Abstract
In the present study we investigate whether the unicellular green alga Micrasterias denticulata is capable of executing programmed cell death (PCD) upon experimental induction and by which morphological, molecular and physiological hallmarks it is characterized. This is particularly interesting as unicellular fresh water green algae growing in shallow bog ponds are exposed to extreme environmental conditions and the capability to perform PCD may provide an important strategy to guarantee survival of the population. The theoretically “immortal” alga Micrasterias is an ideal object for such investigations as it has served as a cell biological model system since many years and details on its growth properties, physiology and ultrastructure throughout the cell cycle are well known. Treatment with low concentrations of H2O2 known to induce PCD in other organisms resulted in severe ultrastructural changes of organelles as observed in TEM. These include deformation and partly disintegration of mitochondria, abnormal dilatation of cisternal rims of dictyosomes, the occurrence of multivesicular bodies, an increase in the number of ER compartments and slight condensation of chromatin. Additionally, a statistically significant increase in caspase-3-like activity could be detected which was abrogated by a caspase-3 inhibitor. Photosynthetic activity measured by fast chlorophyll fluorescence decreased as a consequence of H2O2 exposure whereas pigment composition, except of a reduction in carotenoids, was the same as in untreated controls. TUNEL positive staining and ladder-like degradation of DNA, both frequently regarded as PCD hallmark in higher plants could only be detected in dead Micrasterias cells.
Keywords: caspase-3-activity, programmed cell death, green algae, H2O2, Micrasterias, photosynthesis, ultrastructure
Introduction
Programmed cell death (PCD) an active genetically regulated physiological process, is essential for development and reproduction, differentiation of specialized tissues and defence against environmental impact and pathogens in higher plants (Gray, 2004; Mittler and Cheung, 2004; Nooden, 2004; van Doorn and Woltering, 2005). Although recent studies have indicated that PCD may also take place in primitive single cell eukaryotes (Ameisen, 1996; Lewis, 2000; Sen et al., 2004) and prokaryotes (Berman-Frank et al., 2004; Gordeeva et al., 2004), there is a tremendous lack in information on cell death pathways in unicellular photosynthetic organisms (Golstein et al., 2003). Only a few studies have dealt so far with some aspects of PCD in algae (Pommerville and Kochert, 1982; Vardi et al., 1999; 2002; Debrabant et al., 2003; Segovia et al., 2003; Bidle and Falkowski, 2004; Casotti et al., 2005; Leu and Hsu, 2005; Segovia and Berges, 2005; Moharikar et al., 2006; Zuppini et al., 2007). However, studies on PCD in unicellular photosynthetic organisms may provide important insight into intracellular and molecular cell death pathways which in multicellular organisms and tissues are difficult to investigate. In addition a more detailed knowledge on PCD in unicellular organisms may yield new information on survival mechanisms and could delineate the evolutionary origin of cell death processes.
One of the most intensively investigated cell death modes is apoptosis which in animal cells has been essentially defined by three morphological hallmarks: nuclear and cytoplasm condensation, formation of apoptotic bodies and finally degradation of apoptotic bodies by lytic enzymes (Kerr et al., 1972; van Doorn and Woltering, 2005). In plants, plant cell cultures or unicellular photosynthetic organisms cell death pathways are less precisely defined and seem to be much more divers. Characteristics such as DNA degradation, chromatin condensation, cytochrome c release from mitochondria, involvement of caspase-like enzymes, decrease in photosynthetic activity, vacuolization of cytoplasm, ATP depletion and loss of membrane integrity have been found to accompany cell death upon different kinds of induction (Mittler et al., 1997; Korthout et al., 2000; Polverari et al., 2000; Veldhuis et al., 2001; Yao et al., 2001; Tiwari et al., 2002; Franklin and Berges, 2004; Geitmann et al., 2004; Overmyer et al., 2005; Moharikar et al., 2006; Aranha et al., 2007; He et al., 2007). At the ultrastructural level particularly morphological changes of the nucleus (Levine et al., 1996), changes in mitochondrial structure (Gunawardena et al., 2001; Virolainen et al., 2002; Yu et al., 2002; Selga et al., 2005), tonoplast rupture (Polverari et al., 2000), dictyosome dilatation (McManus et al., 1998; Gunawardena et al., 2001) and formation of membranous bodies (Polverari et al., 2000) have been reported during plant cell death. Although several authors have regarded at least some of these processes as hallmarks for apoptosis their occurrence does not necessarily indicate an apoptotic cell death pathway and it is doubted that apoptosis in the classical sense (Kerr et al., 1972) exists in plants at all (for discussion see also van Doorn and Woltering, 2005).
Unfavourable environmental conditions such as drought, high light intensities, UV-irradiation, salinity, extreme temperature or oxidative stress, the latter induced among others for example by impact of different compounds (e.g. herbicides) may result in various cell death events in plants (Evans, 2004). A key element in both abiotic and biotic stress is the production of H2O2 within the cell. Increases in endogenous oxidants induce cell death e.g. during hypersensitive response, whereas antioxidants block apoptosis (Lam et al., 2001; Gechev and Hille, 2005). As known from various studies reactive oxygen species have a direct effect on mitochondrial morphology probably leading to PCD (for review see Logan, 2006). Moreover, various studies have shown (e.g. Houot et al., 2001; Gechev and Hille, 2005; Gechev et al., 2006; de Pinto et al., 2006) that experimental exposure of plant cells to different concentrations of H2O2 may induce different cell death pathways.
The aim of this study was to find out whether PCD occurs in the unicellular green alga Micrasterias denticulata upon H2O2 induction and by which morphological, molecular and physiological hallmarks it is characterized. Micrasterias has been used as a cell biological model system since many years and numerous data on its cell physiology and ultrastructure are available (Kiermayer, 1981; Meindl, 1993; Oertel et al., 2004; Aichinger and Lütz-Meindl, 2005). The replication mode of Micrasterias, where one semicell is remodelled after each mitosis with the parental semicell remaining unchanged, makes it particularly interesting to study cell death in this generally “immortal” biological system. However, PCD may be of high ecological relevance in a unicellular alga as it may guarantee survival of the population under unfavourable environmental conditions. In the present study we exposed Micrasterias cells at various stages of the cell cycle (see Material and Methods) to different concentrations of hydrogen peroxide and other cell death inductors in order to characterize PCD. We investigated whether typical PCD hallmarks known from other organisms, such as caspase-like activity, changes in chromatin distribution and DNA structure, ultrastructural alterations in morphology of nucleus and organelles as well as changes in photosynthetic activity and pigment composition occur.
Materials and methods
All chemicals have been purchased by Sigma-Aldrich (Vienna, Austria) or Roth (Karlsruhe, Germany) unless stated differently.
Cell cultures and H2O2 treatment
Cells of Micrasterias denticulata were grown in liquid Desmidiaceaen medium in Erlenmeyer flasks (Schlösser, 1982) and were kept at a 14 to 10 h light-dark regime at 20 °C. The cultures were subcultured every 4 to 5 weeks. Under these conditions the algae divide every 3rd to 4th day by mitosis (for details of culture method see Meindl et al., 1989).
H2O2 concentrations in a range between 50 μM and 1 M were tested on Micrasterias cells. As 2.5 mM and 5 mM turned out to be most effective, these concentrations were used for long-term treatments (3, 6, 12, 24 h). For short-term treatment 200 mM H2O2 was employed for 1 h.
Light microscopy
Cells of defined stage (48 h after mitosis) were treated with H2O2 for 3 to 24 h and photographed in a Axioplan microscope using AxioCam camera (Zeiss, Oberkochen, Germany). To define the cell stage Micrasterias cells 1 h after mitosis were collected by a glass pipette and were grown for 47 h under standard culture conditions prior to H2O2 treatment. Due to the highly symmetric cell pattern, cell stages of Micrasterias are easy to determine as each developmental stage is characterized by a particular morphology (see Kiermayer, 1981).
Sample preparation and TEM
Cells 48 h after mitosis were treated with 2.5 mM and 5 mM H2O2 for 3, 6 and 12 h or with 200 mM H2O2 for 1 h, and were then fixed for electron microscopy. To prove that the results are reliable two different fixation methods were used: chemical fixation and high pressure freeze fixation. For chemical fixation, cells were fixed with 1 % glutaraldyhyde (10 min) and 1 % OsO4 (2 h) in cacodylate buffer (Meindl, 1990) and HPF was done in a Leica EMPACT high pressure freezer (Leica Mikrosysteme GmbH, Vienna, Austria). Freeze substitution took place in a Leica EM AFS freeze substitution apparatus in 1 % OsO4 and 0.05 % uranyl acetate in acetone, for 58 h at −80 °C and for 2 h at −30 °C (Aichinger and Lütz-Meindl, 2005). Preparations were embedded in epoxy resin (Embed 812, Araldite 502, DDSA and BDMA). Ultrathin sections were placed on Formvar coated copper grids for TEM analysis. Electron micrographs were captured in a LEO 912 transmission electron microscope (Zeiss, Oberkochen, Germany) equipped with an in-column energy filter, at 80 kV by using a Slow Scan Dual Speed CCD camera TRS Sharpeye (Troendle, Moorenwies, Germany) which was controlled by a EsiVision 3.2 or ITEM Software (SIS, Soft Image System, Münster, Germany).
Measurement of caspase-3-like activity
Cell cultures (3 to 5 weeks after subculturing) were treated with 2.5 mM and 5 mM H2O2 for 3, 6, 12 and 24 h. Cells were harvested by centrifugation (Heraeus Sepatech, Osterode, Germany) at 2000 g for 5 min at 12 °C, washed in ddH2O and centrifuged at 5000 g for 5 min at 12 °C in a microcentrifuge (Centrifuge 5403, Eppendorf, Hamburg, Germany). Cells were incubated in chilled lysis buffer on ice for 10 min. Cells were ground and the lysates were centrifuged at 23.100 g for 20 min at 4 °C to precipitate cellular debris. Clear lysates could only be obtained after centrifugation of cells treated with concentrations up to 2.5 mM H2O2 for 3 h. Therefore we only used 2.5 mM H2O2 for measuring caspase-3-like activity. We used the “Caspase-3 DEVD-R110 Fluorometric & Colorimetric Assay Kit” (Biotium, Hayward, USA) for measuring the activity of caspase-3-like enzymes. For determination of the induction factor, the ratios of the caspase-3-like activity of treated cells vs. control cells from 8 independent experiments were calculated and averaged. The enzyme activity of untreated controls was set to 100 % for each individual experiment. Additionally three independent experiments were performed to show that the caspase inhibitor Ac-DEVD-CHO was able to abrogate caspase-3-like activity in H2O2 treated cells.
The statistical difference between values of control and H2O2 induced (2.5 mM, 3h) caspase-3-like activity was assessed by a paired Student's t test using a SigmaPlot software.
DAPI staining
For depicting changes in nuclear morphology and chromatin distribution we applied the fluorescent dye DAPI. Cells 48 h after mitosis were treated with 2.5 mM to 200 mM H2O2 for 1 to 24 h and fixed in 1 % paraformaldehyd at room temperature (RT) for 15 min and were then washed for 5 min with ddH2O. Fixed cells were stained with 0.2 μg/ml DAPI for 50 min in the dark at RT. Cells were washed with phosphate buffered saline (PBS) for 5 min and briefly rinsed with ddH2O thereafter. Cells were photographed in a fluorescence microscope (Leitz Dialux 20, Wetzlar, Germany) with a digital camera (Canon PowerShot G5, Tokio, Japan).
TUNEL assay
Cells 48 h after mitosis were treated with 2.5 mM and 5 mM H2O2 for 3 to 24 h, and were then fixed with 4 % paraformaldehyd for 20 min. After fixation they were washed with PBS, pH 7.4 for 15 min. Cells were permeabilized with 0.1 % Triton X-100 in 0.1 % trisodiumcitrate for 15 min at RT. Cells were washed again with PBS for 15 min and a TUNEL assay was performed using the “In Situ Cell Death Detection Kit, POD” (Roche Diagnostics, Vienna, Austria). The cells were washed again with PBS and viewed in a Zeiss Axiovert 100M inverted microscope equipped with a confocal laser scanner (Zeiss LSM 510, Oberkochen, Germany). Excitation was generated with an argon laser at 488 nm; emitted light was 505-550 nm band-pass filtered. For statistic evaluation 6 independent experiments were performed for 2.5 mM H2O2, 24 h (54 cells in total) and 7 independent experiments for 5 mM H2O2, 24 h (72 cells in total).
Cell vitality assay with FDA
For measuring cell vitality cells 48 h after mitosis were suspended in 0.23 mM fluorescein diacetate (FDA) for 45 min in the dark (for method see Yamori et al., 2005). Unbound dye was removed by washing with ddH2O and the cells were viewed in a confocal laser scanning microscope (for details see above). Cells were treated with 2.5 mM and 5 mM H2O2 for 3, 6 and 12 h. Each experiment was done with 15 cells and repeated three times.
To find out, whether Micrasterias cells are still alive after a freeze-thawing procedure, cell cultures (3 to 5 weeks after subculturing) were spun down and frozen in liquid nitrogen as described in the DNA isolation section. After thawing, the cells (n = 79) were incubated in FDA and analyzed as described above.
DNA isolation
For all DNA-analysis experiments cultures 3 to 5 weeks after subculturing were used. In order to induce cell death in Micrasterias and to determine whether DNA laddering occurs as a consequence of the induction, cultures were treated with 2.5 mM H2O2 for 3 and 24 h. For comparison with other DNA laddering inductors, cells were also exposed to 100 mM D-mannose for 3 d or heat treated between 37 °C and 50 °C in a water bath (Julabo F 25, Julabo Labortechnik, Seelbach, Germany) for 15 min up to 12 h. In the latter experiment cells were allowed to recover at standard growth conditions for different periods between 1 and 140 h. For ultraviolet (UV)-C irradiation, cells were exposed to 254 nm at a dose of 2500 Jm−2 in a UV Stratalinker 2400 (Stratagene, La Jolla, USA) and recovered in the dark for 18 h. After all treatments, cells were collected by centrifugation at 3291 g in an Omnifuge 2.0RS (Heraeus Sepatech, Osterode, Germany), washed 3 times with ddH2O to remove mucilage and were immediately frozen in liquid nitrogen. For heat treatment of frozen cells, untreated algae were collected and frozen as described above. Then, the cells were exposed to varying temperature ranging from RT to 55 °C for 1 to 12 h and were again frozen in liquid nitrogen. Genomic DNA was isolated from Micrasterias using standard CTAB extraction (Murray and Thompson, 1980; Moharikar et al., 2006) with few modifications. Cells were homogenized to a fine powder. 2x CTAB-buffer (2 % w/v CTAB, 1.4 M NaCl, 1 % w/v PVP-40, 20 mM EDTA, 100 mM Tris-HCl, pH 8.0) and RNase A were added and the mixture was incubated at 65 °C for 30 min and at 37 °C for 60 min. The homogenate was extracted twice with an equal volume of chloroform-isoamyl alcohol (24:1 v/v). The DNA was precipitated with 2/3 volumes of ice-cold isopropanol, centrifuged and the pellet was resuspended in ddH2O. To visualize the DNA, equal amounts of DNA were separated on a 1.5 % TAE-agarose-gel and stained with ethidium bromide.
Determination of pigment composition
Cultures 3 to 5 weeks after subculturing were used and were treated with 2.5 mM and 5 mM H2O2 for 3, 6 and 12 h. Chloroplast pigments were extracted from 1.5 ml suspension of cells (approx. 2000 cells/ml), in 0.5 ml DMF, and the insoluble material was removed by centrifugation. Pigment separation was performed on an Agilent 1100 HPLC with diode-array detection and cooled sample compartment, following the method described by Lütz et al. (1997).
Measurement of photosynthetic activity
Cell cultures 3 to 5 weeks after subculturing were treated as for determination of pigment composition. Assay of photosystem II activity was performed by fast chlorophyll fluorescence according to Strasser et al. (1995), using the Handy-Pea from Hansatech (King's Lynn, England). Ten drops of the cell suspension were placed on small pieces of thick filter paper fixed in the leaf clips of the Handy-Pea and incubated in darkness for 20 min. The cells remained moisture and controls showed normal induction curves (Kautsky-effect) and values of the Fv/Fm ratio of approx. 0.8 (see Results). For controls and treatments, a minimum of 10 parallel measurements each were taken. Mean curves were calculated to compare control and treatments using the biophysical parameters of primary photochemistry according to the formula given by Strasser et al. (1995) and Srivastava et al. (1999; see Results).
Attempts to measure photosynthesis and respiration in H2O2 treated cells by means of polarographic oxygen determination, as performed in Micrasterias in earlier experiments (Lütz et al., 1997, Weiss et al., 1999), failed as the continuous presence of oxygen and radicals abolished an accurate determination of physiological activities.
Results
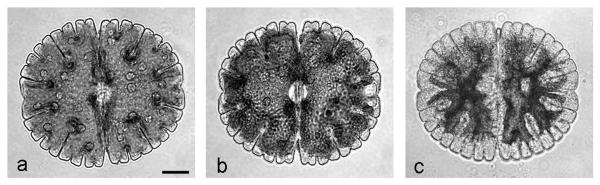
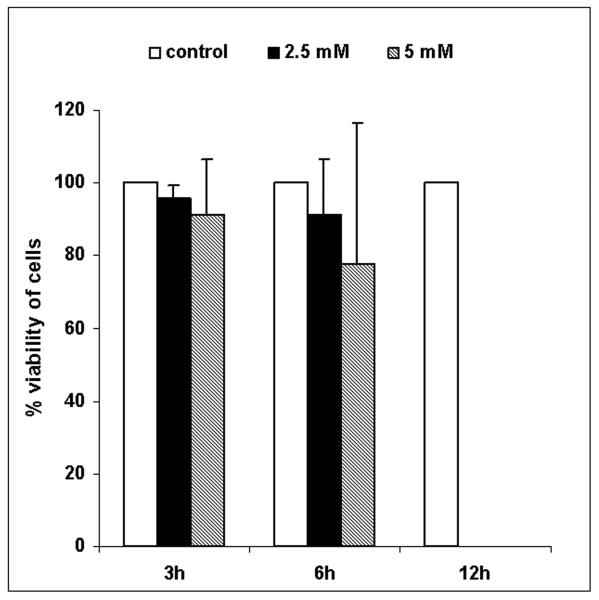
Treatment with 2.5 mM and 5 mM H2O2 evoked the same morphological and ultrastructural changes in Micrasterias cells. After 3 h treatment vacuoles appeared preferentially in polar lobes (Figs. 1 b, compare to control in Fig.1 a) and increased in size after 6 and 12 h (data not shown). After 24 h the chloroplast was condensed, cytoplasmic streaming had ceased and the cells appeared dead (Fig. 1 c). To find out whether the cells after treatment with different H2O2 concentrations and varying duration were alive we used FDA staining as a vitality marker. As shown in Fig. 2, most of the cells (more than 75 %) were alive after 3 h or 6 h treatment with 2.5 mM and 5 mM H2O2. After 12 h 100 % of the cells were dead at both concentrations. This indicates that treatment with 2.5 mM and 5 mM H2O2 leads to cell death between 6 and 12 h after the onset of the incubation. In control cells vitality was 100 % after 3 h, 6 h and 12 h.
Fig. 1.
Light microscopic images of Micrasterias cells. (a) Control cell, (b) cell treated with 2.5 mM H2O2 for 3 h, (c) dead cell after 2.5 mM H2O2 for 24 h. Bar = 50 μm
Fig. 2.
Vitality test with FDA. Cells were treated with 2.5 and 5 mM H2O2 for 3, 6 and 12 h. Values represent the mean of three independent experiments ± SD
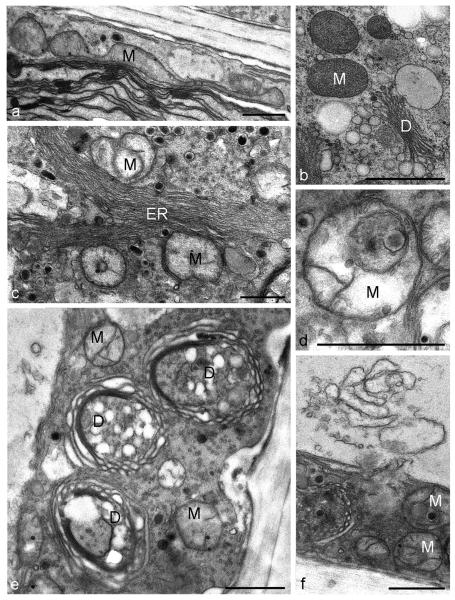
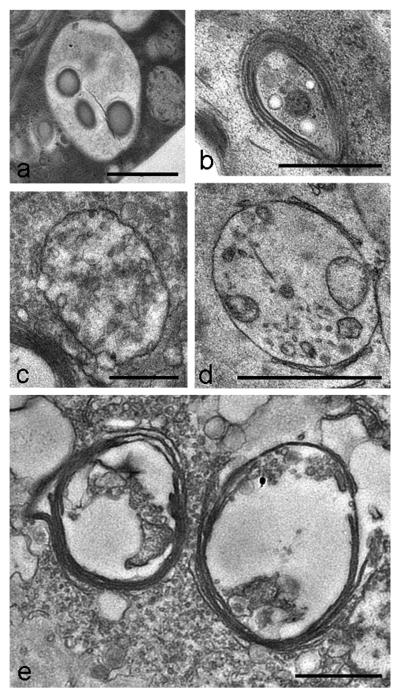
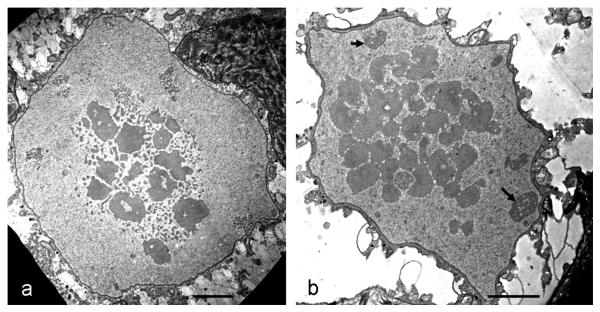
In cells treated with both, 2.5 mM and 5 mM H2O2 for 3 h, we observed severe ultrastructural changes such as deformation and disintegration of mitochondria in TEM (Figs. 3 a, c - f) when compared to untreated controls (Fig. 3 b; for images of untreated Micrasterias cells see also e.g. Meindl et al., 1992). Some mitochondria were abnormally long and some revealed abnormally long cristae (Figs. 3 a, d). Abnormal dilatation of the cisternal rims of dictyosomes (Fig. 3 e), an increase in the number of ER compartments (Fig. 3 c) and tonoplast ruptures (Fig. 3 f) were additionally observed as a consequence of this treatment. After 6 h treatment with the same H2O2 concentrations some dictyosomes were involute (Fig. 4 b). In contrast to dictyosomes, mitochondria did not show any further changes. The number of multivesicular bodies was increased (Fig. 4 a). Structure and morphology of the cell wall was not affected in these cells. Interactions of organelles with lytic compartments as observed recently in untreated controls (Aichinger and Lütz-Meindl, 2005) appeared not to be increased in cells undergoing PCD. After 12 h treatment the ultrastructural appearance indicated that the cells were dead. Disorganization and swelling of mitochondria up to a complete disintegration of their cristae were observed (Fig. 4 c). In comparison to a 6 h treatment more dictyosomes were involute or strongly bent, cisternal membranes were closely attached to each other without revealing any cisternal contents and vesicles were no longer pinched off (Fig. 4 e). The number of membranous bodies (Fig. 4 d) was increased after longer H2O2 exposure. All ultrastructural changes were similar both in chemically fixed and high pressure frozen cells.
Fig. 3.
TEM micrographs showing ultrastructure in control cell, high-pressure freeze fixation (b) and ultrastructural changes (a, c, d, e, f) after 3h treatment with 2.5 mM or 5 mM H2O2, chemical fixation. (a) Abnormally long mitochondrion, (b) mitochondria and dictyosome in untreated control cell. Micrograph by courtesy of Ancuela Andosch (c) unusual large number of ER compartments, (d) deformation and disintegration of a mitochondrion, abnormally long cristae, (e) dilatation of cisternal rims of dictyosomes, (f) tonoplast rupture. M mitochondrion, ER endoplasmic reticulum, D dictyosome. Bar = 1 μm
Fig. 4.
TEM micrographs showing ultrastructural changes during long-term treatment with 2.5 mM or 5 mM H2O2 treatment. (a, b) 6 h, high-pressure freeze fixation (c, d, e) 12 h, chemical fixation (a, d) Multivesicular bodies, (b, e) dictyosomes involute and inactive, (c) disintegrated mitochondrion. M mitochondrion, D dictyosome. Bar = 1 μm
After 3 h to 6 h treatment with 2.5 mM and 5 mM H2O2 nuclear shrinkage and slight chromatin condensation were observed in all cells investigated. Ultrastructure and morphology of the nucleolus changed in a way that the nucleolar portions seemed to be abnormally connected to each other (data not shown). This was observed in the same way at short-term treatment with 200 mM H2O2 for 1 h (Figs. 5 b compare to control in Fig. 5 a). Accordingly, nuclear shrinkage and slight chromatin condensation were also found at the same H2O2 treatment after DAPI staining (Figs. 6 a, b).
Fig. 5.
TEM micrographs showing morphological changes in nuclei of Micrasterias cells. (a) Control cell, (b) treated with 200 mM H2O2 for 1 h. Chromatin condensed (arrows), nucleolar portions abnormally connected. Bar = 2.5 μm
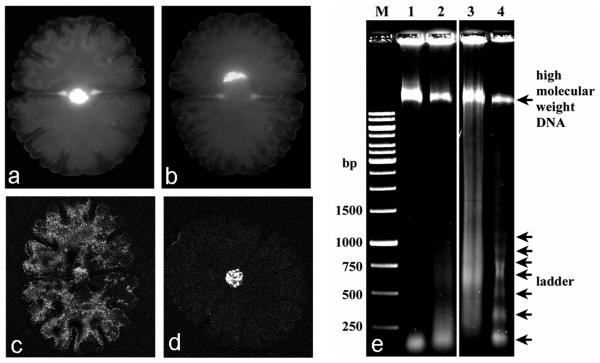
Fig. 6 a-b.
Visualization of changes in nuclear morphology by DAPI staining of Micrasterias cells, (a) untreated control, (b) treated with 200 mM H2O2 for 1 h, nucleus crescent-shaped (c, d) DNA fragmentation in Micrasterias visualized by TUNEL assay. (c) Control, (d) cell treated with 2.5 mM H2O2 for 24 h revealing positive TUNEL staining. (e) DNA degradation in Micrasterias shown by agarose-gel electrophoresis. Genomic DNA extracted from untreated control cells (lane 1), cells treated for 3 h (lane 2) and 24 h (lane 3) with 2.5 mM H2O2. Lane 4 shows DNA laddering from frozen cells incubated at 42 °C for 12 h. Equal amounts (1.3 μg) were loaded in each lane of a 1.5 % agarose gel. M DNA marker.
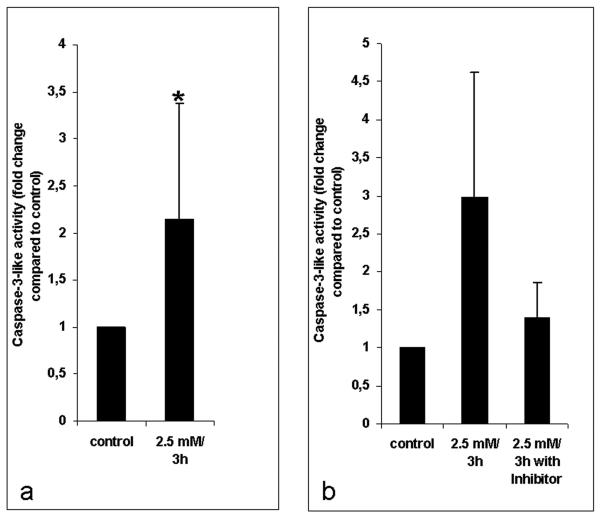
After 3 h exposure to 2.5 mM H2O2 a statistically significant (P < 0.05) increase in the activity of caspase-3-like enzymes by an averaged factor of 2.1 was detected in 8 independent experiments (Fig. 7 a). Additionally it was shown that the caspase inhibitor Ac-DEVD-CHO abrogated caspase activity in H2O2 treated cells almost to control level (Fig. 7 b). H2O2 incubation up to 12 h did not result in DNA degradation as observed by TUNEL assay. A TUNEL positive staining was only detected after 24 h treatment with 2.5 mM H2O2 (Figs. 6 c, d) when the cells were already dead as identified by FDA staining. In this case 64 % of the 2.5 mM H2O2 treated cells and 79 % of 5 mM H2O2 treated cells (data not shown) revealed TUNEL positive staining.
Fig. 7.
(a) Mean values of caspase-3-like activity in Micrasterias calculated from 8 independent experiments ± SD. Cells are treated with 2.5 mM H2O2 for 3 h. Caspase-3-like activity of controls was set to 100%. * indicates that the value is significantly different from the control (P < 0.05) (b) Mean values of caspase-3-like activity after 2.5 mM H2O2 induction and its reduction by the caspase-3 inhibitor Ac-DEVD-CHO calculated from 3 independent experiments.
As a positive TUNEL reaction is not always a reliable marker for PCD (Grasl-Kraupp et al., 1995), the DNA degradation pattern (oligonuceosomal or random) was examined by agarose gel electrophoresis. Genomic DNA was extracted after a 3 h treatment when caspase-3-like activity was increased (Fig. 7). No DNA laddering was visible on the agarose gel (Fig. 6 e, lane 2). In addition, DNA was extracted after 24 h H2O2 treatment, when the TUNEL staining was positive (Fig. 6 d). DNA was slightly degraded as seen as a smear on the agarose gel (Fig. 6 e, lane 3). However, most of the genomic DNA was not degraded, as a high molecular weight DNA-band could still be detected (Fig. 6 e, lane 3).
To find out, whether DNA laddering occurs in Micrasterias at all a screening with different treatments was performed (Table 1). Neither hydrogen peroxide, heat stress with different temperatures, UV-C irradiation nor D-mannose led to a fragmentation of genomic DNA into oligonucleosomes. In some of the treatments only a faint smear was detectable (Table 1). Interestingly, a laddering of the DNA could only be induced by incubating frozen cells for 4 - 12 hours at 37 – 42 °C (Table 1). As determined by FDA staining 100 % of these cells were dead after this harsh treatment. The ladder consisted of bands that are multimers of approximately 175 bp, typical for an oligonucleosomal fragmentation of DNA (Fig. 6 e, lane 4).
Table 1.
Screening in Micrasterias with different treatments known to induce DNA laddering in other plant species (for references see Discussion).
| Treatment | Time | Recovery | Laddering | |
|---|---|---|---|---|
| 1. H2O2 | 2.5 mM | 3 h | − | − |
| 24 h | − | faint smear | ||
| 2. D-Mannose | 100 mM | 3 d | − | − |
| 3. Temperature | RT (frozen cells) | 4 h | − | − |
| 37 °C | 30 min | 1 h | − | |
| 37 °C (frozen cells) | 4h | − | faint smear | |
| 37 °C (frozen cells) | 12 h | − | + | |
| 40 °C (frozen cells) | 4h | − | faint smear | |
| 41 °C (frozen cells) | 4h | − | + | |
| 41 °C (frozen cells) | 8h | − | + | |
| 41 °C (frozen cells) | 12 h | − | + | |
| 41 °C | 12 h | − | − | |
| 42 °C | 4h | − | − | |
| 42 °C | 6h | 16 h | − | |
| 42 °C | 6h | 42 h | faint smear | |
| 42 °C (frozen cells) | 1h | − | − | |
| 42 °C (frozen cells) | 4h | − | + | |
| 42 °C (frozen cells) | 12 h | − | + | |
| 42 °C | 12 h | − | faint smear | |
| 44 °C | 6h | 16 h | − | |
| 44 °C | 6h | 136 h | faint smear | |
| 50 °C | 15 min | 96 h | faint smear | |
| 50 °C | 30 min | 1h | − | |
| 50 °C | 30 min | 140 h | faint smear | |
| 55 °C (frozen cells) | 2.5 h | − | faint smear | |
| 4. UV-C | 2500 Jm−2 | − | 18 h | − |
In order to obtain information whether the physiology of Micrasterias cells after H2O2 exposure indicates PCD, pigment composition of plastids was analyzed and photosynthetic activity was measured. HPLC separation of individual pigments revealed the same results after both 2.5 mM and 5 mM H2O2: No additional compounds like decomposition products of the photosynthetic pigments were found. The formation of the carotenoids antheraxanthin and zeaxanthin was not induced under the growth light condition (approx. 60 μMol photons). Table 2 shows the influence of 5 mM H2O2 on plastid pigments. Neoxanthin and lutein did not respond considerably; but a strong reduction in violaxanthin content down to traces after 12 h incubation under 5 mM H2O2 occurred. An about 20 % decomposition appears also in ß-carotene. Mainly these changes are responsible for the reduction in total carotenoids and the increase in the ratio of chlorophylls to bulk carotenoids.
Table 2.
Changes in pigment composition referred to total chlorophyll a content for different times of exposure to 5 mM H2O2. Neo: neoxanthin; Vio: violaxanthin; Lut: lutein; Chl b: chlorophyll b; ß-Car: ß-carotene; Chl/Car: ratio total chlorophylls to total carotenoids; SD: standard deviation. Mean values of minimum three independent incubations.
| Pigment ratios referred to Chl a (w/w) | |||||||
|---|---|---|---|---|---|---|---|
| Neo | Vio | Lut | Chl b | ß-Car | Car | Chl/Car | |
| Control | 0,053 | 0,021 | 0,116 | 0,101 | 0,034 | 0,223 | 4,92 |
| SD | 0,004 | 0,004 | 0,001 | 0,017 | 0,001 | 0,008 | |
| 3 h | 0,051 | 0,013 | 0,106 | 0,113 | 0,029 | 0,195 | 5,43 |
| SD | 0,004 | 0,001 | 0,006 | 0,012 | 0,002 | 0,014 | |
| 6 h | 0,047 | 0,010 | 0,097 | 0,109 | 0,029 | 0,180 | 6,04 |
| SD | 0,002 | 0,001 | 0,001 | 0,022 | 0,002 | 0,001 | |
| 12 h | 0,047 | n.d. | 0,106 | 0,134 | 0,028 | 0,181 | 6,26 |
| SD | 0,002 | 0,007 | 0,023 | 0,001 | 0,008 | ||
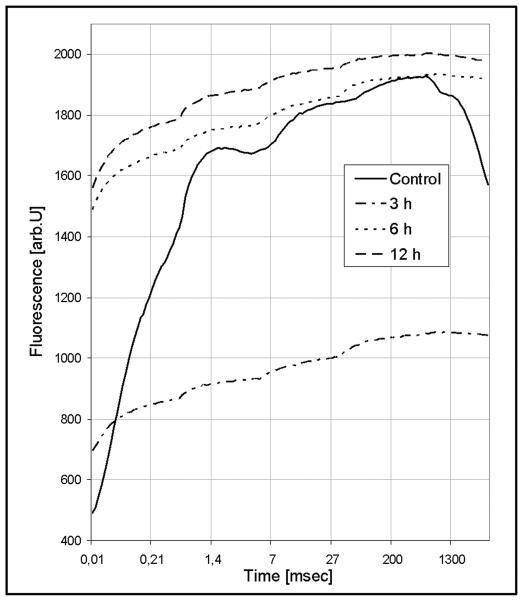
The fluorescence induction curves in Fig. 8 show the typical “O-J-I-P trace” (Strasser et al., 1995) for undisturbed photosystem II activity in controls. However, in all treatments, independent on H2O2 concentration and time, the traces clearly indicate a complete loss of electron transport capability: while the mean value of the ratio Fv/Fm amounts to 0.79 in the control, it is 0.334 for 3 h, 0.209 for 6 h and 0.197 for 12 h 5 mM H2O2 treatment. In addition, the values for the ground fluorescence (= Fo) is low in the control (405, arbitrary units), increases after 3 h to 724, after 6 h to 1530, and after 12 h to 1607. This parameter indicates the loss of thylakoid membrane integrity.
Fig. 8.
Chlorophyll fluorescence induction curves for the 5 mM H2O2 treatment. Arb.u.: arbitrary fluorescence; C: control; 3 h, 6 h, 12 h: duration of treatments.
Discussion
The results of the present study provide evidence that the unicellular green alga Micrasterias denticulata is able to undergo programmed cell death upon experimental induction with hydrogen peroxide, a well known inductor of PCD in plants (Gechev et al., 2006; de Pinto et al., 2006). Several features known to indicate PCD in higher plants or plant cell cultures such as vacuolization, increase in caspase-3-like activity, slight chromatin condensation and particular ultrastructural changes in mitochondria, the endomembrane system, the nucleus and the nucleolus have been found to accompany cell death in Micrasterias whereas other hallmarks commonly linked to PCD, like ladder-like DNA degradation or TUNEL positve staining do not occur. This points towards an individual PCD pathway in the green alga Micrasterias with some similarities to cell death events of higher plants but also to those of other unicellular photosynthetic organisms (for discussion see below).
Treatment with low concentrated H2O2 led to formation of vacuoles which was also observed in tobacco (Mittler et al., 1997) and the dinoflagellate Amphidinium (Franklin and Berges, 2004) during PCD and has been regarded as apoptosis hallmark in these organisms. The same treatment also causes a significant increase in caspase-3-like activity in Micrasterias which can be abrogated by a caspase-3 inhibitor. As the cells stained positively in the FDA vitality assay this indicates an activated metabolism as characteristic for PCD. Similar results have been obtained in the alga Dunaliella where an increase in caspase-like activity occurred during darkness induced PCD (Segovia et al., 2003) and in Peridinium where cystein protease inhibitors blocked PCD evoked by CO2 depletion (Vardi et al., 1999). Additionally Moharikar et al. (2006) have shown the presence of caspase-3-like epitopes in Chlamydomonas. Although an increase in caspase or caspase-like activity is frequently regarded as a hallmark for apoptosis it must be considered that caspases may as well regulate other cell death pathways (van Dorn and Woltering, 2005).
PCD in Micrasterias is also indicated by particular ultrastructural changes in mitochondria and dictyosomes. Such morphological changes in organelles have never been observed in Micrasterias before, although a huge number of inhibitors and other effectors have been used in previous studies (e.g. Meindl, 1990; Höftberger et al., 1995; Salomon and Meindl, 1996; Holzinger and Lütz-Meindl, 2001). However, structural alterations of mitochondria as we found in Micrasterias upon H2O2 induction have been reported also from cells undergoing different forms of PCD in higher plants such as Zea (Gunawardena et al., 2001), Triticum (Virolainen et al., 2002) and in Zinnia (Yu et al., 2002). Elongated cristae were also shown in senescent leaf mesophyll cells of Secale, Cucumis and Pisum (Selga et al., 2005). In neotropical trees two days of anoxia induces almost the same effects as we have observed in Micrasterias cells (Kolb et al., 2004). The shape of mitochondria and the structure of their cristae in Micrasterias cells undergoing PCD are clearly different from those of dead cells. Dictyosomes during PCD in Micrasterias are still active and seem to deliver the entire contents of their cisternae into vesicles. This phenomenon was also observed in primary abscission zones of Phaseolus (McManus et al., 1998) and in ethylene or hypoxia induced “apoptotic” cells of Zea roots where the dictyosome appeared intact with an increased number of secretory vesicles (Gunawardena et al., 2001).
PCD in Micrasterias is accompanied by an increase in the number of ER compartments which is not regarded as a general hallmark for PCD in plants. However, Madeo et al. (1997) observed ER accumulation in a yeast mutant which was supposed to undergo apoptosis. Some studies indicate a role for ER compartments during apoptosis in animal (Häckie et al., 2000) and PCD in plant cells (Zuppini et al., 2004). Also an increased formation of membranous bodies and tonoplast rupture are characteristic for PCD in Micrasterias. Both hallmarks have been also reported from cells of pepper leaves undergoing hypersensitive response (Polverari et al., 2000).
Slight chromatin condensation particularly visible after DAPI staining as well as marked changes in nucleolar structure observed in TEM are further hallmarks of PCD in Micrasterias. However, distinct nuclear and chromatin condensation as revealed in other organisms by DAPI staining (Levine et al., 1996) do only occur in dead Micrasterias cells. The same holds for DNA laddering and TUNEL positive staining. Fragmentation of genomic DNA into discrete fragments of approximately 180 bp, called DNA ladder (Wyllie, 1980) is often used to distinguish necrosis from apoptosis. Whereas a “smear-like” appearance of the DNA on an agarose-gel points to a necrotic event, laddering indicates apoptosis (e.g. Danon et al., 2000; LoSchiavo et al., 2000). In our study, neither different concentrations of hydrogen peroxide, UV-C irradiation, heat treatment nor D-mannose, led to a degradation of the DNA into oligonucleosomes, although these inductors have been found to trigger DNA laddering in other plant species (Danon and Gallois, 1998; Chen et al., 1999; Stein and Hansen, 1999; Tian et al., 2000; LoSchiavo et al., 2000; Houot et al., 2001; Fan and Xing, 2004; Li and Dickman, 2004; Moharikar et al., 2006). In accordance with our results cell death without DNA laddering has also been observed in the unicellular green alga Chlorella after heat stress (Leu and Hsu, 2005). That DNA laddering in principle is possible in Micrasterias has been shown by exposing the cells to extreme conditions by rising the temperature in frozen cells up to 37 – 42 °C. Accordingly, TUNEL positive staining occurs only after incubation with high H2O2 concentrations or prolonged treatment. In both cases FDA assay indicates that the cells are already dead.
In summary we conclude that both DNA laddering and positive TUNEL staining can not be regarded as H2O2 induced PCD hallmarks in Micrasterias. There are some examples, where DNA of cells undergoing PCD even when the cell death pathway is regarded to be apoptotic, did not show an oligonucleosomal ladder on agarose-gels (Collins et al., 1992; Oberhammer et al., 1993). A lack of TUNEL positive staining was also observed during cell death of Dunaliella (Berges and Falkowski, 1998). False positive TUNEL staining has been reported from other organisms and may occur due to non-specific nicking of DNA caused by the preparation (Sloop et al., 1999) or may simply reflect strong nuclease activity without relation to PCD (Fukuda, 2000).
In accordance with results on other photosynthetic organisms (Veldhuis et al., 2001) a decrease in photosynthetic activity was found in Micrasterias cells undergoing PCD as a consequence of H2O2 exposure. By means of fast chlorophyll fluorescence, photosystem II activity expressed as Fv/Fm was shown to be normal in controls and dropped down significantly in all H2O2 treatments. This points towards a complete, irreversible loss of photosynthetic functions. In contrast, pigment composition of H2O2 treated cells was the same as in controls except of a decrease in carotenoids. Obviously, the oxygen-related radicals were not able to affect the bulk of the pigments, probably because violaxanthin and ß-carotene shielded them against further decomposition while being consumed during the defence action. This agrees in part with results by Zuppini et al. (2007) who observed a reduction of carotenoids during PCD of Chlorella which, however, was accompanied by a decrease in chlorophylls as well.
This study shows for the first time that H2O2 induces several structural, physiological and molecular hallmarks in the unicellular alga Micrasterias characteristic for genetically programmed cell death in higher plants and animals. The physiological relevance of PCD in Micrasterias as well as in other eukaryotic algae may lie in an altruistic cell death under unfavourable environmental conditions to allow the whole colony to survive (Welburn et al., 1997; Lee et al., 2002). At their natural habitats, shallow bog ponds, fresh water algae like Micrasterias are exposed to high solar radiation and increased temperature during the growth period which leads to water deficiency and relatively high salt concentrations. Under these conditions PCD of a larger number of cells may be required to guarantee the survival of the population and surviving cells can use dead cells and mucilage produced by them to protect themselves from further environmental impact.
Acknowledgements
We thank Cornelius Lütz for pigment analyses and measurements of the photosynthetic activity as well as for helpful discussions during the evaluation of the data and we are grateful to Ancuela Andosch for her help in cultivating Micrasterias cells. We also gratefully acknowledge funding by the Austrian Science Fund (FWF; grant P18869-B16 to U. L.-M.) and by the Afro-Asiatic Institute.
Abbreviations
- CTAB
cetyltrimethylammonium bromide
- DAPI
4′, 6-diamidino-2-phenylindole
- DMF
dimethylformamide
- FDA
fluorescein diacetate
- HPF
high pressure freezing
- PBS
phosphate buffered saline
- PCD
programmed cell death
- RT
room temperature
- TAE
tris-acetate-ethylenediamine tetraacetic acid
- TEM
transmission electron microscopy
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP nick end-labeling
References
- Aichinger N, Lütz-Meindl U. Organelle interactions and possible degradation pathways visualized in high-pressure frozen algal cells. Journal of Microscopy. 2005;219:86–94. doi: 10.1111/j.1365-2818.2005.01496.x. [DOI] [PubMed] [Google Scholar]
- Ameisen JC. The origin of programmed cell death. Science. 1996;272:1278–1279. doi: 10.1126/science.272.5266.1278. [DOI] [PubMed] [Google Scholar]
- Aranha MM, Matos AR, Mendes AT, Pinto VV, Rodrigues CMP, Arrabaça JD. Dinitro-o-cresol induces apoptosis-like cell death but not alternative oxidase expression in soybean cells. Journal of Plant Physiology. 2007;164:675–684. doi: 10.1016/j.jplph.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Berges JA, Falkowski PG. Physiological stress and cell death in marine phytoplankton: Induction of proteases in response to nitrogen or light limitation. Limnology and Oceanography. 1998;43:129–135. [Google Scholar]
- Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnology and Oceanography. 2004;49:997–1005. [Google Scholar]
- Bidle KD, Falkowski PG. Cell death in planktonic, photosynthetic microorganisms. Nature Reviews Microbiology. 2004;2:643–655. doi: 10.1038/nrmicro956. [DOI] [PubMed] [Google Scholar]
- Casotti R, Mazza S, Brunet C, Vantrepotte V, Ianora A, Miralto A. Growth inhibition and toxicity of the diatom aldehyde 2-trans, 4-trans-decadienal on Thalassiosira weissflogii (Bacillariophyceae) Journal of Phycology. 2005;41:7–20. [Google Scholar]
- Chen H, Yan C, Jiang X, Dai Y-R. Hyperthermia-induced apoptosis and the inhibition of DNA laddering by zinc supplementation and withdrawal of calcium and magnesium in suspension culture of tobacco cells. Cellular and Molecular Life Sciences. 1999;55:303–309. doi: 10.1007/s000180050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RJ, Harmon BV, Gobé GC, Kerr JF. Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. International Journal of Radiation Biology. 1992;61:451–453. doi: 10.1080/09553009214551201. [DOI] [PubMed] [Google Scholar]
- Danon A, Gallois P. UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Letters. 1998;437:131–136. doi: 10.1016/s0014-5793(98)01208-3. [DOI] [PubMed] [Google Scholar]
- Danon A, Delorme V, Mailhac N, Gallois P. Plant programmed cell death: a common way to die. Plant Physiology and Biochemistry. 2000;38:647–655. [Google Scholar]
- Debrabant A, Lee N, Bertholet S, Duncan R, Nakhasi HL. Programmed cell death in trypanosomatids and other unicellular organisms. International Journal for Parasitology. 2003;33:257–267. doi: 10.1016/s0020-7519(03)00008-0. [DOI] [PubMed] [Google Scholar]
- Van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends in Plant Science. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Evans DE. Programmed cell death in response to abiotic stress. In: Gray J, editor. Progammed Cell Death in Plants. CRC Press, Blackwell Publishing; Ohio, U.S.A.: 2004. p. 194. [Google Scholar]
- Fan T, Xing T. Heat shock induces programmed cell death in wheat leaves. Biologia Plantarum. 2004;48:389–394. [Google Scholar]
- Franklin DJ, Berges JA. Mortality in cultures of the dinoflagellate Amphidinium carterae during culture senescence and darkness. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:2099–2107. doi: 10.1098/rspb.2004.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H. Programmed cell death of tracheary elements as a paradigm in plants. Plant Molecular Biology. 2000;44:245–253. doi: 10.1023/a:1026532223173. [DOI] [PubMed] [Google Scholar]
- Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. Journal of Cell Biology. 2005;168:17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssay. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Geitmann A, Franklin-Tong VE, Emons AC. The self-incompatibility response in Papaver rhoeas pollen causes early and striking alterations to organelles. Cell Death and Differentiation. 2004;11:812–822. doi: 10.1038/sj.cdd.4401424. [DOI] [PubMed] [Google Scholar]
- Golstein P, Aubry L, Levraud JP. Cell-death alternative model organisms: why and which? Nature Reviews Molecular Cell Biology. 2003;4:798–807. doi: 10.1038/nrm1224. [DOI] [PubMed] [Google Scholar]
- Gordeeva AV, Labas YA, Zvyagilskaya RA. Apoptosis in unicellular organisms: mechanisms and evolution. Biochemistry (Moscow) 2004;69:1055–1066. doi: 10.1023/b:biry.0000046879.54211.ab. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Gray J. Paradigms of the evolution of programmed cell death. In: Gray J, editor. Programmed Cell Death in Plants. CRC Press, Blackwell Publishing Ltd; Ohio, USA: 2004. p. 1. [Google Scholar]
- Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE. Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.) Planta. 2001;212:205–214. doi: 10.1007/s004250000381. [DOI] [PubMed] [Google Scholar]
- Häcki J, Egger L, Monney L, Conus S, Rosse T, Fellay I, Borner C. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene. 2000;19:2286–2295. doi: 10.1038/sj.onc.1203592. [DOI] [PubMed] [Google Scholar]
- He R, Drury GE, Rotari VI, Gordon A, Willer M, Farzaneh T, Woltering EJ, Gallois P. Metacaspase-8 modulates programmed cell death induced by UV and H2O2 in Arabidopsis. The Journal of Biological Chemistry. 2007 doi: 10.1074/jbc.M704185200. in press. [DOI] [PubMed] [Google Scholar]
- Höftberger M, Url T, Meindl U. Disturbance of the secretory pathway in Micrasterias denticulata by tunicamycin and cyclopiazonic acid. Protoplasma. 1995;189:173–179. [Google Scholar]
- Holzinger A, Lütz-Meindl U. Chondramides, novel cyclodepsipeptides from myxobacteria, influence cell development and induce actin filament polymerization in the green alga Micrasterias. Cell Motility and the Cytoskeleton. 2001;48:87–95. doi: 10.1002/1097-0169(200102)48:2<87::AID-CM1000>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Houot V, Etienne P, Petitot A-S, Barbier S, Blein J-P, Suty L. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose dependent manner. Journal of Experimental Botany. 2001;52:1721–1730. [PubMed] [Google Scholar]
- Kerr JFR, Wylie AH, Currie AR. Apoptosis: a basic biological phenomenon with wilde-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiermayer O. Cytoplasmic basis of morphogenesis in Micrasterias. In: Kiermayer O, editor. Cytomorphogenesis in Plants. Vol. 8. Springer Press, Cell Biology Monographs; Vienna, New York: 1981. p. 147. [Google Scholar]
- Kolb RM, Dolder H, Cortelazzo AL. Effects of anoxia on root ultrastructure of four neotropical trees. Protoplasma. 2004;224:99–105. doi: 10.1007/s00709-004-0048-4. [DOI] [PubMed] [Google Scholar]
- Korthout HAAJ, Berecki G, Bruin W, van Duijin B, Wang M. The presence and subcellular localization of caspase 3-like proteinases in plant cells. FEBS Letters. 2000;475:139–144. doi: 10.1016/s0014-5793(00)01643-4. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death and Differentiation. 2002;9:53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- Leu K-L, Hsu B-D. A programmed cell disintegration of Chlorella after heat stress. Plant Science. 2005;168:145–152. [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Current Biology. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Lewis K. Programmed death in bacteria. Microbiology and Molecular Biology Reviews. 2000;64:503–514. doi: 10.1128/mmbr.64.3.503-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Dickman MB. Abiotic stress induces apoptotic-like features in tobacco that is inhibited by expression of human Bcl-2. Biotechnology Letters. 2004;26:87–95. doi: 10.1023/b:bile.0000012896.76432.ba. [DOI] [PubMed] [Google Scholar]
- Logan DC. The mitochondrial compartment. Journal of Experimental Botany. 2006;57:1225–1243. doi: 10.1093/jxb/erj151. [DOI] [PubMed] [Google Scholar]
- LoSchiavo F, Baldan B, Compagnin D, Ganz R, Mariani P, Terzi M. Spontaneous and induced apoptosis in embryogenic cell cultures of carrot (Daucus carota L.) in different physiological states. European Journal of Cell Biology. 2000;79:294–298. doi: 10.1078/S0171-9335(04)70032-1. [DOI] [PubMed] [Google Scholar]
- Lütz C, Seidlitz HK, Meindl U. Physiological and structural changes in the chloroplast of the green alga Micrasterias denticulata induced by UV-B simulation. Plant Ecology. 1997;128:54–64. [Google Scholar]
- Madeo F, Fröhlich E, Fröhlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. The Journal of Cell Biology. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus MT, Thompson DS, Merriman C, Lyne L, Osborne J. Transdifferentiation of mature cortical cells to functional abscission cells in bean. Plant Physiology. 1998;116:891–899. doi: 10.1104/pp.116.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl U. Effects of temperature on cytomorphogenesis and ultrastructure of Micrasterias denticulata Bréb. Protoplasma. 1990;157:3–18. [Google Scholar]
- Meindl U. Micrasterias cells as a model system research on mohphogenesis. Microbiological Reviews. 1993;57:415–433. doi: 10.1128/mr.57.2.415-433.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl U, Lancelle SA, Hepler PK. Vesicle production and fusion during lobe formation in Micrasterias visualized by high-pressure freeze fixation. Protoplasma. 1992;170:104–114. [Google Scholar]
- Meindl U, Wittmann-Pinegger D, Kiermayer O. Cell multiplication and ultrastructure of Micrasterias denticulata (Desmidiaceae) grown under salt stress. Plant Systematics and Evolution. 1989;164:197–208. [Google Scholar]
- Mittler R, Cheung AY. Biological role of cell death in development and homeostasis. Cell death in plant development and defense. In: Lockshine RA, Zaken A, editors. When Cells Die II. John Wiley & Sons, Inc; Hoboken, New Yersey: 2004. p. 99. [Google Scholar]
- Mittler R, Simon L, Lam E. Pathogen-induced programmed cell death in tobacco. Journal of Cell Science. 1997;110:1333–1344. doi: 10.1242/jcs.110.11.1333. [DOI] [PubMed] [Google Scholar]
- Moharikar S, D'Souza JS, Kulkarni AB, Rao BJ. Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (Chlorophyceae) cells following UV irradiation: detection and functional analyses. Journal of Phycology. 2006;42:423–433. [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooden LD. Plant cell death processes. Elsevier Academic Press; California, USA: 2004. [Google Scholar]
- Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. The EMBO Journal. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel A, Aichinger N, Hochreiter R, Thalhamer J, Lütz-Meindl U. Analysis of mucilage secretion and excretion in Micrasterias (Chlorophyta) by means of immunoelectron microscopy and digital time lapse video microscopy. Journal of Phycology. 2004;40:711–720. [Google Scholar]
- Overmyer K, Brosche M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, Keinänen M, Saarma M, Scheel D, Kangasjärvi J. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 Mutant. Plant Physiology. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pinto MC, Paradiso A, Leonetti P, De Gara L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. The Plant Journal. 2006;48:784–795. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- Polverari A, Buonaurio R, Guiderdone S, Pezzotti M, Marte M. Ultrastructural observations and DNA degradation analysis of pepper leaves undergoing a hypersensitive reaction to Xanthomonas campestris pv. vesicatoria. European Journal of Plant Pathology. 2000;106:423–431. [Google Scholar]
- Pommerville JC, Kochert GD. Effects of senescence on somatic cell physiology in the green alga Volvox carteri. Experimental Cell Research. 1982;140:39–45. doi: 10.1016/0014-4827(82)90153-7. [DOI] [PubMed] [Google Scholar]
- Salomon S, Meindl U. Brefeldin A induces reversible dissociation of the Golgi apparatus in the green alga Micrasterias. Protoplasma. 1996;194:231–242. [Google Scholar]
- Schlösser UG. List of strains. Berichte der Deutschen Botanischen Gesellschaft. 1982;95:181–206. [Google Scholar]
- Segovia M, Berges JA. Effect of inhibitors of protein synthesis and DNA replication on the induction of proteolytic activities, caspase-like activities and cell death in the unicellular chlorophyte Dunaliella tertiolecta. European Journal of Phycology. 2005;40:21–30. [Google Scholar]
- Segovia M, Haramaty L, Berges JA, Falkowski PG. Cell death in the unicellular chlorophyte Dunaliella tertiolecta. A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiology. 2003;132:99–105. doi: 10.1104/pp.102.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selga T, Selga M, Pavila V. Death of mitochondria during programmed cell death of leaf mesophyll cells. Cell Biology International. 2005;29:1050–1056. doi: 10.1016/j.cellbi.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, Rakshit S, Sen T, Majumder HK. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death and Differentiation. 2004;11:924–936. doi: 10.1038/sj.cdd.4401435. [DOI] [PubMed] [Google Scholar]
- Sloop GD, Roa JC, Delgado AG, Balart JT, Hines MO, Hill JM. Histologic sectioning produces TUNEL reactivity. A potential cause of false-positive staining. Archives of Pathology and Laboratory Medicine. 1999;123:529–532. doi: 10.5858/1999-123-0529-HSPTR. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Strasser RJ, Govindjee Greening of peas: parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica. 1999;37:365–392. [Google Scholar]
- Stein JC, Hansen G. Mannose induces an endonuclease responsible for DNA laddering in plant cells. Plant Physiology. 1999;121:71–80. doi: 10.1104/pp.121.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RJ, Srivastava A, Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochemistry and Photobiology. 1995;61:32–42. [Google Scholar]
- Tian R-H, Zhang G-Y, Yan C-H, Dai Y-R. Involvement of poly(ADP-ribose) polymerase and activation of caspase-3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Letters. 2000;474:11–15. doi: 10.1016/s0014-5793(00)01561-1. [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology. 2002;128:1271–1281. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi A, Berman-Frank I, Rozenberg T, Hadas O, Kaplan A, Levine A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Current Biology. 1999;9:1061–1064. doi: 10.1016/s0960-9822(99)80459-x. [DOI] [PubMed] [Google Scholar]
- Vardi A, Schatz D, Beeri K, Motro U, Sukenik A, Levine A, Kaplan A. Dinoflagellate-cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Current Biology. 2002;12:1767–1772. doi: 10.1016/s0960-9822(02)01217-4. [DOI] [PubMed] [Google Scholar]
- Veldhuis MJW, Kraay GW, Timmermans KR. Cell death in phytoplankton: correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. European Journal of Phycology. 2001;36:167–177. [Google Scholar]
- Virolainen E, Blokhina O, Fagerstedt K. Ca 2+-induced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress. Annals of Botany. 2002;90:509–516. doi: 10.1093/aob/mcf221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Lütz C, Lütz-Meindl U. Photosynthesis and heat response of the green alga Micrasterias denticulata (Desmidiaceae) Zeitschrift für Naturforschung. 1999;54c:508–516. [Google Scholar]
- Welburn SC, Barcinski MA, Williams GT. Programmed cell death in trypanosomatids. Parasitology Today. 1997;13:22–26. doi: 10.1016/s0169-4758(96)10076-4. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yamori W, Kogami H, Masuzawa T. Freezing tolerance in alpine plants as assessed by the FDA-staining method. Polar Bioscience. 2005;18:73–81. [Google Scholar]
- Yao N, Tada Y, Park P, Nakayashiki H, Tosa Y, Mayama S. Novel evidence for apoptotic cell response and differential signals in chromatin condensation and DNA cleavage in victorin-treated oats. The Plant Journal. 2001;28:13–26. doi: 10.1046/j.1365-313x.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- Yu X-H, Perdue TD, Heimer YM, Jones AM. Mitochondrial involvement in tracheary element programmed cell death. Cell Death and Differentiation. 2002;9:189–198. doi: 10.1038/sj.cdd.4400940. [DOI] [PubMed] [Google Scholar]
- Zuppini A, Andreoli C, Baldan B. Heat stress: an inducer of programmed cell death in Chlorella saccharophila. Plant and Cell Physiology. 2007;48:1000–1009. doi: 10.1093/pcp/pcm070. [DOI] [PubMed] [Google Scholar]
- Zuppini A, Navazio L, Mariani P. Endoplasmic reticulum stress-induced programmed cell death in soybean cells. Journal of Cell Science. 2004;117:2591–2598. doi: 10.1242/jcs.01126. [DOI] [PubMed] [Google Scholar]