Abstract
BACKGROUND
To estimate the incidence of aneuploidy in relation to patients' characteristics, the type of hormonal stimulation and their response to induction of multiple follicular growth, 4163 first polar bodies (PB1s) were analyzed.
METHODS
Five hundred and forty four infertile couples underwent 706 assisted conception cycles (640 with poor prognosis indications and 66 controls) in which chromosomal analysis of PB1 for the chromosomes 13, 15, 16, 18, 21 and 22 was performed. Results were evaluated in a multivariate analysis.
RESULTS
The proportion of normal oocytes was directly correlated (P < 0.01) with (i) the number of mature oocytes and (ii) the establishment of a clinical pregnancy; and inversely correlated (P < 0.01) with (i) female age, (ii) causes of female infertility (endometriosis, abortions, ovulatory factor), (iii) poor prognosis indications (female age, number of previous cycles, multiple poor prognosis indications), (iv) number of FSH units per oocyte and (v) number of FSH units per metaphase II oocyte. There was a weak significance of frequency (P < 0.05) between type of abnormality (originated by chromatid predivision, chromosome non-disjunction or combined mechanisms in the same oocyte) and groups of the studied variables, rather than to a specific abnormality or a specific chromosome.
CONCLUSIONS
The type of infertility had a significant effect on errors derived from the first meiotic division, whose incidence was significantly higher in the presence of endometriosis or of an ovulatory factor, and in women that experienced repeated abortions. Each aneuploidy event was found to be dependent not on a specific variable, but on groups of variables. In addition, the tendency of chromosomal abnormalities to occur simultaneously implies that the deriving aneuploidies can be of any type.
Keywords: aneuploidy, endometriosis, fluorescence in situ hybridization, polar body biopsy, recurrent abortions
Introduction
Embryo viability is dependent upon many factors, the chromosomal status being one of the most prominent in determining the fate of a conceptus. It has been estimated that up to 30% of human zygotes are aneuploid, this figure being more than double in women with a mean age of 38 years (Kuliev et al., 2005). This high incidence of meiotic errors determines severe clinical consequences with approximately one-third of miscarriages being chromosomally abnormal in origin (Hassold et al., 1996). Studies on male and female gametes have demonstrated that oogenesis is more error-prone compared with spermatogenesis (Gianaroli et al., 2005) and this is possibly a result of the prolonged arrest at the dictyotene stage in a process that begins during fetal life and becomes complete only after ovulation. It has been proposed that the check point regulating the transition from metaphase I to anaphase I is more permissive in oogenesis than in spermatogenesis, for which reason whereas spermatogenesis is blocked when an error occurs in alignment, oogenesis continues yielding aneuploid gametes (Hassold and Hunt, 2001).
Data from clinical pregnancies have documented that the importance of meiosis I versus meiosis II errors varies depending on the different chromosomes, although maternal meiosis I errors highly predominate in the majority of trisomies (Hassold et al., 2007). This could be attributed to the already mentioned peculiar timing and modality of female meiosis, in which the first meiotic division involves homologous chromosome segregation rather than sister chromatids as in meiosis II (Hassold and Hunt, 2001).
Important information on oocyte aneuploidy has been provided by the preconceptional testing of polar bodies (PBs) in which fluorescence in situ hybridization (FISH) has confirmed that 70% of chromosomal abnormalities occur in meiosis I as reflected by an aneuploid first polar body (PB1) (Verlinsky et al., 1999; Kuliev et al., 2003, 2005). These findings supported the validity of proposing the analysis of PB1 to predict the chromosomal status of the oocyte, and to use the derived information as an additional tool for oocyte selection. Situations in which restrictions are imposed on the number of oocytes to be inseminated could benefit from applying this strategy (Munné et al., 2000; Magli et al., 2006; Vialard et al., 2006). On the other hand, the contribution of errors derived from the second meiotic division cannot be disregarded, as a sizeable proportion of aneuploidy occurring in the second PB is expressed at the chromosomal level (Kuliev et al., 2003). It has also been described that errors in the first meiotic division can be compensated by sequential errors in the second meiotic division, generating euploid zygotes (Kuliev and Verlinsky, 2004; Fragouli et al., 2006). Nevertheless, this sort of aneuploidy rescue mechanism seems to predispose to a high frequency of mitotic malsegregation yielding chromosomally abnormal embryos that should not be considered for transfer (Kuliev and Verlinsky, 2004).
The more recent introduction of comparative genomic hybridization (CGH) on PB analysis has started to provide data on all chromosomes and the forthcoming increase in the number of reported cases is expected to clarify the actual incidence of aneuploidy in human oocytes from stimulated cycles (Gutierrez-Mateo et al., 2004; Fragouli et al., 2006; Fishel et al., 2010).
The present study reports the results derived from the FISH analysis of more than 4000 PB1s with the aim of getting all the available information on the chromosomal status of IVF generated oocytes. Special attention was focused on estimating the incidence of aneuploidy in relation to patients' characteristics, the type of hormonal stimulation and their response to induction of multiple follicular growth. The study included patients with a poor prognosis indication, and was extended to a control group represented by young couples at their first or second IVF attempt with no history of clinical abortions.
Materials and Methods
Patients
Between March 2004 and January 2009, 544 infertile couples underwent 706 assisted conception cycles in which chromosomal analysis of PB1 was performed.
Patients' inclusion criteria were maternal age ≥38 years (418 cycles, mean age 41.0 ± 2.3 years, mean number of previous cycles 2.0 ± 2.1), repeated IVF failures (202 cycles, mean female age 34.2 ± 2.5 years, mean number of previous cycles 4.0 ± 1.2) and recurrent abortions (20 cycles, mean female age 33.9 ± 2.4 years, mean number of previous abortions 3.4 ± 0.5). These indications are defined as poor prognosis and are those predisposing the patient to a higher risk of generating aneuploid embryos (Gianaroli et al., 2003). No poor prognosis indications were present in the remaining 66 cycles (mean female age 34.3 ± 2.4 years, 0.7 ± 0.4 previous cycles, no previous abortions), which represented the control group.
Controlled ovarian stimulation was performed as previously described (Ferraretti et al., 1996, 2004; Fasolino et al., 2007). Oocyte retrieval was performed transvaginally via ultrasonography at 34–36 h after HCG administration and oocytes were cultured in HTF medium (SAGE, CooperSurgical Inc., Pasadena, CA, USA) supplemented with HSA (Human Serum Albumin, SAGE), in a 5% CO2 humidified gas atmosphere at 37°C.
PB biopsy
Approximately 1 h after retrieval, oocytes were denuded by hyaluronidase treatment (40 IU/ml, SAGE) and assessed for morphology and nuclear maturation. The removal of PB1 was performed on metaphase II (MII) oocytes, which were manipulated individually in HEPES-buffered medium supplemented with 10% HSA in 0.1 M sucrose, under pre-equilibrated mineral oil (Magli et al., 2006). The opening of a 20–25 µm slit in the zona pellucida was achieved mechanically by passing a glass microneedle through the perivitelline space tangentially to the oocyte, and repeatedly rubbing the microneedle against the holding pipette. A polished glass pipette (12 µm inner diameter) was introduced into the perivitelline space and the PB was removed. The oocyte was then washed and incubated until the time of insemination in Cleavage medium (SAGE) with 10% HSA.
Fluorescence in situ hybridization
After biopsy, the collected PBs were transferred to water using a glass capillar, fixed with methanol and acetic acid (proportion 3:1) on a glass slide and dehydrated in methanol. For the chromosomal analysis, multicolor FISH was used for the simultaneous testing of chromosomes 13, 15, 16, 18, 21 and 22 (Multivision PB Panel, Vysis Inc., Downers Grove, IL, USA; CEP 15 alpha satellite, Spectrum Orange, Vysis). The probe mixture was hybridized to the fixed PBs for 2 h. The slides were then counterstained in antifade solution (Antifade II, Vysis) and observed under a fluorescence microscope (Olympus BX41, Olympus, Tokio, Japan) equipped with a Ludl filter wheel with the following filter sets: dual band pass filters (Red/Green and Aqua/Blue) and single band pass filters (Red, Green, Yellow, Aqua). Images were captured at ×600 magnification using a CCD PVCAM camera associated with image analysis software (Vysis Quips).
The interpretation of the results was based on the consideration that PB1 is the mirror image of the oocyte, and that it normally consists of two chromatids. For this reason, the detection of double-dotted signals (one dot per chromatid) classified the oocyte as normal. Although the presence of a doublet signal is clear when using locus-specific probes, for centromeric probes fluorescence can be concentrated in a large signal or in a doublet with very close signals. Alternatively, the presence or lack of two additional fluorescent dots indicated that the oocyte lost or gained a chromosome, respectively, whereas the presence or absence of a single-dot signal identified an oocyte with a loss or gain, respectively, of a chromatid. In the last case, the meiotic error was caused by premature separation of sister chromatids (Angell, 1991).
The concomitant occurrence of numerical abnormalities involving three or more chromosomes, or the combination of chromatids and chromosome errors was defined as complex abnormality.
Oocyte insemination, control of oocyte fertilization and embryo development
Insemination was performed by ICSI on the basis of FISH results by introducing the injection needle through the breach already opened in the zona pellucida (Magli et al., 2006). Whenever possible, only chromosomally normal oocytes were inseminated and up to a maximum of three per patient as established by the national law on IVF (Benagiano and Gianaroli, 2004), whereas the remaining chromosomally normal oocytes were cryopreserved.
Fertilization check was performed at 16 h post insemination by evaluating the presence and morphology of pronuclei and second PB (Gianaroli et al., 2007). Normally fertilized oocytes were cultured individually in fresh Cleavage medium (SAGE) supplemented with 10% HSA and scored daily at regular time intervals every 24 h. Number and morphology of nuclei and blastomeres (presence of vacuoles, multinucleation, cytoplasmic darkness or inclusions, uneven size of blastomeres), and the percentage of fragmentation were recorded. On the basis of these observations embryos were graded as 1–4, grade I embryos representing those with normal morphology and development according to the time of observation (Magli et al., 2007).
Embryo transfer and pregnancy outcome
Embryo transfer was normally performed on Day 3, except in those cases where the three fertilized oocytes grew normally. In this case, embryos were cultured to the blastocyst stage with the aim of favoring their natural selection in culture, to avoid the transfer of non-viable embryos that were classified as those that arrested in culture for at least 48 h with clear signs of degeneration.
Clinical pregnancies were defined by the presence at ultrasonography of a gestational sac with fetal heartbeat. The implantation rate was expressed as the ratio between number of gestational sacs with fetal heartbeat and total number of embryos transferred. The delivery rate was calculated per transfer, per cycle and per patient.
FISH on oocytes
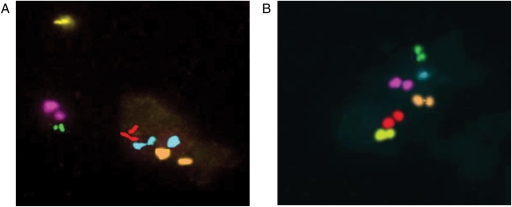
A total of 104 oocytes which had been donated for research by consenting patients were directly tested for the chromosomes 13, 15, 16, 18, 21 and 22. Concomitantly, the same analysis was performed on the corresponding PB1 (Fig. 1). For oocyte fixation, oocytes were incubated for 5–10 min in hypotonic solution (1% sodium citrate) and then fixed with methanol-acetic acid in a proportion 3:1. FISH was performed according to the protocol described above. The study was discussed and approved by our Institutional Review Board.
Figure 1.
FISH analysis with the probes specific for chromosomes 13 (red), 15 (orange), 16 (aqua), 18 (pink), 21 (green) and 22 (yellow). (A) In the first polar body, there are two dots for each signal with the exception of chromosome 16 for which three signals are observed. (B) The corresponding oocyte was analyzed for the same chromosomes. As expected, as the first polar body is the mirror image of the chromosome status of MII oocytes, only one signal for chromosome 16 is detected in the oocytes, whereas two dots are present for the other tested chromosomes. Both images: ×600 magnification.
Statistical analysis
To compare numerical variables in the two groups, data were analyzed in a bivariate analysis by Fisher's exact test and χ2 analysis applying the Yates' correction, 2 × 2 contingency tables.
A multivariate regression analysis corrected for semiquantitative data was performed to investigate the determinants of aneuploidy, expressed as the proportion of euploid oocytes over the number of diagnosed oocytes.
The weight of each different indication to FISH analysis on PBs (FISH–PB) and cause of infertility were estimated by calculation of X variables marginal contribution. Relationships were evaluated for significance using inferential analysis of the regression coefficients of the equation that define relationships.
The multivariate regression analysis among the studied variables and the data of anomalies deriving from chromatid predivison, mixed predivision, chromosomal predivision and aneuploidy of each single chromosome were analyzed by means of stepwise regression analysis. In this way, significances of subgroups were assessed based on the assumption that correlations were regarded as a group and the seven subgroups deriving from it (anomalies of chromatid predivision, mixed predivision, chromosomal predivision and aneuploidies of chromosomes 13, 16, 18, 21 and 22. Chromosome 15 was excluded from the analysis because it was only tested in 2684 oocytes).
To understand whether aneuploidy within the same oocyte is randomly or not randomly generated, the goodness of fit test was applied (Camussi et al., 1995).
Variables were selected at a level risk lower than 5%.
Results
To estimate the efficiency of PB1 testing by FISH and the reliability of PB1 results as predictive of the corresponding oocyte's chromosomal status, 104 oocytes and their corresponding PB1 were fixed and analyzed for the chromosomes 13, 15, 16, 18, 21 and 22. A result was obtained for 99 oocytes, of which 95 gave a result that was compatible with that obtained in the corresponding PB1 (96%). Of the remaining four oocytes that had been diagnosed as abnormal by PB1 analysis, three were apparently normal (3%) and one had a different abnormality (complex abnormality instead of hypohaploidy). For the purpose of this study, the non-concordance of the data from PB1s and the corresponding oocytes were considered to be negligible and the FISH results of PB1s were taken as representative of the chromosomal status of the MII oocytes.
The clinical outcome of the cycles included in the study is described in Table I. A total of 4163 oocytes were biopsied and a diagnosis was obtained in 3816 (92%). In all, 51% (n = 1931) of the oocytes were euploid for the six tested chromosomes, a chromatid error occurred in 36% of them (n = 1386; 13% with a missing chromatid and 23% with an extra chromatid), a chromosome error occurred in 9% of the oocytes (n = 358), with the remaining 4% being of complex origin (n = 141). The overall data regarding the chromosomal status of PB1s in the different patients' categories are detailed in Table II. No differences were detected in the distribution of chromatid or chromosome errors in studied groups.
Table I.
Overall results for clinical outcome of cycles in which chromosomal analysis of the PB1 was performed.
| Age ≥ 38 years | ≥3 IVF cycles | ≥3 abortions | Controls: no indications | Total | |
|---|---|---|---|---|---|
| Cycles | 418 | 202 | 20 | 66 | 706 |
| Patients | 314 | 150 | 15 | 65 | 544 |
| Age (Mean ± SD) | 41.0 ± 2.3 | 34 ± .2 ± 2.5 | 33.9 ± 2.4 | 34.3 ± 2.4 | 38.2 ± 4.1 |
| Previous cycles (Mean ± SD) | 2.0 ± 2.1 | 4.0 ± 1.2 | 0.7 ± 1.0 | 0.7 ± 0.4 | 2.2 ± 2.0 |
| Collected oocytes | 2984 | 1690 | 162 | 601 | 5437 |
| Biopsied oocytes (Mean ± SD) | 2291 (5.5 ± 2.7)a | 1274 (6.3 ± 2.5)b | 121 (6.1 ± 2.3)c | 477 (7.2 ± 2.3)abc | 4163 (5.9 ± 2.6) |
| Diagnosed oocytes | 2093 (91) | 1185 (93) | 109 (90) | 429 (90) | 3816 (92) |
| FISH normal oocytes | 1018 (49)d | 604 (51)e | 63 (58) | 246 (57)de | 1931 (51) |
| Inseminated oocytes (ICSI) | 960 | 533 | 54 | 199 | 1746 |
| Fertilized oocytes | 758 (79) | 422 (79) | 46 (85) | 162 (81) | 1388 (79.5) |
| Generated embryos | 726 (96) | 403 (95) | 45 (98) | 151 (93) | 1325 (96) |
| Transferred cycles | 348 (83)f | 185 (92) | 19 (95) | 63 (96)f | 615 (87) |
| Transferred embryos (Mean ± SD) | 1.9 ± 0.7 | 1.9 ± 0.7 | 2.0 ± 0.6 | 2.1 ± 0.8 | 1.9 ± 0.7 |
| Clinical pregnancies (% per transfer) (% per cycle) (% per patient) | 68 | 50 | 5 (26) | 27 | 150 |
| (19.5)g | (27)h | (25) | (43)gh | (24) | |
| (16)i | (25)j | (33) | (41)ij | (21) | |
| (22)k | (33) | (33) | (41.5)k | (28) | |
| Spontaneous miscarriages | 22* (32)l | 4 (8) | 0 | 4 (15)l | 30 (20) |
| Ectopic pregnancies | 1 | 1 | 0 | 2 | 4 |
| Implantation rate | 76/663 (11.5)m | 57/348 (16.4) | 6/39 (15.4) | 31/133 (23.3)m | 170/1183 (14.4) |
Note: Values are number (percentage) unless otherwise noted. P-values are for comparisons between values with the same superscript letter.
*2 after prenatal diagnosis, normal karyotype.
aP = 0.00001.
bP = 0.009.
cP = 0.049.
dkP < 0.005.
ehP < 0.05.
fjP < 0.025.
gimP < 0.001.
lP = 0.047.
Table II.
Chromosomal status of PB1s in the different categories of patient.
| Age ≥ 38 years | ≥3 IVF cycles | ≥3 abortions | No indications | Total | |
|---|---|---|---|---|---|
| Diagnosed oocytes | 2093 | 1185 | 109 | 429 | 3816 |
| FISH normal oocytes | 1018 (49)a | 604 (51)b | 63 (58) | 246 (57)ab | 1931 (51) |
| FISH abnormal oocytes | 1075 (51)c | 581 (49)d | 46 (42) | 183 (43)cd | 1885 (49) |
| Missing chromatids | 281 (13) | 160 (13) | 7 (6) | 40 (9) | 488 (13) |
| One chromatid | 239 | 144 | 7 | 37 | 427 |
| Two chromatids | 42 | 16 | 0 | 3 | 61 |
| Extra chromatids | 494 (23) | 273 (23) | 29 (27) | 102 (24) | 898 (23) |
| One chromatid | 415 | 228 | 24 | 86 | 753 |
| Two chromatids | 79 | 45 | 5 | 16 | 145 |
| Missing chromosomes | 64 (3) | 22 (2) | 3 (2.5) | 13 (3) | 102 (2.5) |
| One chromosome | 47 | 18 | 3 | 3 | 71 |
| Two chromosomes | 17 | 4 | 0 | 10 | 31 |
| Extra chromosome | 140 (7) | 91 (8) | 3 (2.5) | 22 (5) | 256 (6.5) |
| One chromosome | 121 | 79 | 1 | 18 | 219 |
| Two chromosomes | 19 | 12 | 2 | 4 | 37 |
| Complex abnormalities | 96 (5) | 35 (3) | 4 (4) | 6 (2) | 141 (4) |
acP < 0.005.
bdP < 0.05.
FISH: fluorescence in situ hybridization.
Note: Values are number (percentage) unless otherwise noted.
After ICSI (Table I), normal fertilization occurred in 79.5% of the 1746 inseminated oocytes which originated 1325 embryos (96%). Embryo transfer was performed in 615 cycles yielding 150 clinical pregnancies (24% per transferred cycle) with an implantation rate of 14.4%.
In the group of patients with advanced maternal age, the proportion of euploid oocytes was lower when compared with the controls (49 versus 57%, P < 0.005), and this resulted, in combination with a reduced number of biopsied mature oocytes (5.5 versus 7.2 in the controls, P = 0.00001) in a decreased percentage of transferred cycles (83 versus 96%, P < 0.025). The clinical pregnancy rate was significantly lower when calculated per transfer (19.5 versus 43%, P < 0.001), per oocyte retrieval (16 versus 41%, P < 0.001) and per patient (22 versus 41.5%, P < 0.005). The implantation rate followed the same trend (11.5 versus 23.3%, P < 0.001), whereas the incidence of abortions was higher in the older age group (32%) compared with the controls (15%, P = 0.047).
Regarding patients with more than three IVF cycles, significant differences with the control group were detected in the proportion of mature (6.3 ± 2.5 versus 7.2 ± 2.3, P = 0.009) and chromosomally normal oocytes (51 versus 57%, P < 0.05), as well as in the clinical pregnancy rate when calculated per transfer (27 versus 43%, P < 0.05) and per oocyte retrieval (25 versus 41%, P < 0.025).
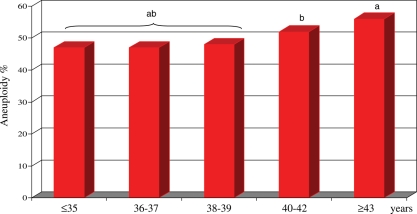
As shown in Fig. 2, the distribution of chromosomal abnormalities was age dependent, with the highest proportion detected in patients aged ≥43 years (56%). A significant increase in the proportion of aneuploid oocytes started at 40 years with an incidence of 52 versus 48% at younger ages (P < 0.01).
Figure 2.
Incidence of aneuploidy in 3816 FISH diagnosed oocytes, in relation to maternal age. The distribution of chromosomal abnormalities was age dependent with a significant increase in the proportion of aneuploid oocytes starting at 40 years (incidence of 52% in the age of 40–42 years, and of 56% at older ages versus 48% at younger ages; P < 0.01). aP < 0.001, bP < 0.01.
To evaluate the variables that could be associated with the proportion of euploid oocytes calculated over the number of diagnosed oocytes, the following dependent variables (x) were analyzed in a multivariate regression analysis: female age, type of stimulation (use of GnRH agonist or antagonist), use of clomiphene citrate, cause of infertility, indication to PGD, number of retrieved oocytes, number of mature oocytes, units of FSH per oocyte, units of FSH per mature oocyte and establishment of a clinical pregnancy.
As reported in Table III, there was a direct and significant correlation between the proportion of normal oocytes and (i) the number of mature oocytes and (ii) the establishment of a clinical pregnancy. An inverse and significant correlation was found between the proportion of normal oocytes and (i) female age, (ii) causes of female infertility (endometriosis, abortions, ovulatory factor), (iii) indications to FISH–PB (female age, number of previous cycles, multiple indications to FISH–PB), (iv) number of FSH units per oocyte and (v) number of FSH units per MII oocyte. No significant correlation was found between the proportion of normal oocytes and (i) the type of stimulating cycle (agonist or antagonist), (ii) the use of clomiphene, (iii) the presence of male infertility, tubal infertility or idiopathic infertility, (iv) the absence of indications to FISH–PB and (v) the number of collected oocytes.
Table III.
Relationships between the proportion of normal oocytes calculated over the number of diagnosed oocytes and the following variables: female age, type of stimulation (GnRH agonist or antagonist), use of clomiphene (yes or no), causes of infertility (male, endometriosis, idiopathic, tubal, ovarian, recurrent abortions), indication to FISH–PB (female age, abortions, previous failed cycles, no indication, multiple indications), number of collected oocytes, number of collected MII oocytes, number of FSH international units per oocyte, number of FSH international units per MII oocyte, clinical pregnancy.
| Relationship | Level of intersection with the Y ordinate axis | Regression coefficients | Significance of each regression coefficients of dependent variables |
|||
|---|---|---|---|---|---|---|
| Determining factors (t) | pρ | |||||
|
Independent variable (predictor) | Normal oocytes/diagnosed oocytes | −9115 | |||
| Dependent variables | Female age | −11.130 | 89.001 | <0.01 | ||
| Type of stimulation (agonist/antagonist) | 0.478 | 1.244 | NS | |||
| Clomiphene (yes/no) | −0.654 | 0.112 | NS | |||
| Cause of infertility: male | 0.111 | 0.112 | NS | |||
| Cause of infertility: endometriosis | −12.223 | 10.117 | <0.01 | |||
| Cause of infertility: idiopathic | 1.212 | 2.117 | NS | |||
| Cause of infertility: tubal | 0.017 | 1.238 | NS | |||
| Cause of infertility: ovarian | −7.116 | 9.118 | <0.01 | |||
| Cause of infertility: abortions | −11.878 | 8.118 | <0.01 | |||
| Indication to FISH–PB: female age | −4.144 | 11.320 | <0.01 | |||
| Indication to FISH–PB: abortions | −5.116 | 8.115 | <0.01 | |||
| Indication to FISH–PB: number of previous cycles | −1.134 | 5.091 | <0.05 | |||
| No indication to FISH–PB | 0.011 | 0.114 | NS | |||
| Multiple indications to FISH–PB | −2.119 | 12.338 | <0.01 | |||
| Number of collected oocytes | −0.012 | 0.002 | NS | |||
| Number of collected MII oocytes | 9.678 | 11.167 | <0.01 | |||
| Number of FSH units/oocyte | −8.156 | 9.118 | <0.01 | |||
| Number of FSH units/MII oocyte | −8.556 | 9.113 | <0.01 | |||
| Clinical pregnancy | 6.445 | 9.667 | <0.01 | |||
NS, not significant; FISH–PB, chromosomal analysis of PB by FISH; IU, international Units; MII oocyte, metaphase II oocyte.
The correlation between the studied variables and the type of chromosome anomalies (type of mechanism generating aneuploidy and type of chromosome involved) was analyzed by means of stepwise regression analysis (Table IV). According to these data, there was a weak significance of frequency (P < 0.05, but lost at P < 0.01) between type of abnormality (originated by chromatid predivision, chromosome non-disjunction or combined mechanisms in the same oocyte) and groups of the studied variables, rather than to a specific abnormality or a specific chromosome. The same was true for the frequency of aneuploidy specifically occurring for each chromosome. In addition, the goodness of fit test indicated that aneuploidies were not randomly distributed within the same oocyte.
Table IV.
Stepwise regression analysis of the studied variables in regard of independent variable (Normal oocytes/diagnosed oocytes [predictor],[Y]): pρ = P < 0.01.
| Subgroups | Number of anomalies | Number highly significant (P < 0.01) relationships in regard of Y | Number of significant relationships (P < 0.05) in regard of Y | Not significant relationships in regard of Y |
|---|---|---|---|---|
| Chromatid predivision errors | 1386 | 0 | 5 | 12 |
|
|
|||
| Combined chromatid and chromosome errors | 141 | 0 | 0 | 17 |
|
||||
| Chromosome non-disjunctions | 358 | 0 | 3 | 14 |
|
|
|||
| Chromosome 13 | 409 | 0 | 3 | 14 |
|
|
|||
| Chromosome 16 | 520 | 0 | 4 | 13 |
|
|
|||
| Chromosome 18 | 377 | 0 | 3 | 14 |
|
|
|||
| Chromosome 21 | 835 | 0 | 5 | 12 |
|
|
|||
| Chromosome 22 | 537 | 0 | 4 | 13 |
|
|
FISH–PB, chromosomal analysis of polar body by fluorescence in situ hybridization; IU, international Units; MII oocyte, metaphase II oocyte.
Discussion
Preconception genetic diagnosis is the earliest form of preimplantation diagnosis and is generally performed by removing the first and second PB in order to have the whole view of the meiotic process in the oocyte (Verlinsky et al., 1990; Verlinsky et al., 1997; Kuliev et al., 2003, 2005). This strategy could not be adopted in the present study owing to the legal restrictions in Italy, according to which no more than three embryos could be generated, with any type of embryo (or zygote) selection being prohibited (Benagiano and Gianaroli, 2004).
Regardless of this limitation, the analysis of PB1 has been routinely used as an additional tool to guide the selection of the three oocytes to be inseminated, in combination with their morphological assessment (Magli et al., 2006; Gianaroli et al., 2007). As presented in Table I, the rates of fertilization, embryo development and implantation were within the normal range, suggesting that PB1 biopsy is not detrimental for oocyte development. If this technique has the final result of improving the oocyte selection, a clinical advantage should be the expected. Actually, data from a prospective randomized study indicated that the selection of euploid oocytes is associated with a reduced abortion rate (Ferraretti et al., 2006).
As expected, the incidence of aneuploidy tended to vary in the different categories of patients. When compared with a control group, patients of advanced maternal age presented the highest level of aneuploidy in oocytes, followed by couples with repeated IVF failures, showing significantly higher values. According to these results, a tendency to chromosomal errors seemed to be related to some conditions of infertility, whereas the figure of 43% aneuploidy in the oocytes from the control group could represent the background level of aneuploidy for the six studied chromosomes in human oocytes derived from stimulated cycles. It is important to keep in mind that numerical chromosomal abnormalities are detected at considerable levels even in embryos generated from natural cycles (36% according to Verpoest et al., 2008), suggesting that the frequent occurrence of aneuploidy could be typical of the human species.
At this point, it was logical to inquire whether the type of infertility, the type of stimulation and the quality of patients' response to gonadotrophins could have an effect on the resumption of oocyte meiosis. In agreement with a previous study (Fasolino et al., 2007), the type of stimulation did not correlate with aneuploidy, but the type of infertility had a significant effect on meiotic errors, whose incidence was significantly higher in the presence of endometriosis or of an ovulatory factor, and in women that experienced repeated abortions (Table III). The hypothesis that oocyte quality might be hampered in women with endometriosis is not new, but no general consensus has been reached on this point (Garcia-Velasco and Arici, 1999). In a recent work based on an experimental model, the effect of endometriosis on oocyte quality was demonstrated to be at the level of the cytoskeleton (Mansour et al., 2010). The authors suggested that endometriosis negatively affects the meiotic spindle and the chromosomes, and this is in agreement with the findings from this study pointing to a link between endometriosis and the formation of aneuploid gametes.
The strong negative correlation between the presence of an ovulatory factor and the proportion of aneuploid oocytes suggest that these ovaries undergo a sort of biological ageing process. Surprisingly, the results coming from the chromosomal analysis of preimplantation embryos do not seem to confirm these findings (Weghofer et al., 2007). It could be postulated that even though the hormonal environment in the ovaries of these patients could predispose to meiotic errors, the high rates of mosaicism characteristic of preimplantation embryos could disguise the oocyte chromosomal status.
Finally, the predisposition in women with a history of previous abortions to generate aneuploid oocytes could result from a sort of biological ageing, a condition for which the hypothesis of the ‘limited oocyte pool’ was formulated (Warburton, 1989; Kline et al., 2000). According to this theory, the age effect might be related to the relative scarcity of oocytes at optimal stages of maturation. This conclusion was based on the observation that women with a trisomic pregnancy entered menopause about 1 year earlier than did those in the control group. As a result, a high incidence of aneuploid oocytes in patients with recurrent miscarriages was found in this study (Table III) and this is also in agreement with the report of high rates of aneuploidy in embryos from young patients with previous aneuploid conceptions (Munné et al., 2004).
The type of response to hormonal stimulation was strictly related to aneuploidy as expressed by the number of mature oocytes and, more precisely, by the number of FSH IU that were necessary to generate an oocytes (Table III). More importantly, a significant inverse correlation was also present between incidence of aneuploidy and clinical pregnancy rate. These data suggest that the retrieval of mature oocytes in response to a limited amount of gonadotrophins is associated with a lower risk of aneuploidy, implying that the quality of response to the hormonal stimulation reflects how the recruited follicles could support oocyte growth and maturation, with the consequent generation of more or less competent oocytes (Fragouli et al., 2009). On the other hand, some data indicate that in cases of very aggressive stimulations, the frequency of aneuploidy in oocytes can be very high even in young patients (Sher et al., 2007), suggesting that results can be distorted when the experimental conditions are too far from the physiological levels. These considerations contributed to the motivation to return to milder ovarian stimulation, that was proven to be associated with a reduced frequency of embryo aneuploidy (Baart et al., 2007).
According to the data reported in this study, premature chromatid separation was by far the most frequent cause of PB1 abnormality. This is in agreement with other studies on PBs by preconception genetic diagnosis (Kuliev and Verlinsky, 2004; Kuliev et al., 2005; Vialard et al., 2006) and CGH (Gutierrez-Mateo et al., 2004), but it is in contrast with the data derived from direct oocyte conventional analysis and from DNA polymorphism (Rosenbusch, 2006). A possible explanation of this inconsistency could reside in the fact that premature chromatid separation seems to be especially related to smaller chromosomes, including chromosomes 13, 15, 16, 18, 21 and 22 (Fragouli et al., 2006). The reliability of FISH diagnosis on PB1 can also be questioned, with the possibility that the error rate on a single cell could jeopardize the conclusions. Nevertheless, the concordance between oocytes and corresponding PB1 was found to be 96%, and this is in agreement with the figure reported by Weier et al. (2005) who found a 92% concordance by using FISH and Spectral Imaging Analysis on 25 oocytes and their PB1. Such a small error rate cannot dramatically affect the results and the consequent conclusions.
Studies using CGH for the analysis of PBs have demonstrated that aneuploidies affect all chromosomes, but those equal or smaller than chromosome 13 are more frequently involved in aneuploid events (Fragouli et al., 2010). As represented in Table IV, the search for preferential susceptibility of single chromosome aneuploidy in relation to different variables demonstrated that the effects of chromosome aberrations were more linked to a generalized oocyte meiotic abnormality rather than to a specific abnormality.
The significance of the associations between the studied variables (patients' characteristics) regarded the typology of the variable (chromosome or chromatid error) and number of the significant variables in the different groups of chromosomal abnormalities, which occurred repeatedly in the different studied categories. In addition, a highly significant correlation was shown between number of chromosomal abnormalities and number of significant correlations. On the basis of two considerations, it can be concluded that the correlation with the studied variables does not depend on the type, but on the frequency of a chromosomal alteration. In other words, the mechanisms generating aneuploidy and the aneuploidy frequency of a single chromosome does not depend on a specific variable (age, for example), but on groups of variables. Therefore, no specific type of aneuploidy is associated with any of the female infertility indications, such as age, endometriosis or an ovarian factor, suggesting that chromosomal errors seem to be linked to a generalized oocyte disorganization rather than to a single/specific oocyte alteration. These considerations might help to explain the contradictory results derived from the FISH analysis of preimplantation embryos when a bivariate analysis is performed.
It was also seen that chromosomal abnormalities tended to be combined and occur simultaneously, suggesting that if the mechanisms entering the meiotic process are malfunctioning, deriving aneuploidies can be of any type. Furthermore, as demonstrated by the goodness of fit test, aneuploidies were not randomly distributed within the same oocyte, suggesting that aneuploidies are probably caused by a dysfunction of the meiotic spindle rather than by the shape and features of the chromosomes. The physiological implications of this condition are unknown but other studies in sperm cells and in preimplantation embryos have reported that chromosomes have a defined, non-random localization within the nucleus, suggesting that the organization of the genome could be functionally important (Foster et al., 2005; Mudrak et al., 2005; Diblik et al., 2007).
In conclusion, despite the high frequency and clinical relevance of aneuploidy in humans, surprisingly little is known about factors that modulate the risk of meiotic non-disjunction, the only factor incontrovertibly linked to human aneuploidy being represented by increasing maternal age. The mechanisms underlying the predisposition to aneuploidy still need to be elucidated, but biological, and not chronological, ageing are probably involved (Hassold and Hunt, 2001). There is increasing evidence supporting the fact that each aneuploidy event is dependent not on a specific variable, but on groups of variables. In addition, the tendency of chromosomal abnormalities to occur simultaneously implies that the deriving aneuploidies can be of any type. More comprehensive information will certainly result from the analysis of the complete chromosomal set in oocytes and embryos (Geraedts et al., 2010).
The findings of the present study confirm that in many cases a poor prognosis condition is associated with chromosome abnormalities, which contributes an additional hurdle to the complex issue of female infertility.
Authors' roles
L.G. formulated the study design and participated in manuscript drafting and critical discussion; C.M. participated in study design, execution, analysis, manuscript drafting and critical discussion; G.C. participated in study analysis, manuscript drafting and critical discussion; A.C. participated in study execution; A.C. participated in study execution; S.R. participated in study execution; F.R. participated in study execution; A.P.F. participated in study design and critical discussion.
Acknowledgements
The authors thank Carlo Cetera, M.D., Director of the Gynecological Department at the Hospital Pieve di Cadore, Italy, for his significant contribution to patient recruitment and care.
References
- Angell RR. Predivision of human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet. 1991;86:383–387. doi: 10.1007/BF00201839. [DOI] [PubMed] [Google Scholar]
- Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. doi:10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Gianaroli L. The new Italian IVF legislation. Reprod Biomed Online. 2004;9:117–125. doi: 10.1016/s1472-6483(10)62118-9. [DOI] [PubMed] [Google Scholar]
- Camussi A, Moller F, Ottaviano E, Gorla M. Statistical metods for experimental biology. 1995 Zanichelli, Bologna, Italy. [Google Scholar]
- Diblik J, Macek M, Sr, Magli MC, Krejci R, Gianaroli L. Chromosome topology in normal and aneuploid blastomeres from human embryos. Prenat Diagn. 2007;27:1091–1099. doi: 10.1002/pd.1834. doi:10.1002/pd.1834. [DOI] [PubMed] [Google Scholar]
- Fasolino MC, Ferraretti AP, Farfalli VI, Feliciani E, Arcarese M, Gianaroli L, Magli MC. Incidence of oocyte aneuploidy by first polar body FISH analysis in relation to the type of GnRH analogue. Hum Reprod. 2007;22(Suppl. 1):i82. [Google Scholar]
- Ferraretti AP, Magli MC, Feliciani E, Montanaro N, Gianaroli L. Relationship of timing agonist administration in the cycle phase to the ovarian response to gonadotropins in the long-down regulation protocols for assisted reproductive technologies. Fertil Steril. 1996;65:114–121. doi: 10.1016/s0015-0282(16)58037-6. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Gianaroli L, Magli MC, D'Angelo A, Farfalli V, Montanaro N. Exogenous LH in COH for ART: when and which? Fertil Steril. 2004;82:1521–1526. doi: 10.1016/j.fertnstert.2004.06.041. doi:10.1016/j.fertnstert.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Gianaroli L, Magli MC, Mattioli M, Cetera C, Feliciani E. Selecting oocytes for insemination by first polar body biopsy. Hum Reprod. 2006;21(Suppl. 1):i5–i6. [Google Scholar]
- Fishel S, Gordon A, Lynch C, Dowell K, Ndukwe G, Kelada E, Thornton S, Jenner L, Cater E, Brown A, et al. Live birth after polar body array comparative genomic hybridization prediction of embryo ploidy-the future of IVF? Fertil Steril. 2010;93:1006.e7–1006.e10. doi: 10.1016/j.fertnstert.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Foster HA, Abeydeera LR, Griffin DK, Bridger JM. Non random chromosome positioning in mammalian sperm nuclei, with migration of the sex chromosomes during late spermatogenesis. J Cell Sci. 2005;118:1811–1820. doi: 10.1242/jcs.02301. doi:10.1242/jcs.02301. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Wells D, Thornhill A, Serhal P, Faced MJW, Harper JC, Delhanty JDA. Comparative genomic hybridization analysis of human oocytes and polar bodies. Hum Reprod. 2006;21:2319–2328. doi: 10.1093/humrep/del157. doi:10.1093/humrep/del157. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Escalona A, Gutierrez-Mateo C, Tormasi S, Alfarawati S, Sepulveda S, Noriega L, Garcia J, Wells D, Munné S. Comparative genomic hybridization of oocytes and first polar bodies from young donors. Reprod Biomed Online. 2009;19:228–237. doi: 10.1016/s1472-6483(10)60078-8. [DOI] [PubMed] [Google Scholar]
- Fragouli E, Katz-Jaffe M, Alfarawati S, Stevens J, Colls P, Goodall N, Tormasi S, Gutierrez-Mateo C, Prates R, Schoolcraft WB, et al. Comprehensive chromosome screening of polar bodies and blastocysts from couples experiencing repeated implantation failure. Fertil Steril. 2010;93:1006.e7–1006.e10. doi: 10.1016/j.fertnstert.2009.04.053. [DOI] [PubMed] [Google Scholar]
- Garcia-Velasco JA, Arici A. Is the endometrium or oocyte/embryo affected in endometriosis? Hum Reprod. 1999;14:77–89. doi: 10.1093/humrep/14.suppl_2.77. [DOI] [PubMed] [Google Scholar]
- Geraedts J, Collins J, Gianaroli L, Goossens V, Handyside A, Harper J, Montag M, Repping S, Schmutzler A. What next for preimplantation genetic screening? A polar body approach! Hum Reprod. 2010;25:575–577. doi: 10.1093/humrep/dep446. doi:10.1093/humrep/dep446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Fiorentino F, Baldi M, Ferraretti AP. Clinical Value of Preimplantation Genetic Diagnosis. Placenta. 2003;24:77–83. doi: 10.1016/s0143-4004(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Ferraretti AP. Sperm and blastomere aneuploidy detection in reproductive genetics and medicine. J Histochem Cytochem. 2005;53:261–268. doi: 10.1369/jhc.4B6434.2005. doi:10.1369/jhc.4B6434.2005. [DOI] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Ferraretti AP, Lappi M, Borghi E, Ermini B. Oocyte euploidy, pronuclear zygote morphology and embryo chromosomal complement. Hum Reprod. 2007;22:241–249. doi: 10.1093/humrep/del334. doi:10.1093/humrep/del334. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mateo C, Benet J, Wells D, Colls P, Bermudez MG, Sanchez-Garcia JF, Egozcue J, Navarro J, Munné S. Aneuploidy study of human oocytes first polar body comparative genomic hybridization analysis. Hum Reprod. 2004;19:2859–2868. doi: 10.1093/humrep/deh515. doi:10.1093/humrep/deh515. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. doi:10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hassold T, Abruzzo M, Adkins K, Griffin D, Merril M, Millie E, Saker D, Shen J, Zaragoza M. Human aneuploidy: incidence origin etiology. Environ Mol Mutagen. 1996;28:167–175. doi: 10.1002/(SICI)1098-2280(1996)28:3<167::AID-EM2>3.0.CO;2-B. doi:10.1002/(SICI)1098-2280(1996)28:3<167::AID-EM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16:203–208. doi: 10.1093/hmg/ddm243. doi:10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- Kline J, Kinney A, Levin B, Warburton D. Trisomic pregnancy and earlier age at menopause. Am J Hum Genet. 2000;67:395–404. doi: 10.1086/303009. doi:10.1086/303009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliev A, Verlinsky Y. Meiotic and mitotic non-disjunction: lessons from preimplantation genetic diagnosis. Hum Reprod Update. 2004;10:401–407. doi: 10.1093/humupd/dmh036. doi:10.1093/humupd/dmh036. [DOI] [PubMed] [Google Scholar]
- Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online. 2003;6:54–59. doi: 10.1016/s1472-6483(10)62055-x. [DOI] [PubMed] [Google Scholar]
- Kuliev A, Cieslak J, Verlinsky Y. Frequency and distribution of chromosome abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:193–198. doi: 10.1159/000086889. doi:10.1159/000086889. [DOI] [PubMed] [Google Scholar]
- Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86:629–635. doi: 10.1016/j.fertnstert.2006.02.083. doi:10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development is dependent on the chromosomal complement. Fertil Steril. 2007;87:534–541. doi: 10.1016/j.fertnstert.2006.07.1512. doi:10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- Mansour G, Sharma RK, Agarwal A, Falcone T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2009.09.043. (in press) [DOI] [PubMed] [Google Scholar]
- Mudrak O, Tomilin N, Zalenski A. Chromosome architecture in the decondensing human sperm nucleous. J Cell Sci. 2005;118:4541–4550. doi: 10.1242/jcs.02581. doi:10.1242/jcs.02581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné S, Sepulveda S, Balmaceda J, Fernandez E, Fabres C, Makenna A, Lopez T, Crosby JA, Zegers-Hochschild F. Selection of the most common chromosome abnormalities in oocytes prior to ICSI. Prenat Diagn. 2000;20:582–586. doi: 10.1002/1097-0223(200007)20:7<582::aid-pd872>3.0.co;2-3. doi:10.1002/1097-0223(200007)20:7<582::AID-PD872>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Munné S, Sandalinas M, Magli MC, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenat Diagn. 2004;24:638–643. doi: 10.1002/pd.957. doi:10.1002/pd.957. [DOI] [PubMed] [Google Scholar]
- Rosenbusch B. The contradictory information on the distribution of non-disjunction and pre-division in female gametes. Hum Reprod. 2006;21:2739–2742. doi: 10.1093/humrep/del122. doi:10.1093/humrep/del122. [DOI] [PubMed] [Google Scholar]
- Sher G, Keskintepe L, Keskintepe M, Ginsburg M, Maassarani G, Yakut T, Baltaci V, Kotze D, Unsal E. Oocyte karyotyping by comparative genomic hybridization provides a highly reliable method for selecting ‘competent’ embryos, markedly improving in-vitro fertilization outcome: a multiphase study. Fertil Steril. 2007;87:1033–1040. doi: 10.1016/j.fertnstert.2006.08.108. doi:10.1016/j.fertnstert.2006.08.108. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Ginsberg N, Lifchez A, Valle J, Moise J, Strom CM. Analysis of the first polar body: preconception genetic diagnosis. Hum Reprod. 1990;5:826–829. doi: 10.1093/oxfordjournals.humrep.a137192. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Rechitsky S, Cieslak J, Ivakhnenko V, Wolf G, Lifchez A, Kaplan B, Moise J, Walle J, White M, et al. Preimplantation diagnosis of single gene disorders by two-step oocyte genetic analysis using first and second polar body. Biochem Mol Med. 1997;62:182–187. doi: 10.1006/bmme.1997.2635. doi:10.1006/bmme.1997.2635. [DOI] [PubMed] [Google Scholar]
- Verlinsky Y, Cieslak J, Ivakhnenko V, Evsikov S, Wolf G, White M, Lifchez A, Kaplan B, Moise J, Valle J, et al. Prevention of age-related aneuploidies by polar body testing of oocytes. J Assist Reprod Genet. 1999;16:165–169. doi: 10.1023/A:1020304621338. doi:10.1023/A:1020304621338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpoest W, Fauser BC, Papanikolaou E, Staessen C, Van Landuyt L, Donoso P, Tournaye H, Liebaers I, Devroey P. Chromosomal aneuploidy in embryos conceived with unstimulated cycle IVF. Hum Reprod. 2008;23:2369–2371. doi: 10.1093/humrep/den269. doi:10.1093/humrep/den269. [DOI] [PubMed] [Google Scholar]
- Vialard F, Petit C, Bergere M, Molina Gomes D, Martel-Petit V, Lombroso R, Ville Y, Gerard H, Selva J. Evidence of a high proportion of premature unbalanced separation of sister chromatids in the first polar bodies of women of advanced age. Hum Reprod. 2006;21:1172–1178. doi: 10.1093/humrep/dei484. doi:10.1093/humrep/dei484. [DOI] [PubMed] [Google Scholar]
- Warburton D. The effect of maternal age on the frequency of trisomy: change in meiosis or in utero selection? Prog Clin Biol Res. 1989;311:165–181. [PubMed] [Google Scholar]
- Weier HUG, Weier JF, Renom MO, Zheng X, Colls P, Nureddin A, Pham CD, Chu LW, Racowsky C, Munné S. Fluorescence in situ hybridization and spectral imaging analysis of human oocytes and first polar bodies. J Histochem Cytochem. 2005;53:269–272. doi: 10.1369/jhc.4B6391.2005. doi:10.1369/jhc.4B6391.2005. [DOI] [PubMed] [Google Scholar]
- Weghofer A, Munné S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88:900–905. doi: 10.1016/j.fertnstert.2006.12.018. doi:10.1016/j.fertnstert.2006.12.018. [DOI] [PubMed] [Google Scholar]