1) | 0.36 | 0.24 |
| Saccadic velocity | 0.93 | 0.29 |
| Mean accommodation (PowerRefractor) | 0.27 | −0.07 |
| Pupil size | 0.44 | −0.13 |
| Ciliary body thickness | 0.03 | −0.003 |
| Amplitude of lens oscillations | 0.35 | −0.30 |
TABLE 3.
Multiple linear regression model for the log of the low-frequency component accommodative microfluctuations as a function of ciliary body thickness and other independent variables in children
| Predictor | p | Parameter estimate |
|---|---|---|
| Intercept | −3.85 | |
| Age (relative to 8 yr) | 0.03 | −0.20 |
| Gender (female = 1) | 0.81 | 0.09 |
| Saccadic velocity | 0.77 | −1.35 |
| Mean accommodation (PowerRefractor) | 0.96 | −0.005 |
| Pupil size | 0.16 | −0.35 |
| Ciliary body thickness | 0.13 | −0.003 |
| Amplitude of lens oscillations | 0.54 | −0.28 |
TABLE 4.
Multiple linear regression model for log root mean square accommodative microfluctuations as a function of ciliary body thickness and other independent variables in children
| Predictor | p | Parameter estimate |
|---|---|---|
| Intercept | −0.79 | |
| Age (relative to 8 yr) | 0.008 | −0.10 |
| Gender (female = 1) | 0.96 | −0.008 |
| Saccadic velocity | 0.95 | −0.10 |
| Mean accommodation (PowerRefractor) | 0.34 | −0.04 |
| Pupil size | 0.16 | −0.14 |
| Ciliary body thickness | 0.20 | −0.001 |
| Amplitude of lens oscillations | 0.33 | −0.18 |
Models of Accommodative Microfluctuations and Refractive Error
There was a positive, statistically significant relationship between the log of the high-frequency component and refractive error (Table 5). This indicates that the more hyperopic refractive errors have larger microfluctuations of accommodation in the high-frequency component. Mean measurement of refraction during accommodation was also significant in this model. Refractive error was also significant in the log low-frequency component model (Table 6). Both refractive error and mean measurement of refraction during accommodation were significant in the log root mean square model (Table 7).
TABLE 5.
Multiple linear regression model for the log of the high-frequency component accommodative microfluctuations as a function of refractive error and control variables in children
| Predictor | p | Parameter estimate |
|---|---|---|
| Intercept | −5.85 | |
| Age (relative to 8 yr) | 0.50 | −0.04 |
| Gender (female = 1) | 0.29 | 0.25 |
| Mean accommodation (PowerRefractor) | 0.006 | −0.18 |
| Pupil size | 0.18 | −0.20 |
| Refractive error | 0.0005 | 0.25 |
TABLE 6.
Multiple linear regression model for the log of the low-frequency component accommodative microfluctuations as a function of refractive error and control variables in children
| Predictor | p | Parameter estimate |
|---|---|---|
| Intercept | −3.88 | |
| Age (relative to 8 yr) | 0.61 | −0.05 |
| Gender (female = 1) | 0.81 | 0.09 |
| Mean accommodation (PowerRefractor) | 0.26 | −0.11 |
| Pupil size | 0.06 | −0.44 |
| Refractive error | 0.02 | 0.25 |
TABLE 7.
Multiple linear regression model for log root mean square accommodative microfluctuations as a function of refractive error and control variables in children
| Predictor | p | Parameter estimate |
|---|---|---|
| Intercept | −0.82 | |
| Age (relative to 8 yr) | 0.43 | −0.03 |
| Gender (female = 1) | 0.91 | −0.01 |
| Mean accommodation (PowerRefractor) | 0.04 | −0.08 |
| Pupil size | 0.06 | −0.17 |
| Refractive error | 0.008 | 0.11 |
DISCUSSION
In this study, greater ciliary body thickness was associated with reduced power in the high-frequency component of accommodative microfluctuations. The power of the high-frequency component accommodative microfluctuations decreases by 86% for every 50 μm increase in ciliary body thickness. The high-frequency component has previously been associated with the arterial pulse.4 Thicker ciliary bodies may dampen the effects of pulse on accommodation. Greater ciliary body thickness has also been associated with a decreased amount of accommodative lag in adults30 and increased amounts of myopia in children.20 Although the role of an increased ciliary body thickness in juvenile-onset myopia is still unclear, it seems that the increased thickness of the ciliary body may actually improve the stability of the high-frequency portion of the accommodative response.
The low-frequency component of accommodative microfluctuations has previously been associated with optical fluctuations in the crystalline lens.8 Because no relationship was found between the low-frequency component and tension on the crystalline lens, it can be hypothesized that the low-frequency component of accommodative microfluctuations is not affected by variation in tension on the lens capsule and/or zonules and must instead originate from some inherent property of the lens itself or feedback control noise in neural input to accommodation.10
More hyperopic refractive error was associated with higher powers of high-frequency accommodative microfluctuations in this study. This differs from what others have previously reported.11–13,19 Because increased myopic refractive error was associated with increasing age in this sample, the myopic subjects were likely to have completed their myopia progression. In addition, some of the younger subjects with refractive error more in the emmetropic range may have just begun the process of developing myopia. This is illustrated in Fig. 5 that shows a normal accommodative lag in most of the myopic subjects, with a few low myopes/emmetropes having higher accommodative lag. Accommodative lag has been shown to increase at myopia onset,31 but the differences in accommodative lag between myopes and emmetropes disappear once myopia progression has ended.32,33 Mutti et al.34 have found that an elevated response AC/A ratio in non-myopic children was associated with a greater risk of myopia development during the next year. Perhaps, accommodative microfluctuations are another feature of accommodation that is transiently affected by myopia progression. If relationship between accommodative microfluctuations and myopia onset were evaluated in a longitudinal study, it would be interesting to determine whether larger accommodative microfluctuations would be predictive for myopia development in a manner similar to the AC/A ratio.
Others have found that accommodative microfluctuations were increased in subjects with late-onset myopia who were assumedly still progressing.12,13 Langaas et al.19 studied subjects with early-onset myopia and although no difference in accommodative lag was found between emmetropic and myopic subjects, myopic subjects were found to have more variable accommodation. The subjects in the study by Langaas et al. were older than subjects in this study and their individual refractive phenotype was likely already expressed. Because accommodative problems may be more likely during active myopia progression, studying accommodative abnormalities is challenging in a cross-sectional study, where children may be at different, unknown stages of myopia development. Thus, accommodative microfluctuations need to be assessed in a longitudinal study to determine how much age, ciliary body thickness, and refractive error progression affect the microfluctuations.
Finally, another source of variance between studies that should be addressed is the different methods in measuring accommodative microfluctuations. Harb et al.11 measured accommodative microfluctuations after prolonged periods of reading when the subject may have been fatigued. It may be important to differentiate anatomical and fatigue-related accommodative microfluctuations. This study did not evaluate accommodative stability under fatigue.
The results of this study provide some insight into how microfluctuations of accommodation change with age. All accommodative microfluctuation measurements decreased with advancing age in this sample of children. In addition, the mean power values for the high-frequency component (0.005 D2/Hz), low-frequency component (0.042 D2/Hz), and root mean square (0.49 D) in this study are smaller than those found by Candy and Bharadwaj25 in infants but larger than those found by Day et al.12 in young adults. The accommodative ability of children in this study was not yet adult-like but more advanced than the infant state. Although it has been suggested that microfluctuations of accommodation may decrease as the neurological system matures,25 there may be an additional explanation. The results of this study suggest that a thicker ciliary body provides a more stable accommodative response. Thus, some of the decrease in accommodative microfluctuations may be also due to the increase in the size of the ciliary body as children become older.35
Accommodative microfluctuations represent an interesting component of accommodation, which is known to be affected by the development of myopia. A longitudinal study is needed to determine whether accommodative microfluctuations increase before, during, or after myopia progression and the role of the ciliary body thickness in the process.
References
- 1.Campbell FW, Robson JG, Westheimer G. Fluctuations of accommodation under steady viewing conditions. J Physiol. 1959;145:579–94. doi: 10.1113/jphysiol.1959.sp006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winn B, Pugh JR, Gilmartin B, Owens H. The frequency characteristics of accommodative microfluctuations for central and peripheral zones of the human crystalline lens. Vision Res. 1990;30:1093–9. doi: 10.1016/0042-6989(90)90117-4. [DOI] [PubMed] [Google Scholar]
- 3.Kotulak JC, Schor CM. Temporal variations in accommodation during steady-state conditions. J Opt Soc Am (A) 1986;3:223–7. doi: 10.1364/josaa.3.000223. [DOI] [PubMed] [Google Scholar]
- 4.Winn B, Pugh JR, Gilmartin B, Owens H. Arterial pulse modulates steady-state ocular accommodation. Curr Eye Res. 1990;9:971–5. doi: 10.3109/02713689009069933. [DOI] [PubMed] [Google Scholar]
- 5.Collins M, Davis B, Wood J. Microfluctuations of steady-state accommodation and the cardiopulmonary system. Vision Res. 1995;35:2491–502. [PubMed] [Google Scholar]
- 6.Owens H, Winn B, Gilmartin B, Pugh JR. Effect of a topical beta-adrenergic receptor antagonist on the dynamics of steady-state accommodation. Ophthal Physiol Opt. 1991;11:99–104. doi: 10.1111/j.1475-1313.1991.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 7.Winn B. Accommodative microfluctuations: a mechanism for steady-state control of accommodation. In: Stark L, editor. Accommodation and Vergence Mechanisms in the Visual System. Boston: Birkhauser Verlag; 2000. pp. 129–40. [Google Scholar]
- 8.van der Heijde GL, Beers AP, Dubbelman M. Microfluctuations of steady-state accommodation measured with ultrasonography. Ophthal Physiol Opt. 1996;16:216–21. doi: 10.1046/j.1475-1313.1996.95000518.x. [DOI] [PubMed] [Google Scholar]
- 9.Toshida K, Okuyama F, Tokoro T. Influences of the accommodative stimulus and aging on the accommodative microfluctuations. Optom Vis Sci. 1998;75:221–6. doi: 10.1097/00006324-199803000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Miege C, Denieul P. Mean response and oscillations of accommodation for various stimulus vergences in relation to accommodation feedback control. Ophthal Physiol Opt. 1988;8:165–71. doi: 10.1111/j.1475-1313.1988.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 11.Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res. 2006;46:2581–92. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day M, Strang NC, Seidel D, Gray LS, Mallen EA. Refractive group differences in accommodation microfluctuations with changing accommodation stimulus. Ophthal Physiol Opt. 2006;26:88–96. doi: 10.1111/j.1475-1313.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Seidel D, Gray LS, Heron G. Retinotopic accommodation responses in myopia. Invest Ophthalmol Vis Sci. 2003;44:1035–41. doi: 10.1167/iovs.02-0264. [DOI] [PubMed] [Google Scholar]
- 14.Stark LR, Atchison DA. Pupil size, mean accommodation response and the fluctuations of accommodation. Ophthal Physiol Opt. 1997;17:316–23. [PubMed] [Google Scholar]
- 15.Niwa K, Tokoro T. Influence of spatial distribution with blur on fluctuations in accommodation. Optom Vis Sci. 1998;75:227–32. doi: 10.1097/00006324-199803000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Winn B, Charman WN, Pugh JR, Heron G, Eadie AS. Perceptual detectability of ocular accommodation microfluctuations. J Opt Soc Am (A) 1989;6:459–62. doi: 10.1364/josaa.6.000459. [DOI] [PubMed] [Google Scholar]
- 17.Charman WN, Heron G. Fluctuations in accommodation: a review. Ophthal Physiol Opt. 1988;8:153–64. doi: 10.1111/j.1475-1313.1988.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 18.Seidel D, Gray LS, Heron G. The effect of monocular and binocular viewing on the accommodation response to real targets in emmetropia and myopia. Optom Vis Sci. 2005;82:279–85. doi: 10.1097/01.opx.0000159369.85285.21. [DOI] [PubMed] [Google Scholar]
- 19.Langaas T, Riddell PM, Svarverud E, Ystenaes AE, Langeggen I, Bruenech JR. Variability of the accommodation response in early onset myopia. Optom Vis Sci. 2008;85:37–48. doi: 10.1097/OPX.0b013e31815ed6e9. [DOI] [PubMed] [Google Scholar]
- 20.Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008;49:4353–60. doi: 10.1167/iovs.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira C, Tello C, Liebmann JM, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005;140:324–5. doi: 10.1016/j.ajo.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Hunt OA, Wolffsohn JS, Gilmartin B. Evaluation of the measurement of refractive error by the PowerRefractor: a remote, continuous and binocular measurement system of oculomotor function. Br J Ophthalmol. 2003;87:1504–8. doi: 10.1136/bjo.87.12.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolffsohn JS, Hunt OA, Gilmartin B. Continuous measurement of accommodation in human factor applications. Ophthal Physiol Opt. 2002;22:380–4. doi: 10.1046/j.1475-1313.2002.00050.x. [DOI] [PubMed] [Google Scholar]
- 24.Choi M, Weiss S, Schaeffel F, Seidemann A, Howland HC, Wilhelm B, Wilhelm H. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor) Optom Vis Sci. 2000;77:537–48. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Candy TR, Bharadwaj SR. The stability of steady state accommodation in human infants. J Vis. 2007;7:4.1–16. doi: 10.1167/7.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh JR, Eadie AS, Winn B, Heron G. Power spectrum analysis in the study of ocular mechanisms. Ophthal Physiol Opt. 1987;7:321–4. [PubMed] [Google Scholar]
- 27.Deubel H, Bridgeman B. Fourth Purkinje image signals reveal eye-lens deviations and retinal image distortions during saccades. Vision Res. 1995;35:529–38. doi: 10.1016/0042-6989(94)00146-d. [DOI] [PubMed] [Google Scholar]
- 28.Zuber BL, Stark L. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science. 1965;150:1459–60. doi: 10.1126/science.150.3702.1459. [DOI] [PubMed] [Google Scholar]
- 29.Neter J, Wasserman W, Kutner MH. Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Designs. 3. Homewood, IL: Irwin; 1990. [Google Scholar]
- 30.Ernst LE, Sinnot LT, Bailey MD. Ciliary body thickness and accommodative lag in adults. Optom Vis Sci. 2008;75 Program #80001. [Google Scholar]
- 31.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–46. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 32.Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res. 1995;35:1299–304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- 33.Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthal Physiol Opt. 1998;18:13–20. [PubMed] [Google Scholar]
- 34.Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000;41:2469–78. [PubMed] [Google Scholar]
- 35.Aiello AL, Tran VT, Rao NA. Postnatal development of the ciliary body and pars plana. A morphometric study in childhood. Arch Ophthalmol. 1992;110:802–5. doi: 10.1001/archopht.1992.01080180074031. [DOI] [PubMed] [Google Scholar]