Abstract
The value assigned to aversive events is susceptible to contextual influences. Here, we asked whether a change in the valuation of negative events is reflected in an altered neuronal representation of their expected aversive outcome. We show that experiencing an aversive event in the past, and choosing to experience it in the future, reduces its aversive value. This psychological change is mirrored in an altered neural representation of aversive value in the caudate nucleus and anterior cingulate cortex. Our findings indicate that subcortical regions known to track expected value such as the caudate nucleus, together with anterior cingulate cortical regions implicated in emotional modulation, mediate a revaluation in expectancies of aversive states. The results provide a striking example of a contextual sensitivity in how the brain ascribes value to events, in a manner that may foster resilience in the face of adversity.
Introduction
Imagine you are at the dentist having your annual checkup when a rotten tooth is discovered. You are now faced with two options: Either the tooth can be pulled or left to rot until it (hopefully) falls out on its own. Both alternatives involve expectations of pain, but after careful consideration you commit to the first option. Does the mere act of making this decision alter your expected aversive outcome from the selected and rejected options? Furthermore, will experiencing the unwanted event, in this case having your tooth pulled, change how you perceive its aversive value? If the answer to both questions is affirmative, then a critical unanswered question is whether, and how, these reevaluations are reflected by changes in the representation by the brain of the expected aversive outcomes.
There is good reason to hypothesize that both past experience, and choice, facilitate a reassessment of the impact of unwanted events. Before becoming ill, we tend to view sickness and disability as states to be avoided, a disposition that is adaptive insofar as it motivates us to shun hardship and avoid unnecessary danger. However, once adversity becomes a reality an overly negative valuation may no longer be in our interest, and there is good evidence to indicate that we perceive such circumstance as less negative than before (Ubel et al., 2005a,b). In fact, across a range of medical conditions, patients report a significantly higher quality of life and well being than predictions derived from the appraisals of the same events from otherwise healthy individuals (Ubel et al., 2005b), a phenomenon known as the “disability paradox” (Albrecht and Devlieger, 1999). One influential account of this phenomenon is that the inescapable reality of many aversive states motivates a rapid reevaluation (Gilbert, 2006).
A striking aspect in reevaluation of negative events is their facilitation by contexts where individuals believe they were instrumental in their occurrence. For example, participants tend to perceive their environment as less intimidating, estimating distance to be traveled as shorter, a hill to be climbed as less steep, if they believe they themselves had selected the task (Balcetis and Dunning, 2007). Indeed, we have recently showed that the act of choosing between positive events (vacation destinations) modulates the expected hedonic outcome of those events, a change that is tracked by caudate nucleus activity (Sharot et al., 2009a). Whether the biological representation of the expected value for an aversive event is also altered by choice is unknown.
We reasoned that, even if the cognitive mechanisms mediating reassessment of negative events that befall us are different than those that we choose ourselves, both are likely to result in a change of the neural representations of expected aversive value. To investigate this hypothesis, we obtained behavioral and functional magnetic resonance imaging (fMRI) data while participants imagined, and predicted, their emotional reactions to medical conditions (e.g. broken nose, kidney stones, deafness) both before and after hypothetically choosing the “lesser of two evils,” and indicated how often they had experienced each illness in the past.
Materials and Methods
Participants
Fourteen volunteer participants were recruited through posted advertisements. One participant was eliminated from the analysis because of an excessive number of trials with no response (>25%). A second participant was eliminated because of a technical error that resulted in partial loss of MRI data, leaving 12 participants in the analysis (males, 6; females, 6; age range, 18–40) (for similar sample sizes, see Phelps et al., 2004; Delgado et al., 2005; Daw et al., 2006; Kable and Glimcher, 2007; Hasson et al., 2008). All participants gave informed consent and were paid for participation. The study was approved by the Institute of Neurology (University College London) Research Ethics Committee.
Stimuli
Stimuli consisted of 80 names of medical conditions (e.g., broken arm, measles, gum disease). Conditions were rated by three independent medical doctors for severity on a scale from 1 (least severe) to 6 (very severe). Doctors were instructed to take into account the likelihood of mortality and morbidity, effect on quality of life, and tolerability of treatments. The interjudge reliability among the doctors was good (Cronbach's α = 0.94). The order in which stimuli were presented was random.
Procedure
Overview
Before the scanning session, participants underwent four practice trials. The session began with a short structural scan, followed by three functional scan sessions (scan 1, prechoice imagining and rating task; scan 2, choice task; scan 3, postchoice imagining and rating task) and an additional longer structural scan. After the scanning session, participants filled out a postscan questionnaire.
Scanning sessions 1 and 3 (imagining and rating task)
Scanning sessions 1 and 3 (imagining and rating task) were 14 min 40 s each and consisted of 80 trials of 11 s. On each trial, a name of a medical condition appeared on screen for 6 s presented via a mirror mounted on the head coil. The participants were instructed to imagine having that condition in 1 year's time. The participant then had 2 s to provide an estimate of how they would feel if they were to have that medical condition (1, neutral; 2, a bit unhappy; 3, unhappy; 4, very unhappy; 5, extremely unhappy). Responses were made using a button box placed in their right hand. If the participant did not respond within the 2 s window, the trial was excluded from data analysis. Finally, a fixation cross was presented for 3 s.
Scanning session 2 (choice task)
Scanning session 2 (choice task) was 6 min long and consisted of 40 trials of 9 s each. On each trial, two names of medical conditions from session 1 appeared on screen side by side for 4 s. Then the word “choose” appeared on screen above the two options for 2 additional seconds. The participants were instructed to indicate which medical condition they would rather avoid and which they would rather have, if they had to have one of the two conditions over the next year, by pressing a button to indicate the condition to be avoided. After making a response, a star symbol appeared next to the rejected medical condition (the one to be avoided). Finally, a fixation cross was presented for 3 s.
Pairs of stimuli were determined by a Matlab program used previously (Sharot et al., 2009a,b) such that more than two-thirds of the trials included two options that were rated the same in session 1 (the critical condition), and the rest (less than one-third of the trials) included two options that were rated differently in session 1 (the noncritical condition). Each stimulus appeared in only one pair. The number of pairs in the critical condition (mean, 26.85; range, 20–31) was more than double than in the noncritical condition (mean, 11.08; range, 8–15) to maximize the power of finding differences prechoice and postchoice in this condition of interest. In the behavioral and fMRI analysis of the effects of choice on expected aversive outcome, only data from the critical condition are included. Only behavioral data from this session were used in data analysis. Specifically, the choices made during this session were used to classify the trials in scans 1 and 3 into trials of subsequently (and previously) rejected and subsequently (and previously) selected stimuli.
Postscanning questions
After the scanning session, participants were asked to rate all stimuli on five scales: past experience (How many times have you had this condition before? From 0 = never to 6 = almost all the time); last experience (When was the last time you had this condition? From 0 = never to 6 = this week); familiarity (How familiar do you feel this condition is to you regardless if you had it before? From 1 = low to 6 = high); vividness (When you imagine having this condition, how vivid is your image? From 1 = low to 6 = high); and arousal (When you imagine having this condition, how emotionally arousing is your image? From 1 = low to 6 = high).
MRI scanning
The study was conducted at the Wellcome Trust Center for Neuroimaging at University College London using a 3T Siemens Allegra scanner equipped with a Siemens head coil. Anatomical images were acquired using magnetization-prepared rapid-acquisition gradient echo scans, which were followed by 1-mm-thick axial slices parallel to the anterior commissure–posterior commissure plane. Functional scans used a gradient echo sequence; repetition time, 2.7 s; echo time, 30 ms; flip angle, 90; matrix, 64 × 64; field of view, 192 mm; and slice thickness, 2 mm. A total of 42 axial slices (−45° tilt) were sampled for whole-brain coverage. The in-plane resolution was 3 × 3 mm.
Imaging data were analyzed for the rating sessions (scans 1 and 3). Statistical parametric mapping (SPM5; Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) was used to analyze the fMRI data. Images were realigned with the first volume (after discarding the first six dummy volumes) and unwarped, normalized to a standard echo-planar imaging template based on the Montreal Neurological Institute reference brain, resampled to 2 × 2 × 2 mm3 voxels, and spatially smoothed (8 mm full width at half-maximum).
Data analysis
Effect of past experience on expected aversive outcome
Behavioral analysis.
Postscanning questionnaire scores for each participant, as well as independent severity ratings of medical conditions (see above, Stimuli), were entered into two linear regressions. Aversive ratings from the prechoice scan were entered as the dependent measure in one linear regression, and aversive ratings from the postchoice scan in another. This analysis allows us to examine the effect of past experience on aversive expectancies while controlling for all other variables. A t test was conducted on standardized βs from these linear regressions to test whether these were significantly different from zero across participants.
For each postscanning scale, average scores were computed for selected and rejected stimuli for each participant. Paired t tests were then conducted on these scores to examine whether rejected and selected stimuli differed on any of the scales.
fMRI analysis: whole-brain general linear model parametric analysis.
For each participant, a time series was generated indicating the temporal position of stimuli onset (the appearance of the medical condition name) to stimuli offset, creating 6sc “mini blocks” as done previously (Sharot et al., 2009a).
All trials from sessions 1 and 3 were included in a whole-brain parametric modulation analysis using random-effects general linear model (GLM). Three parametric modulators were included in the model: the number of past experiences with a specific medical condition, ratings of expected aversive outcome, and their interaction. The interaction regressor was the product of the two ratings on each trial. Entering the product of two variables as a parametric regressor allowed an identification of regions where the interaction between these two variables is expressed in the blood oxygen level-dependent (BOLD) signal (see Pine et al., 2009). For simplicity, aversive scores were transformed such that lower numbers indicated greater aversive reaction. A relatively stringent threshold was used (p < 0.0005, uncorrected; K > 100 contiguous voxels), as the power for this analysis was greater than all subsequent analyses, since all 160 trials were included (Sharot et al., 2009a).
Follow-up GLM analysis.
In a follow-up analysis, we controlled for objective severity of the medical conditions (as rated by medical doctors) by adding those ratings as a covariate in the model described above while examining for regions that expressed the interaction between past experience and aversive ratings (p < 0.0005, uncorrected). Furthermore, to ensure that the effect in regions found to express the interaction between past experience and expected aversive outcome were not confounded by choice, we included choice as a covariate in our parametric modulation analysis by adding choice as a regressor orthogonalized to the interaction regressor in that order. Thus, in this stringent analysis, we modeled all trials with a parametric modulator of past experience and aversive ratings (as done above), and modeled trials in the critical condition with the interaction regressor while controlling for choice (p < 0.001, uncorrected).
Effect of choice on expected aversive outcome
Behavioral analysis.
For each scan, and participant, the mean rating of predicted aversive reaction was calculated, and the distance of each rating from its mean computed. Mean corrected scores, which reflect the relative value of a stimulus, were used so as to control for shifts in the use of the scale over scans, and to standardize scores over participants (Sharot et al., 2009a,b). The average score for selected and rejected stimuli prechoice and postchoice was calculated for each participant and submitted to a 2 (scan: prechoice/postchoice) by 2 (stimuli type: select/reject) ANOVA, followed by planed t tests.
Order.
The order in which stimuli were presented in the prechoice scan was related to participants' subsequent choice. Participants were more likely to reject stimuli that were imagined earlier in the scan than stimuli that appeared later in the scan (p < 0.005). In the second scan, there were no differences in the order in which the stimuli of the different conditions were presented. To control for order effects in the first scan, we added the difference between stimulus positions of rejected and selected trials for each subject as a covariate in all second-order contrasts of the prechoice scan and the interaction analysis.
Reaction time.
Analysis of reaction times (RTs) for ratings in the first and last scan revealed a 2 (scan: prechoice/postchoice) by 2 (stimuli type: select/reject) interaction (F(1,12) = 14.72; p < 0.005). The interaction was characterized by (1) longer RTs during the prechoice scan for trials of stimuli subsequently rejected (0.87 s) relative to those subsequently selected (0.77) (p < 0.005), with no significant difference between RTs of selected and rejected stimuli during the postchoice scan, and (2) longer RTs for trials of stimuli rejected during the prechoice scan (0.87 s) relative to the postchoice scan (0.75) (p < 0.01), with no difference in RTs for stimuli selected between prechoice and postchoice scans. In the decision-making task, reaction times for making the choice in the difficult critical condition were longer than in the easy noncritical condition (p < 0.05).
Note that reaction times are independent from trial duration in fMRI data modeling. All trials were modeled as 6sc mini blocks during which participants imagined the medical conditions. These blocks were modeled from cue onset (name of medical condition) to rating scale onset. The time between rating scale onset and response, which is reaction time, were not modeled as regressors of interest.
fMRI analysis.
Trials were modeled as described previously (Sharot et al., 2009a). Trials from the critical condition in session 1 (prechoice) and session 3 (postchoice) were classified into four groups according to the participants' choice during session 2, resulting in subsequently and previously selected and rejected stimuli. The same procedure was implemented for trials in the noncritical condition.
Anatomically defined region of interest analysis.
To test whether regions that express an interaction between past experience and aversive ratings also express an interaction between choice and aversive ratings, we conducted an anatomical region of interest (ROI) analysis on regions identified in the previous parametric modulation analysis and then anatomically defined [i.e., right and left caudate, right and left putamen, right and left anterior cingulate cortex (ACC)]. We extracted the mean parameter estimates for the different trial types averaging across the whole anatomically defined regions. Differences in these parameter estimates will indicate robust effects that could be attributed to a general trend in that anatomical region. Statistical tests included within-subject t tests and an ANOVA testing for an interaction between time (prechoice/postchoice) and decision (selected/rejected). All anatomical definitions were performed according to the Talairach Daemon atlas (Lancaster et al., 1997) using the SPM WFU PickAtlas tool (Maldjian et al., 2003).
Across participants, we conducted a correlation analysis to examine whether participants who show greater choice-induced change in ratings also show greater change in BOLD signal. This involved contrasting postchoice BOLD signal differences between selected and rejected stimuli with prechoice differences, using each participant's spread in postchoice rating as a covariate in a second-level analysis [p < 0.5, familywise error (FWE) corrected for anatomically defined regions].
Functional connectivity analysis.
To assess whether choice commitment altered patterns of functional connectivity, we conducted a psychophysiological interaction (PPI) analysis in SPM5. For each participant, we extracted the deconvolved time course of activity averaged over the whole anatomically defined right caudate nucleus (i.e., the volume of interest) for the prechoice scan and postchoice scan, separately. The right caudate nucleus was chosen as seed because it has been identified previously as expressing the interaction between choice and reevaluation of expected outcome (Sharot et al., 2009a).
A whole-brain PPI analysis was conducted to identify target brain regions that showed a significant difference in functional coupling with the caudate nucleus before and after a choice was made. The regressors in PPI analysis include two covariates: (1) the activation time course of the volume of interest (i.e., the physiological variable “y”), and (2) a regressor representing the psychological variable of interest (i.e., the time during which participants imagined having medical conditions that will later be included in the difficult decision-making task, or were previously included in the decision making task), and (3) a regressor of interest representing the cross product of the previous two (the psychophysiological interaction term, ppi). Our a priori target region was the right ACC. For each subject, we averaged the parameter estimates of the PPI regressor across the whole right ACC before and after the decision-making task and conducted a t test comparing these parameters. We did the same for the physiological (y) regressor.
Exploratory whole-brain GLM analysis.
Exploratory whole-brain random-effects GLM analyses were conducted on data from all participants (p < 0.001, uncorrected). We contrasted BOLD signal during each imagination scan (prechoice and postchoice) of trials for the critical condition of selected compared with rejected stimuli. These findings are reported in the supplemental material (available at www.jneurosci.org).
Results
Effect of past experience on expected aversive outcome
We found that medical conditions experienced more often and more recently were estimated by participants as likely to elicit a less aversive reaction in the future. Across participants, average βs from the linear regression analysis showed that expected aversive outcome was related to (1) the objective severity associated with the illness (prechoice ratings, p < 0.0001; postchoice ratings, p < 0.001), and inversely related to (2) how often an illness had been experienced in the past (prechoice ratings, p < 0.005; postchoice ratings, p < 0.005); and (3) how recently a condition was experienced (prechoice ratings, p < 0.005; postchoice ratings, p < 0.05).
General familiarity with an illness (from others' experiences, films, books, etc.) and the vividness associated with imagining it, were not related to expected aversive outcome scores (p > 0.3). The degree of emotional arousal associated with an illness was related to ratings of expected aversive outcome prechoice (p < 0.001), but not postchoice (p > 0.5). Furthermore, participants were more likely to select a medical condition for which they had greater personal experience in the past (number of past experience, p < 0.005; time of last experience, p < 0.025) and that were perceived as less emotionally arousing (p < 0.025).
Having established that personal experience with an illness was related to an expectation that the same illness will elicit less of an aversive reaction in the future, we next examined our fMRI data to identify how, and where, this effect was expressed in the brain. An interaction between past experience and predicted aversive outcome was observed in the ACC (BA32/BA10; peak voxels in Talairach coordinates: R: 10, 48, 1) (Fig. 1a) (L: 4, 48, −2) (Fig. 1b), a region previously implicated in generating optimistic predictions (Sharot et al., 2007), extinction of fear conditioning (Phelps et al., 2004), and anxiety reduction (Simpson et al., 2001); and in the striatum (caudate R: 10, 16, 0; caudate L: −4, 10, −6; putamen R: 15, 14, −8; putamen L: −14, 11, −9) (Fig. 1a–c), a region implicated in the anticipation of both pain and reward (for review, see Delgado, 2007). No significant effects were found in any other region, nor were main effects of past experience evident at this threshold. A main effect of aversive ratings was observed in bilateral occipital cortex (L: −6, −85, −2; R: 2, −76, −3), right cerebellum (34, −42, −28), bilateral striatum (caudate R: 4, 10, −4; caudate L: −4, 10, −6; putamen L: −18, 15, −7), and left medial prefrontal cortex (BA24: −4, 4, 44; BA31: 2, −11, 43; BA10: −4, 50, −2; rACC: −12, 48, 1).
Figure 1.
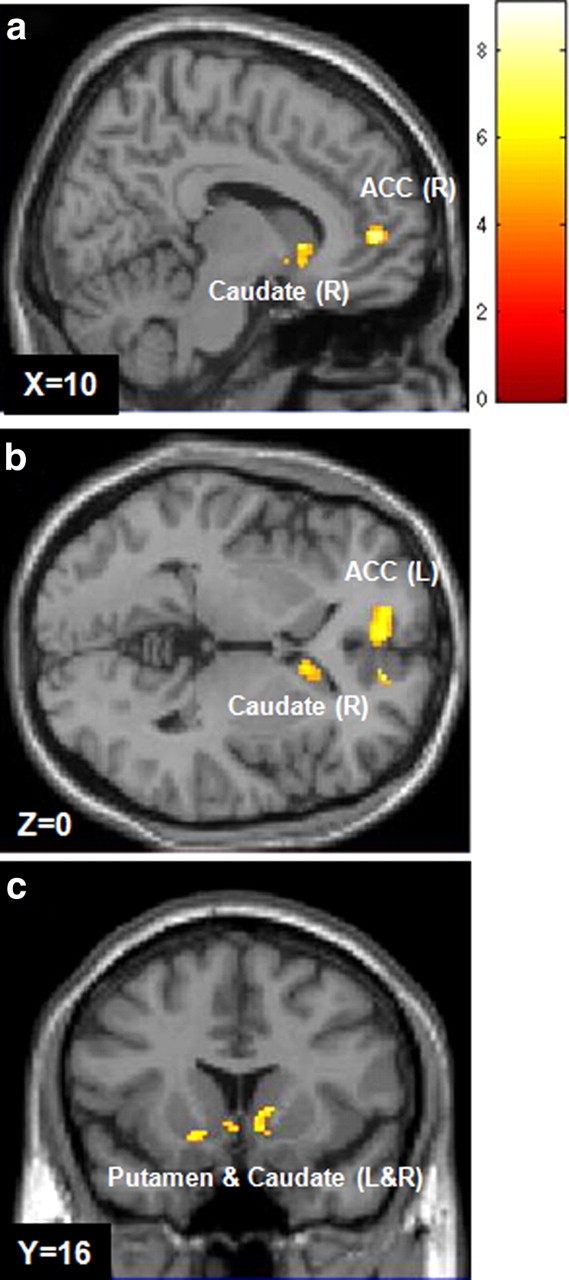
Brain activity mediating the relationship between past experience and estimated aversive outcome. Shown are regions in which BOLD response was positively correlated with the interaction between the number of times an illness was experienced in the past and ratings of estimated future aversive outcome (p < 0.0005, uncorrected; K > 100 contiguous voxels). Behavioral results showed that the more often an illness was experienced in the past, the lower the predicted aversive reaction to it. This interaction was expressed in the right (a) and left (b) anterior cingulate cortex, and the right and left striatum (c).
The above findings indicate that an effect of past experience on expected aversive outcome is reflected in the striatum and ACC. To test whether activity in these regions specifically expresses an interaction between past experience and aversive ratings, without a potential confound of choice, we included choice as a covariate in our parametric modulation analysis. In this stringent analysis, activity in right caudate nucleus and bilateral ACC expressed an interaction between past experience and expectations of aversive outcome even when controlling for effects of choice. However, in this analysis, activity in the putamen (bilateral) and left caudate was no longer significant.
We also conducted an analysis in which we included objective severity of the medical conditions (as rated by medical doctors) as a covariate. Again, the interaction between past experience and aversive rating was still expressed in the ACC (bilaterally) and right caudate (see supplemental figure, available at www.jneurosci.org as supplemental material), even when controlling for objective severity of the medical conditions. Thus, during imagination of a future event, BOLD signal in the right caudate nucleus and bilateral ACC appears to specifically track a physiological change of estimated aversive reaction induced by past experience.
Effect of choice on expected aversive outcome
A crucial question is whether choosing an aversive event lowers its expected aversive outcome and whether, in such instances, this effect is also reflected in altered activity in the striatum and ACC. Indeed, participants rated medical conditions as less aversive after choosing to have them relative to before (t(12) = 3.38; p < 0.005). Ratings for illnesses chosen to be avoided (“rejected” stimuli) did not differ before and after the choice task (t(12) = 1.4; p > 0.18). The interaction between choice (selected/rejected) and time of rating (before choice/after choice) on mean corrected scores of expected aversive outcome was significant (F(1,12) = 4.66; p < 0.0001) (analysis of raw scores also revealed a significant interaction, p < 0.001). Although before choice the mean estimated emotional reaction for selected and rejected stimuli was the same by design, significant differences emerged postchoice (t(12) = 2.62; p < 0.025).
We next turned to our fMRI data to examine whether the striatum and ACC expressed the interaction between choice and aversive expectancies. An anatomically defined ROI analysis on these regions (i.e., right and left caudate nucleus, right and left putamen, and right and left ACC) revealed that parameter estimates over all voxels in the anatomically defined right caudate nucleus (p < 0.05) (Fig. 2a) and right and left ACC (R, p < 0.005; L, p < 0.05) (Fig. 2b,c) during the postchoice scan were greater when participants imagined medical conditions they selected relative to conditions that they rejected. A similar trend was observed in the left caudate nucleus (p = 0.07) (Fig. 2d) (for exploratory GLM analysis in additional regions, see supplemental table, available at www.jneurosci.org as supplemental material).
Figure 2.
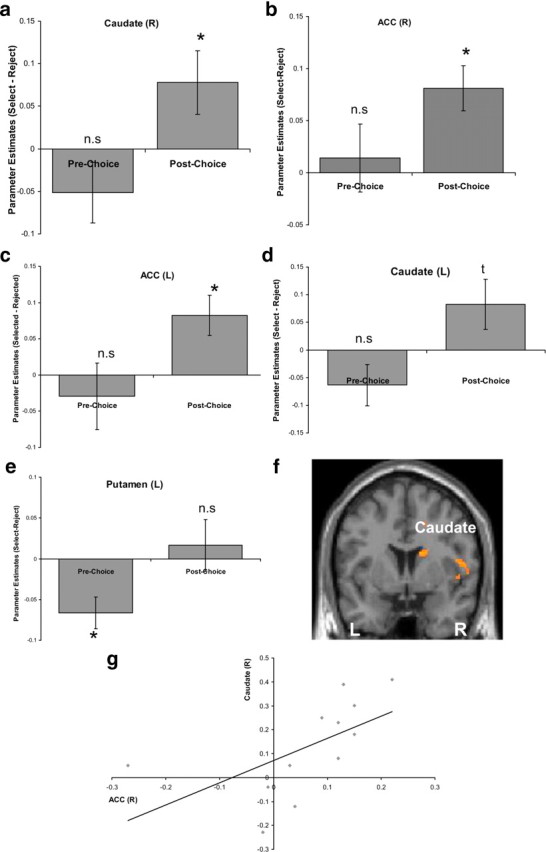
Brain activity mediating the relationship between choice and estimated aversive outcome. a–c, The difference in mean parameter estimate for selected minus rejected stimuli, averaged across anatomically defined right caudate nucleus (a), right ACC (b), and left ACC (c), showing effects significantly different from zero postchoice but not prechoice. d, e, A similar pattern of results was found in the left caudate (d) and the opposite pattern of results in the left putamen (e). f, g, Enhancement in BOLD signal for selected versus rejected stimuli in the caudate nucleus postchoice relative to prechoice, correlated with postchoice spread in expected aversive outcome across individuals (f) (p < 0.05, FWE corrected for anatomically defined right caudate nucleus, displayed at p < 0.005, uncorrected K > 35) and with the parallel change in parameter estimates in the right ACC (g) (r = 0.59; p < 0.05); the correlation is between change in parameter estimates averaged over the whole anatomically defined right caudate nucleus and right ACC. Error bars indicate SEM. *p < 0.05, two-tailed; t = trend = p < 0.1, two-tailed; n.s., not significant.
These findings raise the intriguing question as to whether the identified differences in activity were preexisting or, instead, emerged solely after the decision-making stage. In contrast to results from the postchoice condition, parameter estimates averaged over all voxels in the right and left caudate (Fig. 2a,d), right and left ACC (Fig. 2b,c), and right putamen did not show differences between selected and rejected stimuli prechoice. Rather, an effect was revealed in the left putamen (p < 0.05) (Fig. 2e). This finding suggests that, during the first imagination session, before participants knew they would have to hypothetically commit to specific aversive events, greater activity in the left putamen was associated with stimuli participants later rejected rather than selected as hypothetical illness.
Importantly, we observed a significant interaction of choice (selected/rejected) and time (prechoice/postchoice) on parameter estimates in the right and left caudate nucleus and left ACC (all at p < 0.05) (Fig. 2a,c,d). This suggests that a representation of expected aversive outcome in these regions was altered by the choice task and cannot be attributed to preexisting differences inherent in the stimuli, such as past experience (as those factors do not change before and after a choice is made).
Furthermore, participants who were more inclined to change their expected aversive reaction to stimuli postchoice also showed the highest change in right caudate activity (p < 0.05, FWE corrected for anatomically defined right caudate nucleus; peak voxel: 16, 6, 20) (Fig. 2f). This correlation was not found in any of the other ROIs. Specifically, the difference in BOLD signal between selected and rejected stimuli in the right caudate during the postchoice scan relative to the prechoice scan correlated with participants' postdecision change in ratings. This result replicates our previous findings (Sharot et al., 2009a), in which change in right caudate activity correlated with participants' tendency to modify their valuation of rejected and selected positive stimuli. Note, however, that the correlation in the current study is based on a smaller sample than in our previous study and thus should be treated with caution.
This change in BOLD signal averaged over the whole right caudate nucleus also correlated across participants with the parallel change in parameter estimates averaged over the right ACC (r = 0.59; p < 0.05) (Fig. 2g), but not left ACC. Thus, participants who showed greater choice-induced change in right caudate nucleus activity also showed greater choice-induced change in right ACC activity, suggesting a similar activity change in these two regions as a function of choice.
To directly test for difference in functional connectivity between the right caudate nucleus and right ACC as a function of the decision-making task, we conducted a connectivity analysis (PPI analysis) on the data recorded before and after the choice using the whole anatomically defined right caudate nucleus as seed region. Averaging parameter estimates over the whole right ACC, for trials involving the difficult choice task, we found greater connectivity with the seed after a choice was made relative to prechoice (t(11) = 2.6; p < 0.05). Note that, when examining the connectivity between these regions over the whole time course of the scans (i.e., imagination, rating, and fixation for all trial types), there was no difference in connectivity between the prechoice and postchoice scans (i.e., the simple physiological variable did not differ, t(11) = 0.58, p > 0.5). Instead, the difference was significant before and after the choice only for the ppi regressor that coded for the time period of imagination.
Discussion
When faced with adversity, humans adapt by reevaluating negative events as less aversive, thus retaining a sense of well being (Gilbert, 2006). Our behavioral results demonstrate that people's expectations of negative events are rated as less aversive under two circumstances; first, if they had encountered them in the past and second if they had made a choice to encounter them in the future. Whether the biological representation of the expected aversive value of a stimulus is altered by this type of commitment, and by past experience, was a central question addressed in this experiment. Using fMRI, we show that a modulation in predicted aversive outcome, whether induced by past experience or choice, was mirrored in altered neuronal signal in the caudate nucleus and in a parallel change in the ACC. This suggests that, in the case of an aversive event, both previous experience and choice alter its neural representation.
When participants had previously experienced medical conditions, their predictions of these same events were less aversive, even controlling for factors such as “objective” severity of illness and vividness with which it is imagined. Second, even though participants were making a hypothetical choice between two equally rated aversive conditions, with no apparent consequences, they nevertheless rated the option they committed to as less severe postchoice. The right caudate nucleus emerged as the key brain region showing a modulation in activity that reflected the impact of past experience and choice on predictions of aversiveness. Furthermore, participants more inclined to reevaluate their expected emotional reaction postchoice showed the greatest modulation in right caudate nucleus activity.
The involvement of caudate nucleus is of particular interest given previous evidence that this region is implicated in tracking predicted value (Seymour et al., 2007; Delgado et al., 2008), states of imagination (Sharot et al., 2007; D'Argembeau et al., 2008), and expected emotional outcome during simulation of future life events (Sharot et al., 2009a). We have previously shown that, after choosing between two equally desirable alternatives, activity in the right caudate nucleus increases to chosen, and decreases to rejected, positive stimuli (Sharot et al., 2009a). Our current finding that postchoice change in ratings of aversive stimuli is also reflected in a parallel change in the activity within the caudate nucleus suggests that a physiological representation of an expected aversive outcome is altered by choice. Furthermore, we show that past experience alters aversive expectancies in a similar manner. The pattern of modulation we observe suggests a reference dependency in valuation in which commitment to, and past experience with, an option leads to a relative enhancement in its relative reward value (De Martino et al., 2009).
Choice-induced changes in activity of the right caudate nucleus correlated with that of the right ACC, a region previously identified as mediating optimistic expectations of the future (Sharot et al., 2007), extinction of fear conditioning (Phelps et al., 2004), and anxiety reduction during shock expectancy (Simpson et al., 2001). A general role for the ACC is suggested in assessing the salience of emotional and motivational information, and regulating emotional responses accordingly (Cunningham et al., 2005; Sharot et al., 2007). Consistent with this is evidence that the response by the ACC to positive and negative stimuli can reflect a situational specificity. For example, the ACC is more sensitive to positive stimuli in subjects who focused on obtaining goals (promotional context) and to negative stimuli in subjects who focused on avoiding failure (prevention context) (Cunningham et al., 2005). In accordance with these previous studies, we speculate that activity in the ACC in the current study may reflect a self-regulatory focus that biases attention and vigilance toward less negative aspects of future aversive events. As in previous studies, a change in ACC activity showed situational specificity; being enhanced for conditions experienced in the past, or chosen to be experienced in the future, possibly reflecting a motivational imperative to reduce negative reactions in those cases.
Before the decision-making stage, there was no differential activity in the caudate and ACC when participants imagined conditions they subsequently selected relative to those that they would later reject. This suggests that differences observed in the postchoice scan were consequential on the decision-making task. We have previously shown that, when choosing between two positive stimuli (e.g., vacation destinations), caudate activity, which correlated with ratings of hedonic expectancies, predicted the participants' choice (Sharot et al., 2009a). A critical distinction with the aforementioned study is the fact that here subjects choose between aversive stimuli, rather than pleasant stimuli. Their decision is likely based on the aversive value of the stimuli, rather than on hedonic value. Interestingly, in the present study activity in the left putamen in the prechoice scan was greater when participants imagined conditions they would subsequently decide to avoid relative to those that they would choose, in the absence of knowledge that they would be required to commit to a future choice. This suggests that participants' selection between equally rated medical conditions may not have been arbitrary. Rather, the decision may be based on preexisting differences in the representation of expected aversive outcomes, reflected in level of putamen activity during the initial imagination stage. In keeping with this idea, the putamen has previously been shown to track expected aversive consequences (Seymour et al., 2004, 2005) and, in our case, reflected participants' later choice of the lesser of two evils.
A general perspective on our findings is that they speak to a pervasive human disposition to adopt the most rewarding (or least aversive) perspective on situations. An overwhelming motivation to realize positive states and avoid negative states is likely to influence the way we perceive our future, past, and present. When considering the future, people expect positive events even when evidence provides no support for such expectations, a phenomenon known as an optimism bias (Weinstein, 1980; Sharot et al., 2007). When recalling the past, we tend to remember positive self-relevant events with more details than negative self-relevant events (D'Argembeau et al., 2008). In the here and now, our desires and preferences may even bias visual perception in a manner that best fits our goals (Balcetis and Dunning, 2007). For example, thirsty participants are more likely to perceive transparency, a characteristic of water, in an ambiguous visual stimulus in comparison with hydrated participants (Changizi and Hall, 2001).
We show that people's predicted emotional reaction to future aversive events is altered as a function of whether they have previously encountered them and whether they choose to encounter them in the future. Although all options may seem equally forbidding, choosing a medical condition led participants to rate them as less severe. One theoretical account for this observation is that dissonance arises because choice (to have a medical condition next year) conflicts with previous belief (“being ill is bad and should be avoided”). Such dissonance is then reduced by a less negative reevaluation of the outcome (“having this medical condition is not as bad as I initially thought”) (Festinger, 1957). Another view is that the reevaluation occurs because envisioning competing possibilities provides a new context or reference point from which the stimuli are assessed (Sharot et al., 2009a). Specifically, making a decision highlights the unique aspects of the two alternatives (Tversky, 1972; Houston et al., 1991), providing new weights to features of the stimuli that may not have been considered thoroughly before (for a third interpretation, see Bem, 1967).
Our study highlights a capacity of the brain to reassess expected aversive outcomes in a less severe manner as a function of past and future experiences. Whether we chose an aversive situation, or whether we are passive victims of fate, when encountering the apparently insurmountable, we appear endowed with an ability to quickly adopt a less negative perspective on such events (Gilbert, 2006). Although different cognitive mechanisms may underlie this ability, our findings suggest that subcortical regions implicated in tracking expectations of pleasure and pain, together with the ACC, mediate a positive reevaluation of expectancies of aversive states. As attitudes and values are ultimately subjective, this means that they are easily subverted to ensure an optimal adaptation that fit our ever-changing circumstances and goals.
Footnotes
This work was supported by a Wellcome Trust Programme grant (R.J.D.), a British Academy Postdoctoral Fellowship (T. Sharot), and a Medical Research Council Clinical Research Training Fellowship (T. Shiner). We thank J. McDermott for assistance with programming, K. Friston for advice on design and analysis, G. Flandin for assistance with analysis, M. Symmonds and N. Wright for rating stimuli, and B. Bahrami, S. Bengtsson, S. Fleming, A. Nicolle, and D. Talmi for comments on a previous version of this manuscript.
References
- Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48:977–988. doi: 10.1016/s0277-9536(98)00411-0. [DOI] [PubMed] [Google Scholar]
- Balcetis E, Dunning D. Cognitive dissonance and the perception of natural environments. Psychol Sci. 2007;18:917–921. doi: 10.1111/j.1467-9280.2007.02000.x. [DOI] [PubMed] [Google Scholar]
- Bem DJ. Self-perception: an alternative interpretation of cognitive dissonance phenomena. Psychol Rev. 1967;74:183–200. doi: 10.1037/h0024835. [DOI] [PubMed] [Google Scholar]
- Changizi MA, Hall WG. Thirst modulates a perception. Perception. 2001;30:1489–1497. doi: 10.1068/p3266. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cogn Affect Behav Neurosci. 2005;5:202–211. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005;8:1611–1618. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc Lond B Biol Sci. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Holt B, Dolan RJ. The neurobiology of reference-dependent value computation. J Neurosci. 2009;29:3833–3842. doi: 10.1523/JNEUROSCI.4832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L. A theory of cognitive dissonance. Stanford, CA: Stanford UP; 1957. [Google Scholar]
- Gilbert D. Stumbling on happiness. New York: Knopf; 2006. [Google Scholar]
- Hasson U, Furman O, Clark D, Dudai Y, Davachi L. Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron. 2008;57:452–462. doi: 10.1016/j.neuron.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DA, Sherman SJ, Baker SM. Feature matching, unique features, and the dynamics of the choice process: predecision conflict and postdecision satisfaction. J Exp Soc Psychol. 1991;27:411–414. [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, Dolan RJ. Encoding of marginal utility across time in the human brain. J Neurosci. 2009;29:9575–9581. doi: 10.1523/JNEUROSCI.1126-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Sharot T, De Martino B, Dolan RJ. How choice reveals and shapes expected hedonic reaction. J Neurosci. 2009a;29:3760–3765. doi: 10.1523/JNEUROSCI.4972-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Shiner T, Brown AC, Fan J, Dolan RJ. Dopamine enhances expectation of pleasure in humans. Curr Biol. 2009b;19:2077–2080. doi: 10.1016/j.cub.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A. Elimination by aspects: a theory of choice. Psychol Rev. 1972;79:281–299. [Google Scholar]
- Ubel PA, Loewenstein G, Jepson C. Disability and sunshine: can hedonic predictions be improved by drawing attention to focusing illusions or emotional adaptation? J Exp Psychol Appl. 2005a;11:111–123. doi: 10.1037/1076-898X.11.2.111. [DOI] [PubMed] [Google Scholar]
- Ubel PA, Loewenstein G, Schwarz N, Smith D. Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005b;24(4 Suppl):S57–S62. doi: 10.1037/0278-6133.24.4.S57. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Unrealistic optimism about future life events. J Pers Soc Psychol. 1980;39:806–820. [Google Scholar]


