Abstract
Background
The atopic march is well documented, but the inter-relationship of food allergy (FA) and asthma is not well understood.
Objective
To examine the strength of the association and temporal relationships between food allergy and asthma.
Methods
This analysis included 271 children ≥6 years (older group) and 296 children <6 years (younger group) from a family-based FA cohort in Chicago, IL. Asthma was determined by parental report of physician diagnosis. FA status was determined based on type and timing of clinical symptoms after ingestion of a specific food, and results of prick skin test (Multi-Test II) and allergen specific IgE (Phadia ImmunoCAP). Analyses were carried out using logistic regression accounting for important covariates and autocorrelations among siblings. Kaplan-Meier curves were used to compare the time to onset of asthma by FA status.
Results
Symptomatic FA was associated with asthma in both older (OR=4.9, 95%CI:2.5–9.5) and younger children (OR=5.3, 95%CI:1.7–16.2). The association was stronger among children with multiple or severe food allergies, especially in older children. Children with FA developed asthma earlier and at higher prevalence than children without FA (Cox Proportional hazard ratio=3.7, 95%CI:2.2–6.3 for children ≥6 years and hazard ratio=3.3, 95%CI:1.1–10 for children <6 years of age). No associations were seen between asymptomatic food sensitization and asthma.
Conclusions
Independent of markers of atopy such as aeroallergen sensitization and family history of asthma, there was a significant association between FA and asthma. This association was even stronger in subjects with multiple food allergies or severe food allergy.
Keywords: Food allergy, asthma, child
INTRODUCTION
Food allergy is a prevalent pediatric condition affecting 2–6% of children [1–4] and 1 to 3.2% of adults[4, 5]. Asthma is even more common, with 5 to 8% of the US population suffering from asthma.[6, 7] Over the past decades, both diseases have shown an increasing prevalence in children in the US[8–10] and worldwide.[11, 12] Prior large epidemiological studies[13, 14] have suggested that food allergy precedes asthma in average age of onset. Other studies have suggested that food allergen sensitization[15–19] is a risk factor for the development of asthma. Indeed, these diseases are often co-morbid [20, 21] and the relationship of these atopic diseases has often been described as “the atopic march”.[13, 14]
Most studies have focused on the relationship of food allergen sensitization to either aeroallergen sensitization or asthma.[13, 18, 19] There are fewer studies evaluating the association between clinical food allergy and asthma.[22, 23] These studies have either had smaller numbers of subjects,[22] or only followed the children through preschool years.[23] Notably, these studies have had conflicting results. Similarly, previous primary prevention trials of asthma which have included food avoidance have had equivocal results when taken as a whole.[24–27]
In addition, the underlying reason for the association of food allergy and asthma is not well studied. While food allergy and asthma may share some common risk factors such as atopy, it is also possible that they are causally related. One prior study found the association of food allergy and asthma to be independent of aeroallergen sensitization.[16] This finding, while intriguing, has not been replicated. Studies of oral food challenges have found changes in bronchial hyper reactivity (BHR) or lung function[28, 29] to be associated with clinical reactivity to food.
Finally, study of the inter-relationship of these two diseases is complicated by the fact that the natural history including age of onset for food allergy and asthma appear to be different.[30, 31] As such, studies may be variably affected by older children having outgrown food allergy and by a number of younger children not having developed the outcome of asthma. It is possible that the inter-relationship of food allergy and asthma may differ by age group in children. To date, there is a lack of in-depth analysis on the inter-relationship between food allergy and the development of asthma by age group in a sample of U.S. children.
We studied a sample of well-phenotyped children from a family-based food allergy study cohort enrolled in Chicago. We sought to determine: 1) the strength of the association between food allergy (clinically symptomatic) and asthma, and also between asymptomatic sensitization and asthma, 2) the dose response relationship between numbers of food allergies and severity of food allergy and the odds of having been diagnosed with asthma. As part of this analysis, we sought to determine whether any association was independent of measures of atopy and aeroallergen sensitization. We also carried out a secondary analysis to explore the temporal relationship between these two diseases. A better understanding of the relationship between these two diseases may provide new insight into the natural history of food allergy and asthma, and measures for early intervention.
METHODS
Study population and data collection
This report included a total of 567 children (271 children 6 years of age and older and 296 children less than 6 years of age). These children were enrolled as part an ongoing family-based food allergy study in Chicago, IL, which includes families both with and without food allergy. Families were recruited through general medical and allergy specialty clinics, community support groups, and advertisements in media. Families with food allergy were derived primarily from allergy specialty clinics, community support groups, and advertisements in media. Families without food allergy were primarily derived from general medical clinics, and advertisements in the media. Of the total 567 children included in this study, 410 were seen at Children’s Memorial Hospital, and 136 were patients in the Division of Allergy and Immunology. Eligible families were those having (1) either one or both parents willing to be enrolled and (2) at least one biological child (aged 0 to 21 years) with or without food allergy. The presence of asthma was not an eligibility or exclusion criterion.
Information regarding the food allergies and medical history of each family member was collected through a questionnaire-based interview conducted by trained research staff. A clinical evaluation, including height, weight, blood pressure, and prick skin tests were performed on each participating family member. Venous blood samples were collected from each participating family member. For those children receiving care in the Allergy and Immunology Clinic at Children’s Memorial Hospital, ICD-9 codes were collected and compared to parent report.
The Institutional Review Board of Children’s Memorial Hospital approved the study protocol. All participating families provided written informed consent.
Total and specific IgE measurement
Total serum IgE, specific IgE (sIgE) for 9 food allergens (egg white, sesame, peanut, soy, milk, shrimp, walnut, cod fish, and wheat) and sIgE for 6 aeroallergens (Dermatophagoides pteronyssinus and Dermatophagoides farinae, cat dander, dog dander, German cockroach, and Alternaria alternata) were measured for each subject using the Phadia ImmunoCAP system. The calibration range for total IgE was from 2.0 to 5,000 kU/L. The calibration range for sIgE was from 0.1 to 100 kUA/L. Total and sIgE assays were performed by the Clinical Immunology Laboratory at Children’s Memorial Hospital, a CLIA certified laboratory for the ImmunoCAP assay.
Prick skin test (PST)
Skin testing was performed on the volar surface of the arms on normal skin using Multi-Test II (Lincoln Diagnostics, Decatur, IL). Subjects were tested to 14 allergens, including 5 aeroallergens (Alternaria tenius, house dust mite mix [equal parts mixture of D. farinae and D. pteronyssinus], cat hair, dog epithelia, cockroach mix [American and German cockroach]) and 9 foods (cow milk, egg white, soybean, wheat, peanut, English walnut, sesame seed, fish mix [cod, flounder, halibut, mackerel, tuna], and shellfish mix [clam, crab, oyster, scallops, shrimp]) plus negative (50% glycerinated saline) and positive (histamine, 1.0 mg/mL) controls (Greer, Lenoir, NC). The results were measured 15 minutes after application. The test was considered positive if the mean wheal diameter (MWD) was 3 mm or greater than the saline control. Data was excluded if the saline control was ≥3 mm, the histamine control was <3 mm, or if the difference of histamine minus saline was <3 mm.
Definition of food allergy
Food allergy status was determined by applying a set of clinical criteria to data gathered from the questionnaire-based interview performed by study staff and results of sIgE measurements and PST. Clinical criteria for symptomatic food allergy were met if tests (sIgE or PST) corroborated typical symptoms of an allergic reaction to a food with onset within 2 hours of ingestion. Symptoms included any one of the following: skin (hives or angioedema); respiratory tract (difficulty breathing, shortness of breath, repetitive coughing, wheezing, chest tightness); oropharyngeal (throat tightness, choking, or difficulty swallowing; tongue swelling); cardiovascular (fainting, dizziness, light-headedness, or decreased level of consciousness); or gastrointestinal (vomiting). Tests were considered corroborative if sIgE was present for the reported food allergen (>=0.1 kUA/L for ImmunoCAP) or if there was a positive PST as defined above. Children were considered food allergic if they met the above criteria for at least one specific food. Those with negative PST or Immunocap levels were not included as cases even if the parent reported history was supportive since the prevalence of food allergy by self report is inflated compared to other methods such as food challenge.[32] Also, individuals with both negative immunocap and negative skin tests are very unlikely to have a positive challenge.[33, 34]
Food allergy was also characterized as severe (suggestive of anaphylaxis) or non-severe (all other symptomatic cases meeting our clinical criteria) based on symptoms. The criteria for severe allergy could be met in one of 2 possible ways. First, subjects could have involvement of the skin, mucosal tissue or both and at least one manifestation of respiratory compromise or cardiovascular dysfunction as described above. Second, subjects could have any one of the above listed symptoms along with persistent gastrointestinal symptoms (vomiting).
Asymptomatic food sensitization was defined as either a detectible level (0.1 kUA/L) of sIgE or a positive PST as defined above to a particular food, but no reported clinical symptoms to that food. The earliest age of food allergy symptoms was based on parental report in a questionnaire-based interview performed by study staff. A subset of 129 children received specialty medical care in the Allergy and Immunology Clinic at Children’s Memorial Hospital. In this subset, 100% of our study subjects who were defined as having food allergy by our criteria were also diagnosed as having food allergy in the Allergy and Immunology Clinic.
Ascertainment of asthma
This analysis focused on current asthma, which was defined as parental report of physician diagnosis and being symptomatic in the year prior to the survey. These outcomes and the earliest age of reported asthma symptoms were determined by questionnaire-based interview performed by study staff.
A subset of children (n=69) with parent reported asthma were also followed by the Allergy and Immunology clinic at Children’s Memorial Hospital. Asthma status was verified by physician diagnosis in 94.2% of this subset of study participants.
Statistical analysis
Our primary analyses were carried out in an age group stratified manner (<6 years of age and ≥6 years of age), with a focus on current food allergy and asthma. The rationale for stratified analyses was that the associations between food allergy and asthma may vary by age group due to differences in the age of onset and natural history between food allergy and asthma. Children may develop tolerance to some food allergies as they grow. Younger children with physician diagnosis of asthma may include a proportion with transient wheezing, whereas older children with physician diagnosis of asthma may be more likely to be true asthma cases. [31]
We carried out logistic regression modeling to assess the association between food allergy diagnosis (Table II and Online Table E1), asymptomatic sensitization (Online Table E2A and E2B), number of food allergies (Table II) and severity of food allergies (Table III and Online Table E3) and the risk of asthma, adjusting for covariates as specified below, as well as adjusting for the correlation between siblings in the same family as a random effect.
Table II.
Relationship between the number of food allergies and risk of asthma.*
| Children <6 years old (N=296) | ||||||
|---|---|---|---|---|---|---|
| Number of food allergies | Number with food allergy | Asthma Diagnosis | OR |
95% CI | p |
|
| Yes | Lower bound | Upper bound | ||||
| N=71 | ||||||
| 0=None | 87 | 4(5%) | 1.0 | n/a | n/a | n/a |
| 1 | 119 | 35(29%) | 5.1 | 1.6 | 16.2 | 0.0053 |
| 2+ | 90 | 32(36%) | 5.8 | 1.8 | 18.9 | 0.0033 |
| Children >=6 years old (N=271) | ||||||
|---|---|---|---|---|---|---|
| Number of food allergies | Number with food allergy | Asthma Diagnosis | OR |
95% CI | p |
|
| Yes | Lower bound | Upper bound | ||||
| N=121 | ||||||
| 0=None | 119 | 24(20%) | 1 | n/a | n/a | n/a |
| 1 | 77 | 41(53%) | 3.2 | 1.5 | 6.7 | 0.0021 |
| 2+ | 75 | 56(75%) | 8.6 | 3.8 | 19.3 | <.0001 |
Adjusted for age, gender, ethnicity (white vs non-white), intrafamilial correlations, household income, family history of asthma, and aeroallergen sensitization
Table III.
Relationship between the severity of food allergy and asthma prevalence in children.*
| Severity | Number with food allergy | Asthma Diagnosis | OR |
95% CI | p |
||
|---|---|---|---|---|---|---|---|
| Yes | Lower bound | Upper bound | |||||
| < 6 years old N=296 | None | 87 | 4(5%) | 1.0 | n/a | n/a | n/a |
| Non-severe | 63 | 15(24%) | 3.8 | 1.1 | 13.2 | 0.0322 | |
| Severe | 146 | 52(36%) | 6.2 | 2.0 | 19.3 | 0.0017 | |
| >= 6 years old N=271 | None | 119 | 24(20%) | 1.0 | n/a | n/a | n/a |
| Non-severe | 41 | 21(51%) | 2.9 | 1.2 | 7.0 | 0.0174 | |
| Severe | 111 | 76(68%) | 6.1 | 3.0 | 12.4 | <.0001 | |
Adjusted for age, gender, ethnicity (white vs non-white), intrafamilial correlations, household income, family history of asthma, and aeroallergen sensitization
Cochran-Armitage test for trend (unadjusted) p<.0001 (<6 years old)
Cochran-Armitage test for trend (unadjusted) p<.0001 (>=6 years old)
Potential confounding variables included basic demographic measures, socioeconomic status measures, and factors known from the literature to be associated with asthma or food allergy. We then examined each of these possible confounders individually (univariate modeling) in a model with the dependent variable of asthma and independent variable of food allergy, adjusting for intrafamilial correlation. This included addition of the potential confounder to the univariate relationship between food allergy and asthma, evaluating whether this resulted in a shift in the odds ratio for asthma. Confounders that were significant in univariate models and resulted in a shift in the odds ratio (of greater than 0.2) for the relationship were then adjusted for in the multiple regression analyses.
The confounders that were adjusted for in the multiple regression models were: age (continuous), race (Minority vs. White/Unknown), gender, sensitivity to aeroallergens (positive skin test for alternaria, cat, dog, dust mite or cockroach), household income, and family history of asthma (either mother or father reported current or outgrown asthma). In our initial models, we included total IgE in addition to sensitization to aeroallergens, but this variable was not significant in the model and did not change the results. As such, this variable was not included in final models.
Using a similar approach, we also examined the association between specific food allergies and asthma (Table E1). We also evaluated the association of asymptomatic food allergen sensitization with asthma (Table E2). This secondary analysis included as cases only those subjects who had asymptomatic sensitization (positive skin test or sIgE >0.1 kUA/L) and no clinical symptoms associated with food ingestion. The reference group for the analyses involving asymptomatic sensitization was the same as the main analysis, and included only those individuals with no clinical food allergy and no evidence of sensitization to foods. Due to sample size limitations, online Tables E2A present unadjusted statistics. In Table E2B, where possible, significance was determined adjusting for the limited subset of the covariates. Some models of the individual food allergens did not converge due to small cell sizes. For other food allergens with very few numbers, unadjusted statistics are presented as specified in Table E2B and E3.
We also compared time-to-asthma onset between children with and without food allergy in Figure 1, using Kaplan-Meier estimates implemented in SAS. Significance for the comparison of age of asthma onset between those with and without food allergy was determined using Cox Proportional Hazard modeling, adjusting for the covariates specified above in the main regression model.
Figure 1.
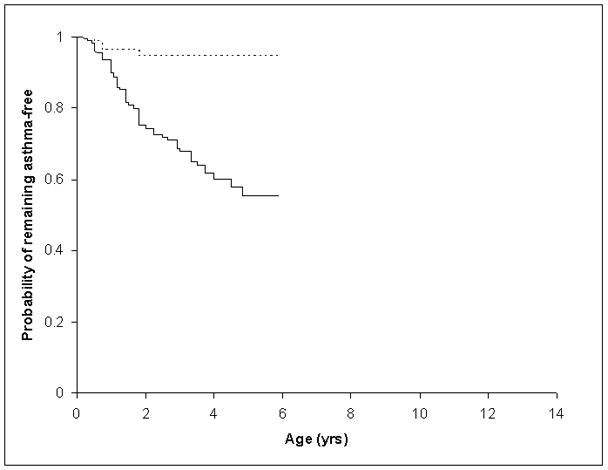
Age of Onset for Asthma for Children <6 years old with and without Food Allergy (FA)
Groups represented are as follows: Dashed line represents children without FA and the solid line represents children with FA.
All statistical analyses were conducted in SAS 9.1 (The SAS Institute, Cary, N.C.). Level of significance used was alpha < 0.05 (two-sided).
RESULTS
This analysis included 271 children who were 6 years of age and older and 296 children who were less than 6 years of age at the time of study entry. The characteristics of these two groups of children are presented in Table I. This is a predominantly Caucasian population ranging from middle to high income families, from urban to suburban Chicago. The prevalence of food allergy and asthma is 71% and 24% in the younger age group, and 56% and 48% in the older age group. Among asthmatic children, 94.4% and 80.2% had coexistence of food allergy in the younger and older age group, respectively. By way of validation, a subset of 129 children were seen in the Allergy and Immunology Clinic at Children’s Memorial Hospital, and 100% who were defined as having food allergy by our criteria also had a corroborative physician diagnosis of food allergy. Likewise, of a subset of 69 children with parent reported asthma seen in the Allergy and Immunology Clinic at Children’s Memorial Hospital, 94.2% had a physician diagnosis of asthma to validate the parent report.
Table I.
Epidemiological and Clinical Characteristics of Study Children, Stratified by Age Groups.
| Total | Children aged < 6 | Children aged 6 or older | |
|---|---|---|---|
| N: Children | 567 | 296 | 271 |
| N: Families | 403 | 234 | 208 |
| # of Children with food allergy | 361 | 209 (71%) | 152 (56%) |
| # of Children with asthma | 201 | 72 (24%) | 129 (48%) |
| age, mean(std) | 3.3 (1.5) | 10.2 (3.2) | |
| Median (range) | 3.3 (0.3 – 6.0) | 9.3 (6.0 – 20.8) | |
| Gender: Male | 66% | 53% | |
| Caucasian | 90% | 85% | |
| Aeroallergen sensitization | 52% | 56% | |
| Family income: | |||
| $0–20,000 | 1% | 7% | |
| $20,000–40,000 | 5% | 7% | |
| $40,000–100,000 | 17% | 15% | |
| $>100,000 | 77% | 70% | |
| Mother’s Education: | |||
| High School or less | 4% | 7% | |
| College degee or less | 55% | 62% | |
| Grad or professional | 41% | 31% | |
| Father’s Education: | |||
| High School or less | 4% | 12% | |
| College degee or less | 60% | 50% | |
| Grad or professional | 36% | 38% | |
| Attended daycare | 64% | 81% | |
| Pet in home | 53% | 63% | |
| Breastfed | 93% | 86% | |
| Exclusively | 24% | 27% | |
| Mother smoked during pregnancy | 5% | 6% | |
| Mother smoked postnatally | 4% | 6% | |
| Child exposed to passive smoke | 13% | 17% | |
| Family history atopy | 86% | 89% | |
| Family history asthma | 44% | 44% | |
The median time difference from time of first reaction to study evaluation was approximately 3 years for all foods. Between initial symptoms and enrollment in the study, the median time lapse and interquartile range for the common foods evaluated were 3.68 y (1.61–6.25) for peanut, 3.35 (1.36–5.84) for egg, and 3.90 y (1.65–6.35) for milk, respectively. The overall median age and interquartile range of the cohort at initial assessment was 5.33 years (3.34–8.53years).
The presence of a symptomatic allergy to any food allergen that met our criteria was strongly associated with having an asthma diagnosis in both the older children (OR=4.9, 95%CI: 2.5–9.5, p <0.0001) and the younger children (OR=5.3, 95%CI: 1.7–16.2, p=0.0034). This association between symptomatic food allergy and asthma persisted even after controlling for age, gender, ethnicity (white vs. non-white), intrafamilial correlations, household income, family history of asthma, and aeroallergen sensitization. In the older children, we also evaluated the associations of asthma with specific type of food allergy as a secondary analysis (Table E1), finding similar associations.
We evaluated the independent contribution of asymptomatic food allergen sensitization to asthma among the group of children that did not have clinical symptoms of food allergy as compared to those without symptoms and without sensitization in both the younger and older children. No statistically significant associations were observed between asymptomatic sensitization as determined by PST and asthma (Table E2A). Similarly, merely having detectable levels of sIgE to food allergens failed to reach statistical significance (Table E2B) except for a marginal association with soy.
Table II shows the relationship between the number of food allergies and asthma in the study population. As compared to children without food allergy, the association with asthma illustrates a striking dose effect in older children as the number of food allergies increases. Older children with one food allergy were found to have 3.2 times greater odds for having an asthma diagnosis than children without food allergy (OR=3.2, 95%CI: 1.5–6.7, p=0.0021). For children with two or more food allergies, the association is even stronger (OR =8.6, 95%CI: 3.8–19.3, p <0.0001). In the children under 6 years of age a dose response was less obvious. Younger children with one food allergy were found to have 5.1 times greater odds for having an asthma diagnosis (OR=5.1, 95%CI: 1.6–16.2, p=0.0053) and for those with 2 or more food allergies the OR increased to only 5.8 (95%CI: 1.8–18.9, p=0.0033).
We also evaluated whether increasing severity of food allergy was associated with a greater odds of having an asthma diagnosis. We found that a severe food allergy to at least one food was strongly associated with the diagnosis of asthma, as shown in Table III. Older children with a severe allergy to any food had a 6.0 fold (95%CI: 3.0–12.4, p<0.0001) increased risk of developing asthma compared to children without food allergy. Whereas, older children with a non-severe food allergy to any food allergen had only a 2.9 (95%CI: 1.2–7.0, p=0.0174) greater odds of having asthma. A similar pattern was seen in the children <6 years of age. Younger children with severe food allergies had a 6.2 fold (95%CI: 2.0–19.3, p=0.0017) increased risk of developing asthma, while those with non-severe food allergies had a 3.8 (95%CI: 1.1–13.2, p=0.0322) increased risk. The associations for specific food allergens were evaluated in older children and for severe allergies to milk, egg, and peanut, all three of these reached statistical significance in a model including age, gender, ethnicity, intrafamilial correlations, family history of asthma and aeroallergen sensitization (Table E3, online supplement). Other food allergens had insufficient numbers for adjusted analyses. These analyses of severity of reaction to specific food allergens did not exclude those with multiple food allergens due to sample size issues.
The time from age of first food allergy onset and age of asthma onset was evaluated for both the older and the younger age groups using time-to-asthma analysis (Figures 1 and 2). In the older age group, for children with food allergy the median age of onset of asthma was 5 years (95 %CI: 4–7). In the younger children, the median age of onset of asthma in children with food allergy was 2.3 years (95 %CI: 1.8 – 3.3). Children in our sample with food allergy developed asthma significantly earlier than children without food allergy. Cox proportional hazards modeling, adjusted for age, gender, ethnicity (white vs non-white), intrafamilial correlations, household income, family history of asthma, and aeroallergen sensitization, demonstrated earlier onset of asthma in children with food allergy than those without. Younger children with food allergy were at 3.3 times of hazard for developing asthma (HR=3.3, 95%CI: 1.1–10.0, p=0.0362) and older children with food allergy were at 3.7 times of hazard for developing asthma (HR=3.7, 95%CI: 2.2–6.3, p<0.0001) than their non-allergic peers.
Figure 2.
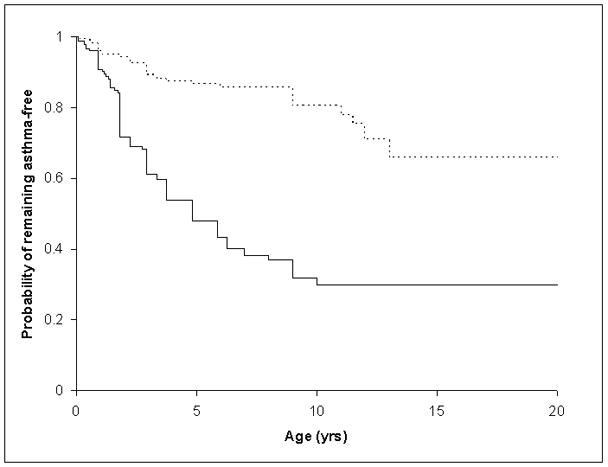
Age of Onset for Asthma for Children >=6 year old with and without Food Allergy (FA)
Groups represented are as follows: Dashed line represents children without FA and the solid line represents children with FA.
DISCUSSION
Asthma and food allergy have been described as part of the “atopic march”; however, there is a lack of in-depth analysis on the inter-relationship between symptomatic food allergy and the development of asthma. This study has several strengths. We examined food allergy both in terms of clinically symptomatic food allergy and asymptomatic sensitization. We examined food allergy as a whole, specific food allergies, as well as number and severity of food allergy. We studied the food allergy-asthma relationship in a large sample of U.S. children stratified by age group (<6 vs. ≥6 years) to account for differences in the age of onset and natural history of these two diseases. Our analysis also accounted for important covariates or confounders such as family history, total IgE, and aeroallergen sensitization.
Our study confirmed a number of findings of other studies. Consistent with a prior report,[15] we found a strong association between symptomatic food allergy and asthma. Furthermore, we found this effect was independent of family history and aeroallergen sensitization, which is corroborated by one other study which also accounted for sensitization to aeroallergens.[16] Both egg and milk allergy have been specifically implicated as risk factors for the development of asthma.[35–38] Our study corroborated this but also found that other allergens such as peanut and tree nut were also strongly associated with the development of asthma.
Our study has contributed several novel observations to the literature as well. We found evidence of a dose effect in terms of both number and severity of food allergies with the likelihood of having a diagnosis of asthma. In contrast, we found no association between asymptomatic sensitization to food allergens and asthma. While one smaller study also had showed no association of individual food allergen sensitization and asthma, [17] this finding is in contrast to other studies which showed an association with sensitization in general.[15, 18, 19] However, compared to these other studies, our analysis of sensitization was limited to children who were sensitized but without any clinical manifestations of food allergy. The implication of our findings is that there may be other links between food allergy and asthma aside from atopy alone, and that this relationship becomes stronger with the presence of greater severity and numbers of food allergies.
While limited by recall bias and the cross-sectional nature of the study, our data suggested temporal relationships between food allergy and asthma, with food allergy being diagnosed earlier than asthma. The Kaplan Meier curve shows that with the presence of food allergy the time to first asthma symptoms is earlier than that for children without food allergy. This is consistent with the prior literature on the atopic march.[13, 35]
Our study was careful to study the older and younger (<6 years of age) children separately for a number of reasons. The older children were ≥6 years of age at enrollment and had a current diagnosis of asthma, making this unlikely to simply be transient wheezing.[31] In the younger children, transient wheezing cannot be excluded. On the other hand, some of the children may outgrow their food allergy by the time they are 6 years of age. Nevertheless, the overall pattern of the food allergy-asthma association was consistent across the age groups.
There are a number of considerations and cautions in interpreting our findings. Food allergies were not confirmed by food challenge but by fulfilling clinical criteria which included having a corroborative allergy test in the presence of typical (self-reported) symptoms of a food allergic reaction within 2 hours of ingesting the food. The symptoms included muco-cutaneous symptoms of urticaria or angioedema, respiratory symptoms, or cardiovascular symptoms. Gastrointestinal symptoms were only included as indicative of a reaction in the presence of one of the other symptoms mentioned.
While this is not equivalent to the results of a challenge, it is better than self-report or physician diagnosis alone. Our approach is consistent with other published studies of food allergy in which food challenge was not required to establish existence of food allergy[39–42] and in some cases our approach is more strenuous as we incorporated serologic criteria in addition to clinical history.[43] Finally, the types of patients described as having food allergy in this study would be consistent with those who would be diagnosed as having food allergy in a specialist clinic as noted by the fact that 100% of the subset of subjects seen in a tertiary care Allergy Immunology Clinic were diagnosed as having food allergy. Certainly, those with severe food allergy by our definition would be classified as having anaphylaxis, and such patients were not challenged in other studies which have utilized challenge as a gold standard.[41]
Furthermore, there may be some concern as to whether some children assessed in our study may have outgrown their food allergy by the time of assessment. The median age of the children in our study was 5.3 years with the median time lapse from initial symptoms to participation in the study assessment being 3 years. While it is possible that some children could have outgrown the allergy by the time of participation, this may be less likely at this age, given some recent studies of food allergic cohorts.[40, 42] Also, there were very few individuals who were excluded from being cases on the basis of negative allergy testing after having met our clinical criteria for symptoms and timing.
With regard to asthma, physician diagnosis in the younger age group may be inclusive of individuals with transient wheezing. This would explain the weaker findings in this age group. There is also a chance that subjects are more likely to be diagnosed with asthma if they have food allergy based on physician judgment. This could be a source of bias in the younger children. Ongoing follow-up of these children will help to ascertain the extent to which this is a problem. Physician diagnosis of asthma in those older than 6 years is unlikely to represent simply transient wheezing in our sample.[31] However, the rate of asthma in the children over 6 years of age was 20%. Thus, there is some oversampling of asthmatic children in the group of children over 6 years of age. As such, our results are biased towards the null hypothesis, implying that the actual effect sizes are even greater than those which we report. Also, the association of the severity of the reaction and the presence of a diagnosis of asthma does not prove that anaphylaxis increases the risk of asthma. As noted in prior literature,[44] [45] asthma itself may be a risk factor for severe food allergy reactions.
There are a few potential sources of selection bias in this study. One point should be noted, the strongest associations were seen in the older age group. One possible reason for this may be due to the fact that this group represents individuals who are more likely to have persistent food allergy. Exclusion of some subjects who may have outgrown their food allergy may have augmented some of these findings and made this sample less representative of the general population with food allergy. However, this is in keeping with our other findings that those with multiple or severe food allergies are more likely to have asthma. A second point is that the controls were aware that the study was evaluating food allergy. While it is possible that the parents in these families may have been more interested if there was a family history of atopic disease, our rates of asthma in this population are not out of keeping with what would be expected with 8.9% of the children over 6 years of age having a diagnosis of asthma in control families. Even if there was a higher incidence of atopic family history (and hence asthma in the offspring) in control families compared to the general population, this would bias our results towards the null hypothesis and not be an explanation for our findings. Another potential source of bias towards a positive association is that a diagnosis of asthma is more likely in the younger age group if the child has recurrent wheezing and has a food allergy as stated above. However, in a subset of 129 children with accessible medical records, a diagnosis was made by pediatric allergists in our tertiary care hospital who use asthma predictive indices in their assessments.[46] A total of 35 children < 6 years of age had asthma by report and were also seen at our institution. The rate of agreement for a specialist diagnosis of asthma in this subgroup under 6 years old with self report of an asthma diagnosis was 30/35 diagnosed as asthma and 5/35 given a symptomatic diagnosis of “wheezing”. Finally, since this is not a population based study and there may be some degree of selection bias as outlined above, caution is needed when extrapolating our findings to the general population.
Our data evaluating the temporal relationship were derived from a cross-sectional analysis and may be subject to recall bias. As such, these findings are suggestive and need confirmation in a large, longitudinal birth cohort study. Notably, longitudinal studies of sensitization have shown a similar temporal pattern for food allergen sensitization and the development of asthma, but did not specifically address clinical food allergy (as opposed to sensitization) as a primary determinant. [15, 18, 19] While similar predictions between food allergy and what was termed asthma at 24 months have been noted in the past,[23] that study had not followed children beyond an age where diagnoses of asthma may be distinguished from transient wheezing Also, a prior study evaluating this issue in a longitudinal fashion may not have found an association with asthma at 7 years of age due to insufficiently large sample size.[22] Therefore, the significantly earlier onset of asthma in those with food allergy needs to be explored in large longitudinal birth cohorts.
Despite the limitations, our data is valuable for hypothesis generation and planning of future studies. The dose effect and the fact that these findings are independent of aeroallergen sensitization and family history of atopy imply that there may be more than just the common link of atopy which may contribute to this association. Indeed, there is some suggestion that those with food allergy may have more of a neutrophilic type of airway inflammation,[47], which is associated with more severe asthma.[48] Our study raised the possibility of certain common environmental and genetic factors underlying both food allergy and asthma, which warrant additional investigations.
Our findings, if replicated by future studies, may have important clinical implications. Food allergy may play a role in patients in whom asthma is poorly controlled with increased levels of health care utilization.[20, 21]Similarly, if food allergy is a risk factor for asthma development, a logical question would be how to reduce the risk of asthma among children with food allergy. One topic that was explored previously was whether infant feeding interventions could modify the risk of asthma. This is particularly relevant given the recent AAP task force statement.[49] This area is highlighted by the contradictory nature of the previous literature including prevention studies with a dietary component,[24–27, 50] and studies evaluating the role of breastfeeding in children of asthmatic or atopic mothers.[51–53] While it is possible that interventions to prevent or mitigate food allergy in early life could reduce the risk of asthma, more prospective studies or intervention trials are needed.
In summary, our data indicated a strong association, a dose effect, and a possible temporal relationship between symptomatic food allergy and asthma. Our findings add to the literature that food allergy may be one of the risk factors for the development of asthma. Since these findings are derived from a cross-sectional analysis and are not sufficient to imply causality[54] especially in a complex disease such as asthma, further prospective birth cohort studies are needed to validate these findings. Also, further studies are necessary to evaluate underlying mechanisms behind this association aside from the common risk factor of atopy.
Supplementary Material
Table E1. Relationship between symptomatic food allergy and asthma in children >=6 years old (n=443)
Table E2A. Relationship between skin test positivity to food allergens and asthma in children >=6 years old with asymptomatic sensitization
Table E2B. Relationship between detectable levels of specific IgE to food allergens and asthma in children >=6 years old with asymptomatic sensitization
Table E3. Severity of food allergy and asthma prevalence in Children >= 6 years (Total subjects=271)
Acknowledgments
Source(s) of support: This study is in part supported by the Food Allergy Project and grant M01 RR-00048 from the National Center for Research Resources, National Institutes of Health, the Chicago Community Trust (C2007-01166); the Sacks Family Fund; and Excellent in Academic Medicine Award by the State of Illinois.
The Authors would like to thank the participating families of the Children’s Memorial Food Allergy Project.
Abbreviations Used
- FA
food allergy
- MWD
mean wheal diameter
- PST
prick skin test
- SES
socioeconomic status
- AAP
American Academy of Pediatrics
Footnotes
Conflicts of Interest: Dr. Kumar has a unrestricted grant (<$10000) from Verus pharmaceuticals for an unrelated project.
References
- 1.Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, Dean T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–24. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 2.Venter C, Pereira B, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study. Pediatr Allergy Immunol. 2006;17:356–63. doi: 10.1111/j.1399-3038.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 3.Pereira B, Venter C, Grundy J, Clayton CB, Arshad SH, Dean T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol. 2005;116:884–92. doi: 10.1016/j.jaci.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 4.Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–73. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 5.Woods RK, Thien F, Raven J, Walters EH, Abramson M. Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Ann Allergy Asthma Immunol. 2002;88:183–9. doi: 10.1016/S1081-1206(10)61994-1. [DOI] [PubMed] [Google Scholar]
- 6.Turkeltaub PC, Gergen PJ. Prevalence of upper and lower respiratory conditions in the US population by social and environmental factors: data from the second National Health and Nutrition Examination Survey, 1976 to 1980 (NHANES II) Ann Allergy. 1991;67:147–54. [PubMed] [Google Scholar]
- 7.Arbes SJ, Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120:1139–45. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arif AA, Delclos GL, Lee ES, Tortolero SR, Whitehead LW. Prevalence and risk factors of asthma and wheezing among US adults: an analysis of the NHANES III data. Eur Respir J. 2003;21:827–33. doi: 10.1183/09031936.03.00054103a. [DOI] [PubMed] [Google Scholar]
- 9.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980–1996. J Asthma. 2007;44:65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- 10.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 11.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuehni CE, Strippoli MP, Silverman M. Food intolerance and wheezing in young South Asian and white children: prevalence and clinical significance. J Allergy Clin Immunol. 2006;118:528–30. doi: 10.1016/j.jaci.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Nickel R, Lau S, Niggemann B, Gruber C, von Mutius E, Illi S, Kulig M, Wahn U. Messages from the German Multicentre Allergy Study. Pediatr Allergy Immunol. 2002;13 (Suppl 15):7–10. doi: 10.1034/j.1399-3038.13.s.15.4.x. [DOI] [PubMed] [Google Scholar]
- 14.Cantani A. The growing genetic links and the early onset of atopic diseases in children stress the unique role of the atopic march: a meta-analysis. J Investig Allergol Clin Immunol. 1999;9:314–20. [PubMed] [Google Scholar]
- 15.Kulig M, Bergmann R, Tacke U, Wahn U, Guggenmoos-Holzmann I. Long-lasting sensitization to food during the first two years precedes allergic airway disease. The MAS Study Group. Germany Pediatr Allergy Immunol. 1998;9:61–7. doi: 10.1111/j.1399-3038.1998.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 16.Penard-Morand C, Raherison C, Kopferschmitt C, Caillaud D, Lavaud F, Charpin D, Bousquet J, Annesi-Maesano I. Prevalence of food allergy and its relationship to asthma and allergic rhinitis in schoolchildren. Allergy. 2005;60:1165–71. doi: 10.1111/j.1398-9995.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 17.Leung TF, Lam CW, Chan IH, Li AM, Tang NL. Sensitization to common food allergens is a risk factor for asthma in young Chinese children in Hong Kong. J Asthma. 2002;39:523–9. doi: 10.1081/jas-120004922. [DOI] [PubMed] [Google Scholar]
- 18.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 19.Nickel R, Kulig M, Forster J, Bergmann R, Bauer CP, Lau S, Guggenmoos-Holzmann I, Wahn U. Sensitization to hen’s egg at the age of twelve months is predictive for allergic sensitization to common indoor and outdoor allergens at the age of three years. J Allergy Clin Immunol. 1997;99:613–7. doi: 10.1016/s0091-6749(97)70021-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner-city children with asthma. J Allergy Clin Immunol. 2005;115:1076–80. doi: 10.1016/j.jaci.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Simpson AB, Glutting J, Yousef E. Food allergy and asthma morbidity in children. Pediatr Pulmonol. 2007;42:489–95. doi: 10.1002/ppul.20605. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy. 2000;55:240–5. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 23.Laan MP, Baert MR, Bijl AM, Vredendaal AE, De Waard-van der Spek FB, Oranje AP, Savelkoul HF, Neijens HJ. Markers for early sensitization and inflammation in relation to clinical manifestations of atopic disease up to 2 years of age in 133 high-risk children. Clin Exp Allergy. 2000;30:944–53. doi: 10.1046/j.1365-2222.2000.00856.x. [DOI] [PubMed] [Google Scholar]
- 24.Becker AB. Primary prevention of allergy and asthma is possible. Clin Rev Allergy Immunol. 2005;28:5–16. doi: 10.1385/CRIAI:28:1:005. [DOI] [PubMed] [Google Scholar]
- 25.Chan-Yeung M, Ferguson A, Watson W, Dimich-Ward H, Rousseau R, Lilley M, Dybuncio A, Becker A. The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol. 2005;116:49–55. doi: 10.1016/j.jaci.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Host A, Halken S, Muraro A, Dreborg S, Niggemann B, Aalberse R, Arshad SH, von Berg A, Carlsen KH, Duschen K, Eigenmann PA, Hill D, Jones C, Mellon M, Oldeus G, Oranje A, Pascual C, Prescott S, Sampson H, Svartengren M, Wahn U, Warner JA, Warner JO, Vandenplas Y, Wickman M, Zeiger RS. Dietary prevention of allergic diseases in infants and small children. Pediatr Allergy Immunol. 2008;19:1–4. doi: 10.1111/j.1399-3038.2007.00680.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95:1179–90. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]
- 28.James JM, Bernhisel-Broadbent J, Sampson HA. Respiratory reactions provoked by double-blind food challenges in children. Am J Respir Crit Care Med. 1994;149:59–64. doi: 10.1164/ajrccm.149.1.8111598. [DOI] [PubMed] [Google Scholar]
- 29.James JM, Eigenmann PA, Eggleston PA, Sampson HA. Airway reactivity changes in asthmatic patients undergoing blinded food challenges. Am J Respir Crit Care Med. 1996;153:597–603. doi: 10.1164/ajrccm.153.2.8564104. [DOI] [PubMed] [Google Scholar]
- 30.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, Arshad SH, Dean T. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2007 doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 31.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–8. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Knight AK, Shreffler WG, Sampson HA, Sicherer SH, Noone S, Mofidi S, Nowak-Wegrzyn A. Skin prick test to egg white provides additional diagnostic utility to serum egg white-specific IgE antibody concentration in children. J Allergy Clin Immunol. 2006;117:842–7. doi: 10.1016/j.jaci.2005.12.1304. [DOI] [PubMed] [Google Scholar]
- 34.Rance F, Abbal M, Lauwers-Cances V. Improved screening for peanut allergy by the combined use of skin prick tests and specific IgE assays. J Allergy Clin Immunol. 2002;109:1027–33. doi: 10.1067/mai.2002.124775. [DOI] [PubMed] [Google Scholar]
- 35.Cantani A, Micera M. Natural history of cow’s milk allergy. An eight-year follow-up study in 115 atopic children. Eur Rev Med Pharmacol Sci. 2004;8:153–64. [PubMed] [Google Scholar]
- 36.Saarinen KM, Pelkonen AS, Makela MJ, Savilahti E. Clinical course and prognosis of cow’s milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol. 2005;116:869–75. doi: 10.1016/j.jaci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Kotaniemi-Syrjanen A, Reijonen TM, Romppanen J, Korhonen K, Savolainen K, Korppi M. Allergen-specific immunoglobulin E antibodies in wheezing infants: the risk for asthma in later childhood. Pediatrics. 2003;111:e255–61. doi: 10.1542/peds.111.3.e255. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720–5. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 39.Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–51. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120:1413–7. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 41.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–6. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 42.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120:1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. 2000;106:53–6. doi: 10.1067/mai.2000.108105. [DOI] [PubMed] [Google Scholar]
- 44.Berns SH, Halm EA, Sampson HA, Sicherer SH, Busse PJ, Wisnivesky JP. Food allergy as a risk factor for asthma morbidity in adults. J Asthma. 2007;44:377–81. doi: 10.1080/02770900701364031. [DOI] [PubMed] [Google Scholar]
- 45.Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003;112:168–74. doi: 10.1067/mai.2003.1569. [DOI] [PubMed] [Google Scholar]
- 46.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Halonen M, Taussig LM, Martinez FD. Relation of two different subtypes of croup before age three to wheezing, atopy, and pulmonary function during childhood: a prospective study. Pediatrics. 2001;107:512–8. doi: 10.1542/peds.107.3.512. [DOI] [PubMed] [Google Scholar]
- 47.Wallaert B, Gosset P, Lamblin C, Garcia G, Perez T, Tonnel AB. Airway neutrophil inflammation in nonasthmatic patients with food allergy. Allergy. 2002;57:405–10. doi: 10.1034/j.1398-9995.2002.13527.x. [DOI] [PubMed] [Google Scholar]
- 48.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–43. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 49.Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 50.Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, Kraemer U, Herbarth O, Behrendt H, Wichmann HE, Heinrich J. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008;121:e44–52. doi: 10.1542/peds.2006-3553. [DOI] [PubMed] [Google Scholar]
- 51.Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Poulton R. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. 2002;360:901–7. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 52.Wright AL, Holberg CJ, Taussig LM, Martinez FD. Factors influencing the relation of infant feeding to asthma and recurrent wheeze in childhood. Thorax. 2001;56:192–7. doi: 10.1136/thorax.56.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guilbert TW, Stern DA, Morgan WJ, Martinez FD, Wright AL. Effect of breastfeeding on lung function in childhood and modulation by maternal asthma and atopy. Am J Respir Crit Care Med. 2007;176:843–8. doi: 10.1164/rccm.200610-1507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kundi M. Causality and the interpretation of epidemiologic evidence. Environ Health Perspect. 2006;114:969–74. doi: 10.1289/ehp.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table E1. Relationship between symptomatic food allergy and asthma in children >=6 years old (n=443)
Table E2A. Relationship between skin test positivity to food allergens and asthma in children >=6 years old with asymptomatic sensitization
Table E2B. Relationship between detectable levels of specific IgE to food allergens and asthma in children >=6 years old with asymptomatic sensitization
Table E3. Severity of food allergy and asthma prevalence in Children >= 6 years (Total subjects=271)


